Method Article
Multimodal Study of Murine Cardiovascular Remodeling: Four-Dimensional Ultrasound and Mass Spectrometry Imaging
* These authors contributed equally
In This Article
Summary
Here, we describe a protocol to use in vivo four-dimensional ultrasound imaging and ex vivo mass spectrometry imaging to assess biomechanical and biomolecular alterations in the murine cardiovascular system. This technique is applied to analyze cardiac remodeling in surgically induced myocardial infarction and vascular changes in aging animals.
Abstract
Cardiovascular disease (CVD) is the leading cause of death in the United States. Damage in the cardiovascular system can be due to environmental exposure, trauma, drug toxicity, or numerous other factors. As a result, cardiac tissue and vasculature undergo structural changes and display diminished function. The damage and the resulting remodeling can be detected and quantified with ultrasound (US) imaging at the organ level and mass spectrometry imaging (MSI) at the molecular level. This manuscript describes an innovative methodology for studying murine cardiac pathophysiology, coupling in vivo four-dimensional (4D) ultrasound imaging and analysis with ex vivo matrix-assisted laser desorption/ionization (MADLI) MSI of the heart. 4D ultrasound can provide dynamic volumetric measurements, including radial displacement, surface area strain, and longitudinal strain throughout an entire cardiac cycle. In the vasculature, MSI and ultrasound are used to assess vessel wall compositions, hemodynamics, and vessel wall dynamics. The methodology can be tailored to study a myriad of CV diseases by adjusting functional metrics of interest and/or varying MALDI MSI protocol to target specific molecules. MALDI MSI can be used to study lipids, small metabolites, peptides, and glycans. This protocol outlines the use of MALDI MSI for untargeted lipidomic analysis and the use of ultrasound imaging for cardiovascular hemodynamics and biomechanics.
Introduction
Cardiovascular disease (CVD) is a leading cause of mortality worldwide1. Prevention and treatment of CVD require an in-depth understanding of molecular adaptations to biomechanical forces and the resulting changes in mechanical properties. Throughout the entire cardiovascular system, biomechanical forces play an important role in the function and structure of the tissue2. The mechanical properties of cardiovascular (CV) tissue are influenced by these forces, making them indicators of health and disease3,4,5,6. To prevent, diagnose, and treat CVD, it is crucial to develop methods for understanding and observing the processes of disease initiation and progression. Biomedical imaging has been key in generating physiologic and mechanistic insights, and new imaging technologies and analysis techniques are constantly being developed. This protocol demonstrates a methodology for combining two cardiovascular imaging and analysis techniques to validate the potential for these imaging modalities in ischemic cardiac disease and vascular aging.
Researchers in the biomechanical field often approach the study of biomechanics via a combination of in vivo, ex vivo, and in silico methods. Previous research in molecular biomechanics has focused primarily on proteins7 (particularly extracellular matrix proteins collagen and elastin because of their impact on biomechanical properties), and work to combine in vivo imaging biomechanics with molecular studies has been limited to histology and immunohistochemistry. Although these approaches can yield many molecular indicators and have yielded proposed mechanisms of remodeling of ECM and cells, they are typically limited to the currently available stains or antibodies, respectively. This field of research is missing large classes of molecules, e.g., lipids. While these molecular classes may or may not be mechanistically involved, the resulting molecular adaptations are important to understand because these molecules could be potential targets for both diagnostic markers and therapeutics. Analytical chemistry techniques, such as liquid chromatography-mass spectrometry (LC-MS), can be applied; however, the spatial orientation of the molecules in these techniques is lost. With mass spectrometry imaging (MSI), the spatial distribution of molecules remains intact, and multiple analyte types (classes of molecules) can be imaged with serial sections. MSI is a powerful analytical tool to investigate the spatial distributions of nearly all types of molecules in biological tissue, including metabolites, lipids, glycans, peptides, and small molecular weight drugs8. Matrix-assisted laser desorption/ionization (MALDI) MSI is a type of MSI that is well suited for discovery-based analysis of molecular weights in the range of 50-8000 Da. MALDI-MSI is an ionization technique that applies a laser energy-absorbing matrix to the sample to aid in the ionization of the analytes of interest. This approach prevents being limited to one molecular target and can use bioinformatics tools to determine which molecules have an impact on biomechanical properties and remodeling.
Four-dimensional ultrasound (4DUS) is a non-invasive in vivo method useful for both temporal and spatial characterization of the heart. 4DUS utilizes a series of high frame rate cine loops from different planes, compiling them into a 3D dataset that includes temporal information. This allows for direct visualization and quantification of the complex 3D shape changes of the heart chambers over the cardiac cycle without relying on geometric assumptions as required for traditional 2D echocardiography. 4DUS allows in vivo functional metrics to be calculated from the complex shape and movement of the heart9,10, and MALDI MSI permits the spatial study of biological molecules within the cardiac tissue ex vivo11. To fully understand alterations in the heart with CVD, both mechanical and molecular mechanisms need to be investigated. Thus a combined methodology is proposed for studying murine heart pathophysiology, coupling 4DUS imaging and analysis with MALDI MSI of lipids in the heart. This methodology is demonstrated in a murine model of myocardial infarction.
Vascular biomechanics also play a critical role in regulating cardiovascular function2. Vascular stiffening, which is associated with aging, is a risk factor for CVD12. The biomechanical and hemodynamic changes in the vessels can be imaged using ultrasound. The molecular compositions of the vessel walls are important components of biomechanics and are also exquisitely sensitive to hemodynamic forces. For example, oscillatory wall shear stress has been implicated in atherosclerotic plaque development3. The preliminary data of vessel mechanics and hemodynamics in aged animals will be presented subsequently.
The team is interested in the relationship between biomechanics and molecular composition in various disease states. Preclinical ultrasound imaging and MSI are used to determine the spatial distribution of molecular changes in a tissue and the associated biomechanical changes that occur during disease progression. This report describes these methodologies in detail and presents preliminary data on the heart and the vasculature of the head/neck.
Protocol
The described animal experiments are carried out with the University of Tennessee, Knoxville Institutional Animal Care and Use Committee approval.
1. Ultrasound imaging13
- Setup and animal preparation
- Prior to starting the experiment, check the isoflurane anesthesia level and refill if necessary. Check oxygen tank levels before turning on the gas. Weigh the charcoal and record it on the container.
- Set up the imaging space by placing supplies near the imaging platform. Supplies include precut tape, depilatory cream, gauze, cotton swabs, autoclaved water, corneal lubricant, lubricating gel, rectal temperature probe, electrode leads, ultrasound gel, electrode gel (if applicable), and heat lamp (Figure 1A).
- Turn on the ultrasound machine and initialize the motor (the system is also capable of collecting 4DUS image data). Set up a new experiment on the ultrasound system: select New Study or New Series and fill out mouse information. Turn on the tablet for physiological monitoring.
- Check the stopcock to make sure the flow is open to the induction box and closed to the nose cone. Pre-fill the anesthesia induction box by turning on oxygen to 1 L/min on the vaporizer and turning the dial to 3%-5%.
- Remove the mouse from the housing cage.
- Place the mouse in the induction box until the animal is fully anesthetized with isoflurane between 3%-5%.
- Switch the stopcock to change the flow to the nose-cone on the ultrasound imaging plate. Turn the isoflurane dial between 1%-2%.
- Remove the mouse from the induction box and weigh the animal. Record the animal's weight.
- Place the mouse in the supine position on the imaging plate.
- Apply corneal lubricant to the animal's eyes.
- If fur is present, remove the fur from the imaging area using a commercially available depilatory cream and cotton swabs. Wipe the cream off the skin with water on sponges to remove any excess depilatory cream from the skin and prevent burns.
- Insert the electrocardiography (ECG) leads subcutaneously on either side of the chest and hindlimb, depending on the positioning of the animal.
- Once inserted, hold the ECG leads in place by tape. If ECG leads are not used (i.e., ECG leads are not inserted), monitor ECG via the animal imaging plate.
- For electrical conduction from skin to plate, spot the conducting gel on the plate and hold animal limbs in position.
- Hold the animal limbs in place with either tape or an elastic band placed around the plate and over the limbs (Figure 1B).
- Use lubricating gel to insert the rectal temperature probe and secure the probe with tape.
- Check ECG, respiration, and temperature signals on the tablet. Ensure that the heart rate is ~350-600 bpm, respiration ~50-100 breaths/min, and rectal temperature between 35-37 °C. Adjust isoflurane levels as needed.
- If required, turn on a heat lamp (e.g., 250 W Infra-Red warming bulb)14 and adjust the height to maintain core temperature (Figure 1C).
- Place the heat lamp no closer than 12 inches from the animal. For initial studies to determine the optimal distance between bulb and animal, adjust the height and record the skin temperature using an infrared thermometer, which should not exceed 42 °C15.
- Cardiac 4D ultrasound
- Place the transducer in the holder in a semi-locked position.
- Orient the raised dot on the transducer with the blue dot on the screen. The convention is to place the dot towards the animal's right side.
- Turn the transducer so it is oriented along the sagittal plane of the mouse with a raised notch pointing in the caudal direction.
- Use the lever at the base of the ultrasound plate to tilt the animal. Use the lever at the base of the transducer to adjust the transducer angle.
- Apply a liberal amount of ultrasound gel on the ventral surface of the thoracic cavity for acoustic coupling between the skin surface and the transducer. Ensure there is no remaining fur prior to coupling.
- Lower the transducer to make contact with the ultrasound gel.
- Make micro adjustments with X/Y knobs at the base of the plate, or move the entire plate for large adjustments.
- Ensure that a parasternal long-axis view includes the apex, left ventricular outflow tract, and aorta aligned horizontally on the screen for a more accurate short-axis image (Figure 2A, B).
- Select Name Image in the bottom corner to save the image to the current series.
- Rotate the transducer 90° clockwise to switch to a parasternal short-axis view (Figure 2C).
NOTE: For best 4D image quality, users should try to align the stepper motor along the parasternal long axis of the ventricle which is not always directly parallel to the sternum. The heart/LV often sits at a left downward angle. - Ensure the left ventricle is visible on the right side of the screen and the right ventricle is on the left side of the screen (Figure 2D).
- If the mouse has a stable respiratory rhythm, hit the EKG box in the top left corner of the image to turn on respiratory gating for the 4D image. If not stable, complete this step during post-processing.
- Select the cube in the top left corner of the screen to set up a 4D image.
- Reset the transducer prior to adjusting the Start and Stop positions of the transducer.
- Adjust the Start position to just below the apex and the Stop position to the aortic arch.
- Set Step Size to 0.08-0.13 mm and Frame Rate to 200-300 Hz.
NOTE: Smaller step sizes may provide better image resolution for analysis but will increase acquisition time. Larger step sizes are acceptable if the data is collected with nearly perfect left ventricular alignment and minimal/no re-orientation is needed during analysis. Consider increasing the frame rate when working with animals with higher heart rates (>450 bpm). - Ensure vital signs and EKG signal are consistently stable (>350 bpm and respiration above 50 resp/min) before hitting the scan.
- Once the scan and processing are complete, turn Save EKV/4D data for post-processing and Respiration Gating ON.
- Select Name image in the bottom right and include the mouse ID in the name.
- To check image quality, hit More Controls and select Load into 4D.
- Review each plane view of the heart, making sure the center of the heart does not move throughout the cardiac cycle. Movement of the center indicates variability in EKG and/or respiratory gating and thus complicates the analysis process. Re-adjust monitors and repeat the scan if necessary.
- Cardiac 4D ultrasound analysis
- Navigate to the study browser. Export all B-Mode 2D images to Vevo Lab and Export 4D data as other file type, selecting ".raw" data format to a hard drive.
NOTE: The 4D graphical user interface toolbox used in this work is currently not publicly available. To request access and detailed instructions, reach out to Dr. Craig Goergen, Purdue University. - Accomplish an alternative analysis of 4D data using VevoLab software. To use this framework, export 4D data from the machine to Vevo Lab files.
- Navigate to the study browser. Export all B-Mode 2D images to Vevo Lab and Export 4D data as other file type, selecting ".raw" data format to a hard drive.
- Vascular Imaging
- For B-mode images of the carotid arteries, place the ultrasound transducer parallel to the carotid arteries, near the midline.
- Once the carotid artery is visible, move the transducer superior to find the carotid bifurcation.
- When a clear image of carotid bifurcation is found, adjust the gain setting to 35 dB and capture the B-mode image.
- Angling the transducer to the left or right may improve the image. Adjust the angle of the transducer or stage so the vessel of interest is not directly parallel to the transducer. This allows for the Doppler angle to remain below 60°. Otherwise, a +/- 15° shift may be needed.
- Switch to pulsed-wave Doppler mode to obtain the velocity measurements.
- Place the sample volume in the center of the vessel. Adjust the angle of the cursor so that it is parallel to the carotid artery. Then, hit play to start the velocity measurements and save to record them.
- For the B-mode image of the jugular veins, place the ultrasound transducer parallel to the jugular veins. The jugular veins are anterior and lateral to the carotid arteries.
- Once the jugular vein is located, move the transducer to the location where the internal and external jugular merge. When a clear image is found, adjust the gain setting to 35 dB and capture the B-mode image.
- Switch to the pulsed-wave Doppler mode to obtain the velocity measurements.
- Place the sample volume in the center of the vessel. Adjust the cursor angle so that it is parallel to the jugular vein. Then, hit play to start the velocity measurements and save to record them.
- Vascular ultrasound analysis
- After collecting the B-mode images and pulsed-wave Doppler measurements, measure the diameters and velocities using the software.
- Once the images are loaded into the software, select vascular package.
- Select each side of the vessel to get the full diameter.
- For the carotid artery, make diameter measurements in systole and diastole using the diameter option in the vascular package.
- Make velocity measurements using the velocity option in the vascular package.
- For the carotid artery, select the highest peak for the velocity measurement in systole and the lowest peak for velocity measurement in diastole.
- Use the values of the diameters to calculate the circumferential component of the Green Lagrange strain tensor using the following equation,
 (1)
(1)
where Ds represents the diameter during systole and Dd represents the diameter during diastole. - For the jugular vein, select any points across the cycle to obtain the velocity measurement.
2. Euthanasia and tissue harvesting
- Prepare aluminum foil boats for flash-freezing tissue (Figure 3).
- At the end of a study, euthanize the animal by isoflurane overdose at 5% concentration and either bilateral pneumothorax or cervical dislocation (cardiac study only).
- Using forceps to tent the skin, cut the tented skin with scissors over the neck for vasculature or just below the sternum for cardiac.
- Cut the skin and muscle layers to expose the vasculature or cut through the bone to expose the heart.
- Using blunt dissection with cotton swabs, isolate the heart or vasculature from surrounding tissues, including fat. Be sure to separate the carotid vessel from the nerve. Remove the heart and vessels using surgical tools.
NOTE: Sutures may be used to remove vasculature by suturing proximal and distal before removing the vessels. - Place tissue on a pre-labeled aluminum foil boat and place the boat in liquid nitrogen (Figure 3).
- Store tissue at -80 °C until time for cryosectioning. Transport tissue with dry ice to maintain temperature.
3. Mass spectrometry imaging
- Cryosectioning and optimal cutting temperature (OCT) mounting on glass slides
- Set the cryostat temperature to -25 °C and insert the blade.
- Prepare a metal chuck by applying OCT and allowing it to freeze in the cryostat.
- Fix the base of the heart to the prepared metal chuck using OCT (Figure 4A). Do not allow OCT to touch the sample region of interest because OCT will contaminate the mass spectra (polyethylene glycol [PEG] contamination) and depress lipid signals of interest.
NOTE: Option to perform water mounting to avoid specimen contamination. See the additional protocol below for instructions. - Based on the importance of spatial alignment in this methodology, use the scaled digital rendering of the heart given by the MATLAB GUI to guide sectioning (Figure 4B).
- Count each revolution (10 µm) while sectioning and note the depth in millimeters for each slice to be mounted on the slide.
NOTE: It is necessary to keep track of specific locations in the heart or vasculature for an accurate match to the digital rendering of the ultrasound data provided by MATLAB. - Thaw mount tissue sections (10 µm thick) onto microscope slides.
NOTE: The type of slides will depend on the mass spectrometer used. Each slide includes one section from each region of the heart. A minimum of three slides are required (positive ion mode, negative ion mode, extra). - For n = 1/group, section the heart via the long axis to visualize molecular changes from apex to base.
- For vasculature, embed the tissue in gelatin and flash-freeze before sectioning11.
- Store the slides in slide mailers at -80°C until mass spectrometry imaging experiments.
- If conducting liquid chromatography-mass spectrometry (LC-MS) to further identify and quantify lipids or metabolites, section serial tissue sections of ~60-100 µm and collect them into 0.5-2 mL tubes before freezing.
- Cryosectioning and water mounting tissue on glass slides
- Fill a beaker with high-performance liquid chromatography (HPLC) water and set aside with 5 mL and 1 mL syringes.
- Set the cryostat temperature to -25 °C and insert the blade.
- Place a pair of forceps into the cryostat to cool prior to mounting.
- Draw up 5 mL of HPLC water in a syringe and place it in the cryostat.
- Just before the syringe is fully frozen, empty water onto the metal chuck and allow it to fully freeze.
- Draw up 1 mL of HPLC water and place the syringe in the cryostat.
- After approximately 30-60 s, place a small dot of partially solidified water onto the center of the chuck. Immediately grab the heart with forceps and place it in the dot before it fully freezes. Hold the heart in place until the surrounding water has fully frozen.
NOTE: Heart may be mounted by apex or base, depending on the region of interest. For this study, the base will be mounted with the apex facing out. - Perform the remainder of the steps for this method as those described in step 3.1.
- Matrix application
- Remove the slide from the freezer and place it in a desiccator until the slide is dry.
- Turn on the HTX M3+ sprayer, open the HTX app on the laptop, and select method in the middle of the screen. Standard matrix concentrations can be found in the stored methods (methods on the left side of screen > OI_usermethods > matrix).
- For this work, set the nozzle temperature to 75 °C, the flow rate to 100 µL/min, and the pressure to 10 psi.
- Write the sample name, polarity, matrix, solvent, and concentration down in the lab notebook. Calculate the amount of matrix needed per concentration (e.g., 5 mL at 10 mg/mL = 50 mg of matrix).
- Make solvent (e.g., 70% MeOH).
- For this work, prepare 40 mg/mL 2,5-dihydroxybenzoic acid (DHB) matrix solution for positive mode and 10 mg/mL 9-aminoacridine (9AA) matrix solution for negative mode. Make both matrices with a 70% MeOH solvent. For other common matrices and spray parameters, follow the steps 3.3.2.
- Weigh the matrix and add the matrix to a 15 mL conical tube. Ensure that the amount is close to the required amount calculated in step 3.3.3 but does not have to be exact. Calculate the solvent amount based on the measured mass.
- Add solvent to the conical tube using a pipette.
NOTE: The volume is based on the mass measured in step 3.3.5. - Sonicate the mixture for 10 min. While the matrix is being sonicated, remove the slide from the desiccator.
- Open the sprayer tray, place the slide in the bottom left corner, and tape down the edges.
NOTE: Fold one end of the tape for easy removal. - Select sample spray region. Close the tray.
- Use a syringe and a filter and pour the matrix into the syringe then filter the matrix through the syringe into the vial with the black lid on the left side of the sprayer.
- Place the vial back into its spot on the sprayer and insert the D-line tubing into the vial.
NOTE: Make sure the tube is not touching the bottom of the vial, and is fully immersed in the liquid. - Turn on the inert gas and make sure the gauge on the sprayer reads 10 psi. Press start. Once the sprayer is at the desired temperature, select the flashing start.
- After the spray is complete, open the tray, remove the sample, and place it in the MALDI slide holder or back in the desiccator (Figure 4C).
- Scan the MALDI slide holder and slide on the scanner, or take a picture with a phone. Save the picture on a flash drive for use with MSI.
- Select the appropriate wash or next spray on the sprayer. Move the D-line from the matrix vial into the waste beaker if washing.
- Spray methanol onto the tray and wipe to clean. Turn off nitrogen.
- MALDI MSI
- On the computer, navigate to Synapt, check that the polarity is changed to the mode needed, and switch if not.
- Load the slide in the instrument on the MALDI source and click the load button on the computer using the software.
- Move the image (step 1.2.17) to the Images folder in the Project folder.
- Navigate to HD Imaging and open the previous project (one that uses the same matrix).
- In a laboratory notebook, record mass range, trap and transfer collision energy, laser energy, neg/pos mode.
- In HD Imaging, navigate to the white page icon. Then click the dropdown arrow and select new plate.
- Browse to find the image in Project folder > Images Folder and open it.
- Change plate type to MALDI Standard and define edges of plate (Choose 4 corners that are shown below with red plus signs).
NOTE: The top left box is for navigating to each corner. The top right box is for selecting the corners. The bottom box is just for visualizing the whole slide. - Navigate to the small pattern tab in the top left corner and click pencil or other rectangle or circle options to outline tissue. Outline tissue using left clicks and right click to finish.
- The pixel size defaults to 50 µm. Change if needed.
- Click Save as and navigate to Project Imaging AcqFolder and label as slide name (YYYYMMDD format). Then, save.
- Click the Mass Lynx button in the top middle to export to Mass Lynx.
- Navigate to Mass Lynx software.
- Click on File > open project folder. Edit file name and text name to the slide (YYYYMMDD format).
- Right-click on the MS file, browse and choose the current slide.
- Right-click again on MS file and click Edit. Double left-click on the blue box.
- Navigate to scan conditions. Find source settings and change to user-defined. Then navigate to the correct project folder > acquired folder > current slide > OKAY.
- Save by clicking on Save as and include the slide name.
- Calibrate the instrument using a reference compound (e.g., red phosphorous)
- Navigate to Synapt (Calibrate). Click the play button to start acquiring data. Label as YYYYMMDD_cal1 (ex: 20230614_cal1).
- Make sure it has the same mass range 50 - 2000. Laser energy can be about 175.
- Hit fire laser play, then click the next button. Let it run to about 15 or 20. Click red stop.
- Navigate to W Console.
- Create calibration and click start. Under calibration profile editor, click file > new. Type YYYYMMDD_cal1.
- Edit mass range to desired mass range for the project (e.g., 50-2000).
- Choose manual calibration. Choose resolution mode and click edit. Select red phosphorus as a reference compound.
- Click the raw data file, and scroll to the very bottom (it should be what was just acquired).
- Go to history at bottom, select Acc mass > okay > okay.
- Keep clicking next and okay until the start button is seen, and hit start.
- Navigate to green check and look at what peaks were not found.
- Navigate to Mass Lynx (Start the actual run for experimental samples).
- Hit the play button and check that the number of samples is correct
- Navigate to HD imaging.
- Check to make sure the pixel size is still what was originally set to
- Navigate to Synapt to check polarity.
- Click the camera button to make sure it is moving and firing the laser.
- Check and make sure that it is collecting data. The acquired mass spectrum should be displayed on the Synapt page.
- MS analysis
- Use HD Imaging software and Mass Lynx for analysis.
- Process imaging data using the Process tab in HD Imaging.
- Visualize molecular ion images, also called MS images, in HD Imaging in the Analysis tab. A heat map of a molecular ion image depicts the intensity of that m/z ion over a region (Figure 5). Use the overlays of MS images to show the spatial distribution of molecules (Figure 6 and Figure 7).
- Normalize data using TIC or appropriate normalization for imaging.
- Identify m/z peaks that spatially correlate with regions of interest. Run a spatial correlation (R > 0.65) on HD Imaging to identify co-localized peaks.
- Search for putative lipid identification of these peaks with LipidMaps16 and METASPACE17.
- Confirm lipid annotations with subsequent LC-MS data. Use Metaboanalyst for statistical analysis of LC-MS data.
Results
The imaging protocols described above were used for two preliminary studies: myocardial infarction (MI) remodeling and vascular aging. For the cardiac experiments, a permanent coronary artery ligation surgery was done in order to induce acute myocardial infarction18,19. 4D ultrasound and MALDI MSI were performed progressively on the same tissue, unveiling physiological and molecular changes. Representative molecular ion images in an infarcted heart are shown in Figure 5. The m/z 577.52 has been putatively assigned as Cohibin C or D. Although further analysis will be required for analyte identification (LC-MS or tandem MS), cohibin has been found in bovine hearts20 and could also be indicative of remodeling21,22. Changes in ventricular structure and function can also be associated with up and downregulation of target lipids in the respective region (Figure 6). In the 4DUS image, the green-yellow end of the color spectrum represents tissue with a surface area strain magnitude of less than 20%, which corresponds with infarcted tissue23. The yellow end of the spectrum represents infarcted regions in the MS image as well, corresponding with lipids known to be upregulated in infarcted tissue24. However, the exact location was not co-registered between the US image of the heart and the sectioned tissue. To compare in vivo and ex vivo data, the user must count the number of cryosection revolutions to cut to a specific depth from the apex, as described above. To couple the biomechanical measurements from US to MSI, it is crucial that delocalization of the analytes is minimized in the MSI25. An example of the delocalization of lipids in a long-axis view of the heart is shown in Figure 7.
For vascular aging, two age groups of C57BL/6 mice were studied: young (10-12 weeks) and aged (12 months) for males and females. A one-way ANOVA and Tukey's Honest Significance Difference (HSD) post-hoc test was performed to compare sex and age. All data reported as mean ± standard deviation. Velocity and vessel diameter were measured in the carotid artery and jugular vein. The circumferential strain values were calculated using Equation 1. Representative results are shown in Figure 8. The strain values between young males (n =5) and aged males (n = 5) were not statistically significant and, therefore, were combined as one (n = 10) group to compare to young (n = 10) and aged (n = 10) females (Figure 8A). There was no statistical difference between groups for circumferential cyclic strain (CCS). For the carotid systolic velocity, young females had higher velocities compared to aged females (p = 0.02) and males (p = 0.01, Figure 8B). Figure 8C shows a spatial representation of three different molecules present in the young female C57BL/6 NHsd (Envigo) mouse carotid. Putative assignments for these molecules are heme with m/z 616.18, PC(36:2) with m/z 808.58, and lyso PC(18:0) with m/z 546.35. These lipids are important indicators of CV health, particularly for atherosclerosis: PC(36:2) is increased in atherosclerotic mice26and lysophosphatidylcholines are a phospholipid component of atherogenic lipoproteins27.

Figure 1: Experimental setup. (A) Ultrasound setup with necessary supplies in place before beginning experiments. (B) Animal and electrodes/rectal probe secured in place with tape. (C) Heat lamp used to maintain core body temperature. Please click here to view a larger version of this figure.
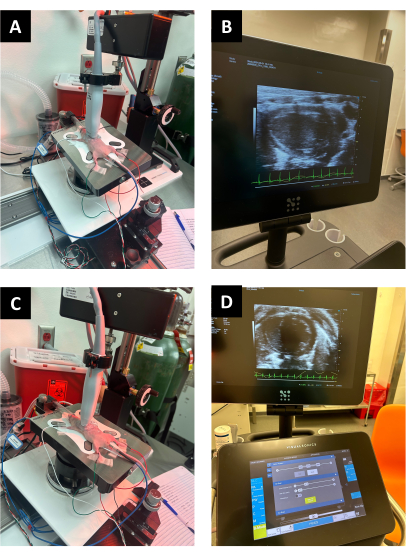
Figure 2: Representative animal set-up. (A,B) PSLAX setup and the respective PSLAX US image, and (C,D) US set-up and US image for the short-axis. The location and orientation of the heart can vary between animals, so adjustments may need to be made. Please click here to view a larger version of this figure.

Figure 3: Flash-freeing using aluminum foil boats. (A) Aluminum (Al) foil boats. (B) Al boats with a heart placed within the boat. (C) Al boats floating on liquid nitrogen. Please click here to view a larger version of this figure.

Figure 4: Cryosectioning and tissue preparation for MALDI. (A) Mouse heart mounted on cryostat chuck with OCT. Apex is embedded in OCT, but other tissue remains free of OCT contaminate. (B) Scaled digital 3D rendering of the left ventricle obtained using the 4D US MATLAB GUI is used to guide sectioning. Scale is in mm. (C) Glass slide with thaw-mounted heart tissue, sprayed with matrix and placed in MALDI slide holder. Please click here to view a larger version of this figure.

Figure 5: Molecule ion image (m/z 577.52) for three short-axis sections of an MI heart in positive ion mode with DHB matrix. The heatmap shows the relative intensity (i.e., abundance) across the tissue sample. For this putative assignment (Cohibin C or D), the analyte has a higher abundance in the left ventricular wall (yellow and red pixels) compared to other regions of the heart. Please click here to view a larger version of this figure.

Figure 6: Ventricle image on the left is from the same heart depicted in the molecular ion image from MSI shown on the right. Note that the 4D US image depicts only the LV, and MS images show a cross-section of the entire heart. Regions imaged with MALDI MSI are labeled A-F. Here, regions B, C, and D were represented. Overlaid heat maps depict up- and down-regulated lipids. Please click here to view a larger version of this figure.
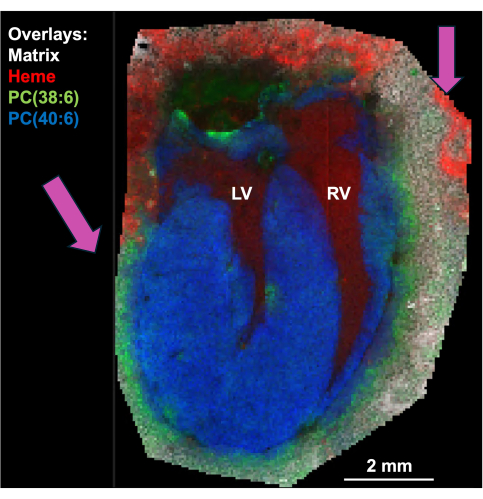
Figure 7: Overlay of four m/z images of a murine heart (matrix, heme, PC(38:6), and PC(40:6)). The pink arrows indicate the delocalization of the heme and PC(38:6) as these analytes overlap with the matrix peak outside of the heart region. Please click here to view a larger version of this figure.

Figure 8: Representative results. (A) Carotid artery circumferential strain between three groups with no statistical difference between means. (B) Carotid systolic velocity was higher in young females compared to aged females (p = 0.02) and males (p=0.01). (C) Molecular overlay of three different molecules in the carotid artery. Red represents heme with m/z 616.18, white represents molecule with m/z 808.58, and purple represents a molecule with m/z 546.35. This image shows the localization of lipids with high signal intensity in the young C57BL/6 female carotid tissue. M: males (combined young and aged), YF: young female, AF: aged female. N=30 Please click here to view a larger version of this figure.
| Problem | Solutions |
| Rolling | Move anti-roll bar down/up |
| Adjust speed of cut | |
| Adjust axis of cut | |
| Hold down anti-roll bar by the handle | |
| Use paintbrush to unravel and then transfer onto slide | |
| Slice is bunching up at blade | Move anti-roll bar up towards blade |
| Slice is not going onto metal tray | Move anti-roll bar down away from blade |
| Slice is sticking to razor/anti-roll bar/metal tray (Condensation) | Leave the lid closed and allow everything to cool. Avoid breathing into the hood if possible (see below) |
| Incomplete profile cut | Increase cut thickness and slice until full profile is obtained then return to original thickness |
| Static | Cut slice, press finger to roll bar then transfer onto slide |
| Freeze artifacts (Tearing) | Increase cut thickness (be sure to note in notebook); Slightly increase temperature of the cryostat. |
| Sample breaks | Prep collection (folded paper or foil) below sample and try to drop sample onto it with cut, do NOT back out of cut as sample may fall. |
Table 1: Common issues and troubleshooting steps for cryosectioning samples.
Discussion
US imaging can be operator-dependent, but the use of anatomical landmarks and adequate training can limit user bias. 2D ultrasound is particularly susceptible to inter-user variability because views are angle-dependent, whereas 4DUS is less susceptible as acquisition encompasses the entire volume and is angle-independent. It was also determined that image reproducibility is easier to achieve because of the adjustable animal platform and transducer holder. US data collection should ideally be conducted by the same researcher throughout a study to prevent technique-derived data alterations.
Maintaining core body temperature is important since changes in temperature can alter cardiovascular hemodynamics and biomechanic measurements28,29,30,31. In addition to the heated plate for imaging, it is also advised to use an external heater, such as a heat lamp, as shown in Figure 1. This heat lamp is adjusted by the user to maintain a rectal temperature of 37 °C.
For ultrasound imaging, study/series naming is important for large datasets. For 4D images, the naming convention should be consistent, and the mouse ID should be included when naming the 4D image prior to saving. Due to the different file types, the 4D image will not automatically be saved with the other images in the study. Therefore, if the mouse ID is not included in the 4D image name, it will be difficult to differentiate which image corresponds to the animal being analyzed. For image analysis, to minimize bias, the researcher can be blinded to animal groups.
Additional resources for ultrasound imaging and analysis can be found on the VisualSonics Learning Hub: https://www.visualsonics.com/learning-hub-online-video-training-our-users
For imaging with a previous version of the Vevo ultrasound system, see the previously published article13.
For tissue harvesting and freezing, the tissue may crack if the aluminum foil boats sink. Be sure to handle frozen samples gently because the frozen samples are very brittle. Do not force the frozen tissue into small tubes. We recommend 50 mL conical tubes for transport and storage. For sectioning the tissue, Table 1 includes modifications that were found to be helpful starting places for troubleshooting. Be sure not to allow OCT to contaminate the section that is thaw-mounted. OCT contains PEG, which is a contaminant in mass spectrometry. When observing the spectrum, a common repeat of 44 Da indicates a PEG contamination. PEG is also in many detergents, so glassware should not be cleaned with detergents and instead be cleaned with ethanol before being autoclaved. While more tedious, water mounting eliminates the limitation of OCT specimen contamination.
For MALDI MSI, the application of matrix is crucial for adequate laser desorption and for minimization of analyte delocalization25. If new matrix protocols are desired, they should be tested before applying to the experimental tissue. Additionally, tissue on the slides can be stained for histology after the MSI data acquisition11 or a multiplex image can be acquired with repeated imaging32.
A limitation of this protocol is the lack of co-registration of the datasets which is the focus of our future work. However, by counting the revolutions in sectioning, the user can determine which slice location corresponds to the functional regions analyzed from the 4DUS, allowing the user to compare mass spectrometry and ultrasound metrics at specific locations in the heart. For this protocol, the goal is to determine the molecular composition (MSI) in locations of the heart that correlate to the changes in functional metrics in the 3D strain data (US). This protocol does not co-register pixel data between the ex vivo and in vivo data because the 4D US provides functional biomechanical data. However, other researchers have begun to develop computational techniques for co-registration of ex vivo imaging with in vivo modalities that provide more molecular information in pixels/voxels such as photoacoustic imaging33, magnetic resonance imaging (MRI)34, MRI with ultrasound35, or positron emission tomography-computed tomography (PET-CT)36,37.
This current protocol could contribute to the identification of molecular biomarkers of disease and associate them with physiological phenomena that result in functional biomechanical changes of the left ventricle. The methodology established here can be tailored to study a myriad of physical phenomena by adjusting functional metrics of interest and/or varying MALDI MSI protocol to target specific molecules. Though lipids were studied in the development of this protocol, the same framework could be used for a multiomic approach, studying proteins, glycans, metabolites, etc. in relation to the physiological and functional changes identified with 4D US imaging and analysis.
In summary, a multimodal imaging protocol was developed to assess cardiovascular function and molecular structure. This technique may allow researchers to use non-invasive in vivo imaging and ex vivo molecular imaging to identify new imaging biomarkers and evaluate novel therapies.
Disclosures
Craig J. Goergen is a paid consultant of FUJIFILM VisualSonics.
Acknowledgements
Allison Jones is supported by the University of Tennessee, Mechanical, Aerospace & Biomedical Engineering Department Graduate Fellowship. Research reported in this publication (Conner Earl) was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health F30HL162452. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Materials
| Name | Company | Catalog Number | Comments |
| 2,5-dihydroxybenzoic acid (DHB) | Supelco, >99.0% (HPLC) | 85707-10MG-F | DHB matrix substance for MALDI-MS; https://www.sigmaaldrich.com/US/en/product/sigma/85707?cm_sp=Insite-_-wimsShippingEmailRecs_wims EmailAPI_wimsGruCrossEntropy-_-wimsEmailAPI10-3 |
| 9-aminoacridine (9AA) | Supelco, ≥99.5% (HPLC) | 92817-1G | 9-Aminoacridine matrix substance for MALDI-MS; https://www.sigmaaldrich.com/US/en/product/sial/92817?srsltid=AfmBOooiQjQ4pWv_XxITkU 4Lkm0UnHXKekGS_ dFl7V40V9QLWoPpNLoc |
| Aquasonic Ultrasound Gel | Parker Laboratories | Parker 01-02 | Ultrasound Gel; https://www.parkerlabs.com/products/aquasonic-100-ultrasound-transmission-gel/ |
| Benchtop Dewar Flasks | ThermoScientific | 4150-2000 | Container for liquid nitrogen; https://www.thermofisher.com/order/catalog/product/4150-4000?gclid=Cj0KCQjwpvK4BhDUARIsA DHt9sQVc2f-NxN04Nb5Mv F6TZ7GLHWWDEeqDYmEvtKJSQ YHDeVgZ9qylvYaAs27EALw_wcB &source=google_shopping&ISO_ CODE=us&LANG_CODE=en&ef_id =Cj0KCQjwpvK4BhDUARIsADHt9 sQVc2f-NxN04Nb5MvF6TZ7GLHWWDE eqDYmEvtKJSQYHDeVgZ9qylvYa As27EALw_wcB:G:s&s_kwcid=AL!3652 !3!716188292869!!!g!2366243726129 !!21787513085!171591181194&ev_chn =shop&cid=0se_gaw_30092024_ PBYTXL&source=google_shopping &ISO_CODE=us&LANG_CODE= en&gad_source=1 |
| Cryostat | Leica Biosystems | CM Series | https://www.leicabiosystems.com/us/histology-equipment/cryostats/ |
| Dessicator | VWR | 89054-052 | https://us.vwr.com/store/product/9104882/desiccator-plastic-ace-glass-incorporated |
| Epredia MX35 Premier Disposable Low-profile Microtome Blades | Fisher Scientific | 3052835 | Cryostat blade; https://www.fishersci.com/shop/products/mx35-premier-disposable-low-profile-microtome-blades/3052835 |
| Falcon 15 mL Conical Centrifuge Tubes | Fisher Scientific | 14-959-53A | Conical Tubes; https://www.fishersci.com/shop/products/falcon-15ml-conical-centrifuge-tubes-5/1495953A?gclid=Cj0KCQjwpvK4BhDUARIsA DHt9sSBcy5n-lhShligJUOX5KKVGn0bt87 8AB2_muOD2PPTue1phpZgeqwa AqgiEALw_wcB&ef_id=Cj0KCQjw pvK4BhDUARIsADHt9sSBcy5n-lhS hligJUOX5KKVGn0bt878AB2_muO D2PPTue1phpZgeqwaAqgiEALw_ wcB:G:s&ppc_id=PLA_goog_20861 45674_81843405034_1495953A__ 386247001345_165426395473886 37329&ev_chn=shop&s_kwcid=AL!4428!3 !386247001345!!!g!856907751004!& gad_source=1 |
| Flex-Tubes Microcentrifuge Tubes | Eppendorf | EP022364120 | Centrifuge tubes; https://www.sigmaaldrich.com/US/en/product/sigma/ep022364120?utm_source=google%2Cgoogle&utm _medium=organicshopping%2Ccpc&utm _campaign=21043330280&utm_ content=&gclid=Cj0KCQjwpv K4BhDUARIsADHt9sTHRD35k CHPtfI2A41axodnMVr6a1eBKk zM4bSUYQAyfEKo3UgTAEQa Ap7wEALw_wcB |
| Gas Nitrogen | Airgas | N/A | |
| Glass microscope slides | Electron Microscopy Sciences | 71873-02 | https://www.emsdiasum.com/positive-charge-microscope-slides |
| Liquid Nitrogen | Airgas | N/A | |
| Mass Spectrometer | Waters | Synapt G2-Si | https://www.waters.com/waters/en_US/SYNAPT-G2-Si-Mass-Spectrometry/nav.htm?locale=en_mkcid=1000251Foodety%3C/a%3E&cid=134740653&bcid= 134528734 |
| Matrix Sprayer | HTX Technologies | M3+ | https://www.htximaging.com/htx-m3-sprayer |
| Methanol (HPLC), >99.9% | Fisher Chemical | A4524 | Methanol; https://www.fishersci.com/shop/products/methanol-hplc-fisher-chemical-9/A4524?crossRef=A4524#?keyword=A4524 |
| Preclinical Ultrasound System | FUJIFILM VisualSonics | Vevo 3100 | https://www.visualsonics.com/product/imaging-systems/vevo-3100; Vevo F2 has replaced the Vevo 3100 in production. System includes isoflurane vaporizer and induction box. |
| Reynolds Wrap | N/A | N/A | Aluminum foil |
| Signagel Electrode Gel | Parker Laboratories | Parker 15-60 | Electrode Conducting Gel; https://www.parkerlabs.com/products/signagel-electrode-gel/ |
| Sterile Lubricating Jelly | Medline | MDS032273Z | Lubricating Gel; https://www.medline.com/ce/product/Sterile-Lubricating-Jelly/Lubricating-Jelly/Z05-PF03664?sku=MDPMDS032273H |
| Surgical instruments: scissors, forceps/tweezers, sutures | Fine Science Tools | 11252-00, 11050-10, 14016-14, 14084-08, 15000-08 | info@finescience.com |
| Surgical Sponges 200 Pack –Gauze Pads Non sterile -First Aid Wound Care Dressing Sponge –Woven Medical Nonstick, Non Adherent Mesh Scrubbing | Medpride | B08RZGQ5GW | Gauze; https://www.amazon.com/Medpride-Surgical-Sponges-200-Pack/dp/B08RZGQ5GW/ref=asc_df_B08RZGQ5GW/?tag=hyprod-20&linkCode=df0&h vadid=693270340506&hvpos= &hvnetw=g&hvrand=960915122 2290977669&hvpone=&hvptwo= &hvqmt=&hvdev=c&hvdvcmdl=& hvlocint=&hvlocphy=9192978&hv targid=pla-1245491514869&psc= 1&mcid=33f4d647c88630c79116 888d565a63b0 |
| Tissue-Plus O.C.T. Compound | Fisher Scientific | 23-730-571 | OCT; https://www.fishersci.com/shop/products/tissue-plus-o-c-t-compound-2/23730571 |
| Wood Handled Cotton Swabs and Applicators | Fisherbrand | 22-363-160 | Cotton swab; https://www.fishersci.com/shop/products/wood-handled-cotton-swabs-applicators-8/p-7146852 |
References
- Tsao, C. W., et al. Heart disease and stroke statistics-2022 update: A report from the American Heart Association. Circulation. 145 (8), E153-E639 (2022).
- Kassab, G. S. Biomechanics of the cardiovascular system: The aorta as an illustratory example. J R Soc Interface. 3 (11), 719-740 (2006).
- Moore, J. E., Xu, C., Glagov, S., Zarins, C. K., Ku, D. N. Fluid wall shear stress measurements in a model of the human abdominal aorta: Oscillatory behavior and relationship to atherosclerosis. Atherosclerosis. 110 (2), 225-240 (1994).
- Nerem, R. M. Vascular fluid mechanics, the arterial wall, and atherosclerosis. J Biomech Eng. 114 (3), 274 (1992).
- Cecchi, E., et al. Role of hemodynamic shear stress in cardiovascular disease. Atherosclerosis. 214 (2), 249-256 (2011).
- Good, B. The influence of blood composition and loading conditions on the behavior of embolus analogs. J Mech Behav Biomed Mater. 140, 105738 (2023).
- Bao, G., et al. Molecular biomechanics: The molecular basis of how forces regulate cellular function NIH public access. Mol Cell Biomech. 3 (2), 91-105 (2010).
- Schwamborn, K., Caprioli, R. M. Molecular imaging by mass spectrometry-looking beyond classical histology. Nat Rev Cancer. 10 (9), 639-646 (2010).
- Soepriatna, A. H., Kevin Yeh, A., Clifford, A. D., Bezci, S. E., O'Connell, G. D., Goergen, C. J. Three-dimensional myocardial strain correlates with murine left ventricular remodelling severity post-infarction. J R Soc Interface. 16 (160), 20190570 (2019).
- Damen, F. W., et al. High-frequency 4-dimensional ultrasound (4DUS): A reliable method for assessing murine cardiac function. Tomography. 3 (4), 180-187 (2017).
- McDonald, R., Poulos, D., Gutzwiller, L., Sheth, R. A., Good, B., Crouch, A. C. A MALDI mass spectrometry imaging sample preparation method for venous thrombosis with initial lipid characterization of lab made and murine clots. J Am Soc Mass Spectrom. 34 (9), 1879-1889 (2023).
- Benetos, A., et al. Influence of age, risk factors, and cardiovascular and renal disease on arterial stiffness: Clinical applications. Am J Hypertens. 15 (12), 1101-1108 (2002).
- Pistner, A., Belmonte, S., Coulthard, T., Blaxall, B. C. Murine echocardiography and ultrasound imaging. J Vis Exp. (42), v2100 (2010).
- Bellantuono, I., et al. A toolbox for the longitudinal assessment of healthspan in aging mice. Nat Protoc. 15 (2), 540-574 (2020).
- Henriques, F. C., Moritz, A. R. Studies of thermal injury: I. The conduction of heat to and through skin and the temperatures attained therein. A theoretical and an experimental investigation. Am J Pathol. 23 (4), 530 (1947).
- Conroy, M. J., et al. LIPID MAPS: Update to databases and tools for the lipidomics community. Nucleic Acids Res. 52 (D1), D1677-D1682 (2024).
- Palmer, A., et al. FDR-controlled metabolite annotation for high-resolution imaging mass spectrometry. Nat Methods. 14 (1), 57-60 (2016).
- Michael, L. H., et al. Myocardial ischemia and reperfusion: A murine model. Am J Physiol. 269 (6 Pt 2), H2147-H2154 (1995).
- Clark, J. E., Flavell, R. A., Faircloth, M. E., Davis, R. J., Heads, R. J., Marber, M. S. Post-infarction remodeling is independent of mitogen-activated protein kinase kinase 3 (MKK3). Cardiovasc Res. 75 (3), 523-530 (2007).
- Hattori, Y., Konno, H., Abe, M., Miyoshi, H., Goto, T., Makabe, H. Synthesis, determination of the absolute configuration of tonkinelin, and inhibitory action with bovine heart mitochondrial complex I. Bioorg Med Chem. 15 (8), 3026-3031 (2007).
- Golam Mostofa, M., et al. CLIP and cohibin separate rDNA from nucleolar proteins destined for degradation by nucleophagy. J Cell Biol. 217 (8), 2675-2690 (2018).
- Chan, J. N. Y., Poon, B. P. K., Salvi, J., Olsen, J. B., Emili, A., Mekhail, K. Perinuclear cohibin complexes maintain replicative life span via roles at distinct silent chromatin domains. Dev Cell. 20 (6), 867-879 (2011).
- Dann, M. M., et al. Quantification of murine myocardial infarct size using 2-D and 4-D high-frequency ultrasound. Am J Physiol Heart Circ Physiol. 322 (3), H359-H372 (2022).
- Kaya, I., Sämfors, S., Levin, M., Borén, J., Fletcher, J. S. Multimodal MALDI imaging mass spectrometry reveals spatially correlated lipid and protein changes in mouse heart with acute myocardial infarction. J Am Soc Mass Spectrom. 31 (10), 2133-2142 (2020).
- Tressler, C., et al. Factorial design to optimize matrix spraying parameters for MALDI mass spectrometry imaging. J Am Soc Mass Spectrom. 32 (12), 2728 (2021).
- Zhang, L., et al. Vascular lipidomics analysis reveals increased levels of phosphocholine and lysophosphocholine in atherosclerotic mice. Nutr Metab. 20 (1), 1-16 (2023).
- Kohno, M., et al. Induction by lysophosphatidylcholine, a major phospholipid component of atherogenic lipoproteins, of human coronary artery smooth muscle cell migration. Circulation. 98 (4), 353-359 (1998).
- Crouch, A. C., Batra, A., Greve, J. M. Hemodynamic response to thermal stress varies with sex and age: A murine MRI study. Int J Hyperthermia. 39 (1), 69-80 (2022).
- Crouch, A. C., Scheven, U. M., Greve, J. M. Cross-sectional areas of deep/core veins are smaller at lower core body temperatures. Physiol Rep. 6 (16), e13839 (2018).
- Crouch, A. C., Castle, P. E., Fitzgerald, L., Scheven, U. M., Greve, J. M. Assessing structural and functional response of murine vasculature to acute β-adrenergic stimulation in vivo during hypothermic and hyperthermic conditions. Int J Hyperthermia. 36 (1), 1136-1145 (2019).
- Crouch, A. C., Manders, A. B., Cao, A. A., Scheven, U. M., Greve, J. M. Cross-sectional area of the murine aorta linearly increases with increasing core body temperature. Int J Hyperthermia. 34 (7), 1121-1133 (2018).
- Clift, C. L., Mehta, A., Drake, R. R., Angel, P. M. Multiplexed imaging mass spectrometry of histological staining, N-glycan and extracellular matrix from one tissue section: A tool for fibrosis research. Methods Mol Biol. 2350, 313-329 (2021).
- Salehi, H. S., et al. Coregistered photoacoustic and ultrasound imaging and classification of ovarian cancer: Ex vivo and in vivo studies. J Biomed Opt. 21 (4), 046006 (2016).
- Verbeeck, N., et al. Connecting imaging mass spectrometry and magnetic resonance imaging-based anatomical atlases for automated anatomical interpretation and differential analysis. Biochim Biophys Acta. 1865 (7), 967-977 (2017).
- Yang, E. Y., et al. Real-time co-registration using novel ultrasound technology: Ex vivo validation and in vivo applications. J Am Soc Echocardiogr. 24 (7), 720 (2011).
- Maris, L., et al. Method for co-registration of high-resolution specimen PET-CT with histopathology to improve insight into radiotracer distributions. EJNMMI Phys. 11 (1), 1-20 (2024).
- Lin, B. J., et al. MSIr: Automatic registration service for mass spectrometry imaging and histology. Anal Chem. 95 (6), 3317-3324 (2023).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved