Method Article
Robotic-based Experimental Procedure for Colorimetric Gas Sensing Development
In This Article
Summary
Here, we present a protocol to develop colorimetric gas sensors using a robotic-based Design-Build-Test-Learn (DBTL) approach. This protocol integrates high-throughput automation, machine learning, and multi-objective optimization to efficiently discover and optimize sensor formulations for detecting gases like CO2, enabling rapid, cost-effective, and precise sensor development.
Abstract
This paper presents a robot-based experimental program aimed at developing an efficient and fast colorimetric gas sensor. The program employs an automated Design-Build-Test-learning (DBTL) approach, which optimizes the search process iteratively while optimizing multiple recipes for different concentration intervals of the gas. In each iteration, the algorithm generates a batch of recipe suggestions based on various acquisition functions, and with the increase in the number of iterations, the values of weighted objective function for each concentration interval significantly improve.
The DBTL method begins with parameter initialization, setting up the hardware and software environment. Baseline tests establish performance standards. Subsequently, the DBTL method designs the following round of optimization based on the proportion of recipes in each round and tests performance iteratively. Performance evaluation compares baseline data to assess the effectiveness of the DBTL method. If the performance improvement does not meet expectations, the method will be performed iteratively; if the objectives are achieved, the experiment concludes. The entire process maximizes system performance through the DBTL iterative optimization process.
Compared to the traditional manual developing process, the DBTL method adopted by this experimental process uses multi-objective optimization and various machine learning algorithms. After defining the upper and lower limits of component volume, the DBTL method dynamically optimizes iterative experiments to obtain the optimal ratio with the best performance. This method greatly improves efficiency, reduces costs, and performs more efficiently within the multi-formulation variable space when finding the optimal recipe.
Introduction
The practical applications of gas sensors are very extensive and have been used in various fields such as environmental monitoring, aerospace, and waste gas treatment1,2,3. The working principle of gas sensors typically relies on multiple mechanisms, such as electrochemistry, gas chromatography, and optical. Among many detection mechanisms, one based on color change has evolved into an acid-base mechanism that stands out uniquely. Due to its low cost and simple application, it is widely used in the design of many portable and disposable gas sensors, such as CO2 sensors1,4,5. This type of sensor uses the color change of certain chemicals to detect gas concentrations. When the gas concentration changes, it causes the sensor material to experience chemical reactions such as ionic complexation or indicator color changes, leading to the change in color of the gas-sensitive dye6. By detecting and analyzing the changes in color, the gas concentration can be measured indirectly. Meanwhile, despite the advantages of low cost and portability, this type of sensor still has some shortcomings, such as a long development cycle and low efficiency7,8,9. At the same time, traditional methods of sensor design struggle to meet multiple sensing characteristics simultaneously, such as achieving the required response time, reversibility, and detection limit. Under the traditional research and development paradigm, these difficulties severely hinder the production and widespread application of colorimetric gas sensors.
In response to the above-mentioned challenges in on-demand research and development, the colorimetric sensor technology developed through this experimental process can address some of the shortcomings of traditional gas sensing. By employing an iterative Design-Build-Test-learning (DBTL) approach10,11, the efficiency of sensor development can be significantly improved, thereby reducing the research and development time and effectively meeting the needs of the research and development1,12. In a typical DBTL development setup, the development of new materials is taken as an iterative feedback loop. The loop contains four key steps: 1. Design of the optimization parameters, targets, and sample the parameter space for a trial experiment; 2. Build the samples of the selected parameters; 3. Test the target value for the built samples; 4. Machine Learning analysis of the target feedback to guide the selection of next batch parameters. In this iterative process, the high-throughput experiment platform that allows for fast building and testing of samples, and the machine learning algorithms are the key components. The automated high-throughput testing platform can simultaneously test up to 384 sensing units, collecting a large amount of high-quality response data. By utilizing machine learning algorithms13,14,15,16,17, such as multi-objective Bayesian optimization, multiple sensing metrics of the sensing units (e.g., sensitivity, response time, and reversibility) can be simultaneously and automatically optimized, thereby improving the overall performance of various sensing characteristics. The sensing unit recipes generated by the optimization algorithm can achieve quantitative CO2 concentration detection without individual calibration, and the root mean square error (RMSE) metric can also meet the required indicators.
Our program is an experimental procedure developed based on colorimetric gas sensing (see Figure 1 for the flowchart). With the development of self-driven labs, the automated DBTL approach has shown excellent prospects due to its high efficiency, speed, and repeatability5,12. The traditional manual development process involves the adjustment of one variable at a timeThe traditional manual development process involves adjusting one variable at a time, followed by modifying another variable to optimize the target parameter and achieve the desired outcome. The primary drawbacks of this process include low efficiency in manual experiments, susceptibility to human error, difficulty in managing multi-dimensional variables in complex high-dimensional scenarios, and a tendency to get stuck in local optima. Compared to the manual development process, the DBTL method adopted in this experimental program uses robotics combined with advanced active learning algorithms such as multi-objective Bayesian optimization. Bayesian optimization is a probabilistic approach for optimizing expensive-to-evaluate objective functions15,18. It builds a surrogate model, often a Gaussian process, to approximate the objective function and uses an acquisition function to decide the next point to sample. The acquisition function balances exploration (searching less-sampled regions) and exploitation (refining known high-performing regions) to efficiently find the global maximum or minimum. This method is particularly useful in high-dimensional, non-convex search spaces where traditional optimization techniques struggle. After roughly defining the upper and lower limits of component content, it dynamically optimizes experiments to obtain the optimal ratio with the best performance iteratively. This method greatly improves efficiency and reduces costs and performs more efficiently within the multi-variable space for developing the optimal recipe5,12.
The overall goal of this article is to establish an experimental procedure based on the automated DBTL method through various computer technologies such as machine learning, multi-object Bayesian optimization, and experimental testing platforms, including the automated liquid handling platform and high-throughput gas testing platform. This will enable the design and research of colorimetric gas sensors. The customized "Opentrons OT-2" liquid handling robot platform is used to complete experiments according to program settings, automatically carrying out steps such as recipe synthesis, mixing, and dipping. The homemade high-throughput gas testing platform is used for gas testing and colorimetric sensor reading in a high-throughput manner, precisely controlling the concentrations of target gases and recording the color changes of the sensing units in real time. Compared with other experimental systems designed based on DBTL, this system has a relatively low hardware cost. Simultaneously, we have partially addressed the aspects of the task that involve human error through a semi-automated approach. providing the maximum marginal benefit while retaining the advantages of DBTL design.
Protocol
1. Preliminary experiment (feasibility test)
NOTE: Based on Zhang's paper8, the relevant variables of chemical colorimetric sensors for the target gas, such as carbon dioxide, can be selected. Before performing the on-demand optimization of the colorimetric sensor formulations, a preliminary experiment using the following procedures can be conducted to establish the variable space.
- Determine the concentration range of the target gas and establish a gas test configuration.
NOTE: The concentration of the target gas within the gas test configuration increases linearly or exponentially. - Before and after the flowing of each concentration of the target gas, purge the testing system with nitrogen. Keep the ratio of the flowing time for nitrogen to the target gas at 1:1.
NOTE: If the concentration of the target gas is <1 ppm, the flowing time is ~10 min; if the target gas concentration is ≥1 ppm, the flowing time is ~5 min. - Prepare the source solutions of variables with solution-appropriate concentrations based on factors such as saturation and viscosity.
NOTE: It is generally advisable to maintain the highest possible concentration of the source solutions. - Set the total volume of the solution of the colorimetric sensor formulation to 400 µL; then, set the volume range for each source solution of the variables according to the literature.
NOTE: The volume ranges for the source solutions of dyes are generally between 0 µL and 200 µL, while the volume ranges for other source solutions are generally between 0 µL and 100 µL. The source solution sampling interval is ~25 µL. - Generate a batch of 96 formulations through random sampling functions to verify the feasibility of detecting the target gas using chemical colorimetry.
- Load the colorimetric sensor formulation file, source solution, tips, 96-well plate, and PTFE membrane into the liquid handler,and sequentially generate independent identity information numbers (Figure 2).
- Set the liquid handler to simulation mode to mimic the operation of synthesizing colorimetric sensor formulations, such as liquid aspirating, dispensing, shaking, and dripping (Figure 3).
- If there are no errors in the simulation state, set the liquid handler to the experiment and start automating the synthesis of colorimetric sensors.
NOTE: The code for automating the process is developed based on the "Opentrons" open-source software package. The synthesis of colorimetric sensors is expected to take 3-6 h. - Place the colorimetric sensors in an oven at 40 °C and heat for 50 min.
- Place the dried colorimetric sensors in the gas chamber and check the uniformity of the lighting in the testing environment and the airtightness of the testing chamber. Once confirmed to be without issues, use the Mass Flow Controllers (MFC) to automatically control the flow rates of the analyte gas (with concentration ca) and nitrogen (concentration cn), executing the gas testing configuration. Suppose the overall gas flow rate is S in volume/min, and the target analyte concentration is c. the flow rates for the analyte gas MFC and nitrogen MFC in volume/min are
 (1)
(1)
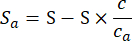 (2)
(2) - During the flowing process, positiona camera above the gas chamber to take a photo every 5 s to record the color changes of the colorimetric sensors (Figure 4).
NOTE: The flowing test is expected to be completed in approximately 2 h. - Note that the computer loads the captured images in chronological order, extracts the RGB color values from each colorimetric sensor in each image, and then calculates the differences relative to the baseline color measured prior to exposure to the target gas, thereby plotting a color variation figure of the colorimetric sensors over the flowing time. The formula for calculating the color difference ΔE is shown as Eq (1):
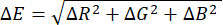 (3)
(3) - Observe whether there are colorimetric sensors exhibiting significant color changes in the target gas and whether the color change values increase in conjunction with an increase in the target gas concentration. If so, the feasibility of using chemical colorimetric sensors to detect the target gas is verified.
- Based on the pre-experimental results, adjust the upper and lower limits of source solutions of the variables and remove source solutions that have an insubstantial effect.
2. Utilize a robotic experimental platform to conduct the Design-Build-Test-Learn (DBTL) iterative optimization process
- Design: Set up multi-objective functions and generate colorimetric sensor formulations.
- Set up a multi-objective function to calculate the weighted evaluation score for multiple figures of merit of the colorimetric sensors (Figure 5).
NOTE: The detail of the weighted evaluation score can be found in the reference1. - If the DBTL optimization is in the initial round, then randomly generate 96 initial colorimetric sensor formulations and create a campaign ID for this optimization task.
- If the DBTL is in the second or later rounds, design the next batch of 96 colorimetric sensor formulations using different acquisition functions (such as Upper Confidence Bound, Probability of Improvement, and Expected Improvement). Additionally, fine-tune the hyperparameters of the acquisition function in each round. Generally, κ as the hyperparameter for UCB, with a value not exceeding 5 and
 serves as the hyperparameter for EI and POI, which gradually decreases to near 0 as the optimization iterations increase. The following three equations (4), (5), and (6) respectively illustrate the acquisition functions for UCB, EI, and POI:
serves as the hyperparameter for EI and POI, which gradually decreases to near 0 as the optimization iterations increase. The following three equations (4), (5), and (6) respectively illustrate the acquisition functions for UCB, EI, and POI:
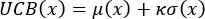 (4)
(4)
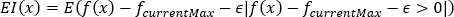 (5)
(5)
 (6)
(6)
Where μ(x) is the mean; σ(x) is the variance; κ, are parameters that balance exploration and exploitation, controlled by the iteration round and decay rate; f(x) is the weighted evaluation score; fcurrentMax is the current highest weighted evaluation score.
are parameters that balance exploration and exploitation, controlled by the iteration round and decay rate; f(x) is the weighted evaluation score; fcurrentMax is the current highest weighted evaluation score. - After generating colorimetric sensor formulations in each non-initial round, observe whether these colorimetric sensor formulations exhibit high expectations or high uncertainty. If not, repeat the operation of step 2.1.3. If so, perform the build step.
- Set up a multi-objective function to calculate the weighted evaluation score for multiple figures of merit of the colorimetric sensors (Figure 5).
- Build: Automatically synthesize colorimetric sensors. See details in steps 1.7-1.10.
- Test: Let the automated testing of colorimetric sensors proceed. See details in steps 1.11-1.13.
- Learn: Fit Surrogate model
- Use the colorimetric sensor formulation variables and their weighted evaluation scores as the input and output of the surrogate model, respectively.
- Use a Gaussian process regression with the mean function for k(xi, xj) and the covariance function (kernel) k(xi, xj) to fit the surrogate model.
NOTE: Figure 6 shows the surrogate model for one- and two-dimensional variables. In the developed Gaussian process fitting algorithm, an anisotropic Matérn kernel combined with a white noise kernel (see following two equations, Eq (7) and Eq (8)) is chosen to ensure the generality of the kernel function.
 (7)
(7)
 (8)
(8)
- Optimization termination criteria: Note that the DBTL optimization stops when the number of iterations reaches a preset value, or when there is no significant improvement in the weighted scores of the generated colorimetric sensors.
3. Construction and characterization of the optimal colorimetric sensor array
- For the six concentration intervals of the target gas, carry out n DBTL optimization campaigns to discover six globally optimal or quasi-optimal colorimetric sensor formulations. Within each concentration interval, the optimized colorimetric sensor formulation exhibits the highest weighted evaluation score in the target gas testing.
- Utilize the liquid handler to construct approximately the colorimetric sensor arrays composed of the six optimized colorimetric sensor formulations. Steps 1.7-1.10 detail the specific operation using the liquid handler.
- Shelf-life testing:
- Construct 14 colorimetric sensor arrays and divide them into two groups. Store one group in an open condition at 25 °C and the other in a vacuum.
- Maintain consistent testing conditions and perform daily response tests over a 7 day period to evaluate the impact of the two storage conditions on the performance of the colorimetric sensor array, thereby inferring the shelf life under both storage strategies.
4. Calibration of the colorimetric sensor array
- For colorimetric sensor array construction, see details in steps 3.1-3.2.
- Data sampling:
- Select 5-10 concentration values at equal intervals for each concentration range of the target gas, with a total of no less than 20 different concentrations.
- Expose the colorimetric sensor array to the target gas according to the concentration value in ascending or descending order and record the response values of the colorimetric sensor array.
NOTE: Five to 10 CO2/N2 cycles were needed for each CO2 concentration.
- Sensing data recording: After exposing to the target gas at various concentrations for 5 or 10 min, extract R, G, and B channel values of n colorimetric sensors from the colorimetric sensor array as features to input into the calibration model. The feature vector contains 3 × n dimensions.
- Dataset division: Based on the number of concentrations of the target gas, divide the response data at different concentrations into the training set, validation set, and test set in a 7:1.5:1.5 ratio.
- Model training: First, use the Python packages, such as scikit-learn and torch, to construct four machine learning regression models for calibration: Ridge, Random Forest, Xgboost, and Deep Neural Network. Then, set the root mean square error as the loss function. Finally, load the dataset and start training the calibration model.
- Model selection: Validate the performance of the four machine learning regression models using the validation set and select the model with the smallest root mean square error as the final calibration model.
- Model testing: Use the test set to perform a performance test on the final calibration model, evaluating the performance of the colorimetric sensor array and the calibration model for quantitative analysis of the target gas.
Results
A typical example of this experimental setup is the "Wide-Range High-Sensitivity Colorimetric CO2 Sensor Array"12. First, the experiment generates a chart based on the change in ΔE over time at a fixed CO2 concentration after Bayesian multi-objective optimization (Figure 7A). Based on its poor response time, ΔE, and reversibility, unnecessary 1 (slow response time), unnecessary 2 (non-responsive), and unnecessary 3 (baseline drifting) should be eliminated by the multi-objective Bayesian optimization function where the goal is set to achieve the recipe of highest sensitivity satisfying the required response time. However, if the objective values are directly added together, as displayed in the left part of Figure 7B, the differences between unwanted samples and preferred samples are not distinct, which may delay the algorithm-guided optimization. The sigmoid method was hence applied in the development of the sensor to prioritize balanced performance across the different objectives. The sigmoid function is used as a smooth translation mechanism to evaluate and filter sensor recipes. This function ensures that the score for any target characteristic (e.g., response time, reversibility, or ΔE) approaches 0 when below a defined threshold and approaches 1 when above it. By multiplying the scores for all targets, the final weighted score significantly decreases if any characteristic fails to meet the threshold, effectively filtering out suboptimal recipes regardless of strong performance in other areas. The final scores after analysis were plotted on a chart (Figure 7B), ultimately verifying the better ratio "preferred" and completing the screening optimization in each round. Due to this sensing, units demonstrate the best sensitivity while responding under 100 s.
After several rounds of optimization and screening, the overall score of the overall score of the optimized recipe significantly improved at a fixed CO2 concentration, greatly enhancing the colorimetric sensing performance (Figure 7C). After four iterations, the highest score is likely to remain essentially unchanged, indicating that the quasi-global maximum has been reached. At this point, selecting the top six formulations with the highest scores in each CO2 concentration interval can form the optimal product array. By optimizing and combining the optimal product array, a wide-range, high-sensitivity colorimetric gas sensing array can be realized.
Finally, to evaluate the sensitivity of the target sensing array, take the average RGB value of all sensing units in each identical formulation. Then, connect the average RGB values of the six sensing formulations in the format of a response vector. To predict the CO2 concentration reversibly from the response vector, fit the data using a Ridge regression model. Ultimately, it is possible to calculate that the mean absolute error of carbon dioxide concentration can reach 0.27% (Figure 7D).

Figure 1: Flowchart. The DBTL cyclical experiment begins with parameter initialization and setting up the hardware and software environment. The baseline test establishes performance standards. Subsequently, the DBTL method identifies the real performance of the different sensor recipes through testing and guiding the optimization adjustment of memory layout. Performance evaluation compares baseline data to assess the effectiveness of the DBTL method. If the performance improvement does not meet expectations, the method is readjusted and iterative testing is conducted; if the target is achieved, the experiment ends. The entire process is optimized iteratively to find the globally best recipe. Please click here to view a larger version of this figure.
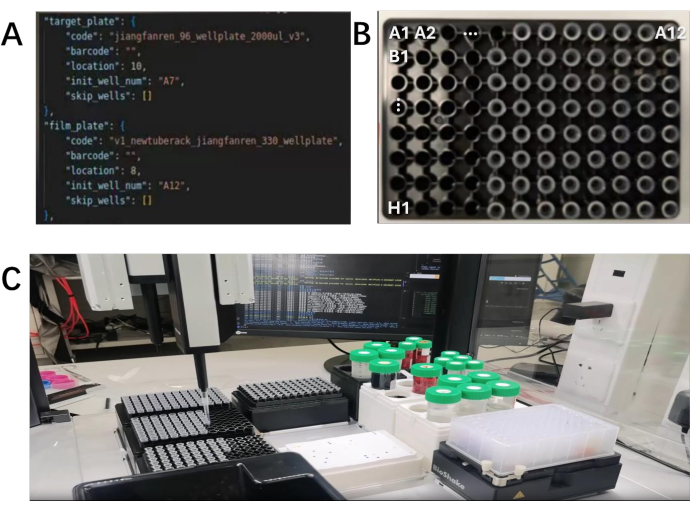
Figure 2: Parameter adjustment and experiment scenario for experimental preparation. (A) Coordinate settings: The positions of tip cartridges with different calibers are set at coordinates 2, 3, 6, and 9. The variable `location` is used to record the different needle tray positions. The coordinate positions of the needle trays are divided into X and Y axes. The variable "init_well_num" is used to record the coordinate positions of the needle trays. (B) The tip cartridges are placed at the top left corner position of the liquid handler, the X-axis is divided into 1-12, and the Y-axis is divided into A-H. When recording coordinates, the Y-axis coordinate is recorded first, followed by the X-axis coordinate. (C) Simulation Experiment: No actual liquid extraction is performed. Check if the configuration file operates normally, and if there are any bugs in communication, database, or errors in the Log. Please click here to view a larger version of this figure.

Figure 3: Pipetting operation. (A) Liquid aspiration: Using the robotic arm with a tip cartridge, aspire the required solution from the source solution tube located at the pre-set coordinate position. (B) Mixing: The deep well plate is shaken at a pre-set frequency to mix the extracted solution evenly. (C) Dispensing: After mixing, dispense the solution from the small well into pre-defined spots on the testing Polytetrafluoroethylene membrane, repeating the process for all 96 wells. Please click here to view a larger version of this figure.

Figure 4: Recording of experimental data and box selection of dipping spots. (A) Membrane Number and gas test ID, which include date\timing information of the gas test: To ensure traceability and accuracy of experiments, create a list containing the venting codes and membranes used in recent experiments for subsequent analysis. (B) Box Selection: The left half of the figure is the area without samples; the right half of the figure is the area on the membrane with colorimetric sensors. During the process of selecting the box, it is crucial to ensure comprehensive coverage of each sensing point to guarantee the precision of the sensor. After completing the box selection, the system will automatically take a photo and perform subsequent colorimetric analysis. Please click here to view a larger version of this figure.

Figure 5: Configuration of the multi-objective function. The system generates a new solution recipe based on Bayesian multi-objective optimization and displays the identity information of the new recipe. Dynamically adjust the amount required for each recipe based on the upper and lower bounds set in the configuration file to prepare for the next iteration cycle. Please click here to view a larger version of this figure.
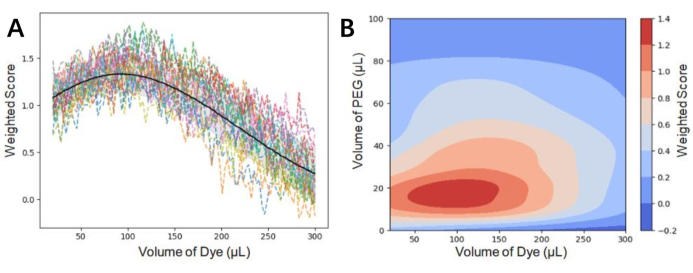
Figure 6. Visualization of The Surrogate Model. (A) The surrogate model for a one-dimensional variable (here, the volume of dye) with other variables held constant. (B) The surrogate model for two-dimensional variables (here, the volume of dye and the volume of polyethylene glycol) with other variables remaining unchanged. Abbreviation: PEG = polyethylene glycol. Please click here to view a larger version of this figure.

Figure 7: Data analysis for an example usage of the setup to develop wide-range CO2 sensors. (A) Response curves of a preferred sensing unit and three suboptimal sensing units within a certain CO2 concentration range. The grey curve is the CO2 concentration at different times and the colored curves are the response color changes. (B) Scores for the four objectives (Sensitivity, Reversibility, Response Time, and Response Intensity) of the preferred sensing unit and three suboptimal sensing units before and after using the multi-objective function. After Sigmoid multi-objective function, the preferred recipe was separated from unwanted recipes with regard to the objective function. (C) Score distributions of sensing units' scores within five rounds of Bayesian optimization experiments for the 0.04%-0.2% CO2 concentration range. The algorithm recommended recipes' target values increased showing the performance increase for the sensing units. (D) Calibration curve of a single sensor and its test data. This figure was taken from Chen et al.1. Please click here to view a larger version of this figure.
Discussion
This article proposes an experimental design that can develop colorimetric gas sensors more quickly and accurately. This experimental process can be used to develop colorimetric sensors for various gases, such as humidity, CO2, and ammonia1,4,5. Through the method of this platform, it can meet the needs of users with various preferences, such as high sensitivity, low detection limit, required response time, considering the presence of interfering gases, humidity, and other different experimental scenarios. It dynamically adjusts the tunable parameters of sensor recipes to meet the prerequisite target requirements. For example, when designing target sensors that require higher sensitivity and reversibility, the weight parameters of sensitivity and reversibility in the sensing performance can be increased during each round of machine learning screening for the optimal ratio, thereby achieving on-demand research and development optimization. The performance of sensors is often related to whether we can find the globally best recipe in the multi-dimensional search space. Conventional experimental designs require long experimental optimization cycles, large manual workload, and introduce large errors19. However, the design of colorimetric gas sensors has rigorous requirements for experimental efficiency and accuracy, so experimental designs for high-dimensional parameter space gases are considered significant obstacles to such experiments20,21.
This experiment addresses this obstacle by utilizing an automated experimental platform, integrating functions such as automated liquid handling, program-controlled mass flow controllers, and high-speed cameras, allowing for the preparation and testing of up to 96 sensing units in a single batch. Through this automated experimental platform and the DBTL method, through multiple rounds of cyclical iterations, the dosage ratios of various source solutions for gas sensor formulations were gradually adjusted to global optima within the multivariate space, achieving multi-objective global optimization. This significantly improved the quality of experiments, efficiency of research and development, and shortened the optimization cycle12.
For different colorimetric gas sensor designs, targeted design is needed in the pre-experimental stage at step 0. Variables that may affect the experimental results need to be screened to identify which source agents has strong impacts on the colorimetric effect for subsequent experiments.
In addition, the experimental content can be modified to design colorimetric liquid sensors. For example, according to the properties of the target liquid, suitable indicators, solvents, and other additives; the gas testing platform can be re-engineered to adapt to the liquid environment for testing, developing new testing protocols and equipment to simulate the liquid environment in practical applications; selecting appropriate substrate materials, such as porous films or special coatings, to enhance absorption and reaction with the liquid22,23. After adjusting the testing platform and experimental process, development experiments for colorimetric liquid sensors can be conducted.
The advantages of using the DBTL loop primarily lie in saving time, offering a broader detection range, and achieving higher sensitivity. In the machine learning ('L' in DBTL) phase, the integration of machine learning algorithms for parameter navigation enables this development approach to effectively discover valuable information in high-dimensional spaces, thereby improving the efficiency of sensor development24,25. Additionally, the DBTL loop can optimize the performance of multiple sensor units simultaneously, with each unit optimized for specific concentration intervals, thus achieving a broader detection range and higher sensitivity. Multi-objective dynamic optimization in multi-parameter dimensions also helps improve the overall performance of the sensor, avoiding falling into local optima5,26.
The DBTL approach is not limited to the development of colorimetric gas and liquid sensors. With sufficient funding, the above methods can also be extended to the research and development of other materials, such as new drug synthesis and high-value materials27,28,29. For different material properties and application requirements, modify the specific processes and details of the experimental platform (build) and (test) parts. After adjusting the optimization algorithm and parameter settings, it is possible to conduct research and development for other high-value materials. Further, its potential applications extend to a wide range of scientific fields. For example, in the field of mechanical structure design, the DBTL approach can be transformative, especially for optimizing complex structures such as lightweight components for aerospace, automotive, or robotics applications. The integration of automation and machine learning ensures high-throughput experimentation and data-driven decision-making, significantly reducing the time and cost associated with traditional R&D processes. By adapting the design, build, and test components to the specific requirements of various fields, the DBTL methodology offers a versatile and transformative approach for advancing innovation across disciplines.
Disclosures
The authors have no conflicts of interest to declare.
Acknowledgements
This work is supported by the Natural Science Foundation of Zhejiang Province (LQ24F040006) and startup fund of Shenzhen University of Advanced Technology.
Materials
| Name | Company | Catalog Number | Comments |
| 96-Well Deep Well Plate | NEST | NEST 2 mL 96-Well Deep Well Plate, V Bottom | |
| 96-Well PCR Plate | NEST | NEST 0.1 mL 96-Well PCR Plate | |
| cresol red | sigma aldrich | 1.05225 | Dyes for colorimetric reagents |
| Ethyl cellulose | sigma aldrich | 200689 | Dyes for colorimetric reagents |
| Ethyl cellulose | Aladdin | E110670-100g | Additive |
| Industrial Camera | HKVision | MV-CS060-10UM/C-PRO | used for recording color changes |
| Liquid handler | Opentrons | OT2 | liquid handler |
| Mass Flow Controller | ASERT | AST10-DLCMX-500C-042-A2B2-48VY | used in controlling analytes gas mixtures |
| m-cresol purple | sigma aldrich | 1.05228 | Dyes for colorimetric reagents |
| Opentrons OT-2 Tips | Opentrons | OT-2 Tips, 300µL | |
| Opentrons OT-2 Tips | Opentrons | OT-2 Tips, 20µL | |
| phenol red | sigma aldrich | 1.07241 | Dyes for colorimetric reagents |
| polyethylene glycol | sigma aldrich | P1458 | Dyes for colorimetric reagents |
| PTFE film | Interstate Specialty Products | PM15M | PTFE mambrane |
| Tetrabutylammonium hydroxide | sigma aldrich | 86854 | Base for colorimetric reagents |
| thymol blue | sigma aldrich | 1.08176 | Dyes for colorimetric reagents |
References
- Chen, Y. et al. Robot-accelerated development of a colorimetric CO2 sensing array with wide ranges and high sensitivity via multi-target Bayesian optimizations. Sensors and Actuators B: Chemical. 390, 133942 (2023).
- Cho, S. H., Suh, J. M., Eom, T. H., Kim, T., Jang, H. W. Colorimetric sensors for toxic and hazardous gas detection: A review. Electron Mater Lett. 17 (1), 1-17 (2021).
- Li, Z., Askim, J. R., Suslick, K. S. The optoelectronic nose: Colorimetric and fluorometric sensor arrays. Chem Rev. 119 (1), 231-292 (2019).
- Ai, Z. et al. On-demand optimization of colorimetric gas sensors using a knowledge-aware algorithm-driven robotic experimental platform. ACS Sens. 9 (2), 745-752 (2024).
- Ai, Z. et al. Customizable colorimetric sensor array via a high-throughput robot for mitigation of humidity interference in gas sensing. ACS Sens. 9 (8), 4143-4153 (2024).
- Evyapan, M., Dunbar, A. D. F. Improving the selectivity of a free base tetraphenylporphyrin based gas sensor for NO2 and carboxylic acid vapors. Sensors and Actuators, B: Chemical. 206, 74-83 (2015).
- Liu, B., Zhuang, J., Wei, G. Recent advances in the design of colorimetric sensors for environmental monitoring. Environ Sci: Nano. 7 (8), 2195-2213 (2020).
- Zhang, Y., Lim, L.-T. Colorimetric array indicator for NH3 and CO2 detection. Sensors and Actuators B: Chemical. 255, 3216-3226 (2018).
- Xu, W. et al. Non-destructive determination of beef freshness based on colorimetric sensor array and multivariate analysis. Sensors and Actuators B: Chemical. 369, 132282 (2022).
- Abolhasani, M., Kumacheva, E. The rise of self-driving labs in chemical and materials sciences. Nat Synth. 2 (6), 483-492 (2023).
- Hickman, R. J., Bannigan, P., Bao, Z., Aspuru-Guzik, A., Allen, C. Self-driving laboratories: A paradigm shift in nanomedicine development. Matter. 6 (4), 1071-1081 (2023).
- Chen, Y. et al. Robot-assisted optimized array design for accurate multi-component gas quantification. Chem Eng J. 496, 154225 (2024).
- Antonova, R., Rai, A., Li, T., Kragic, D. Bayesian optimization in variational latent spaces with dynamic compression. (2019).
- Balandat, M. et al. BoTorch: A framework for efficient Monte-Carlo Bayesian optimization. http://arxiv.org/abs/1910.06403 (2020).
- Frazier, P. I. A tutorial on Bayesian optimization. (2018).
- Zhang, L. et al. Navigating the complexity of hybrid materials without structural dependency: PerovGNN as a map. Acta Materialia. 281, 120437 (2024).
- Wang, H. et al. Uplift modeling based on Graph Neural Network combined with causal knowledge. Proceedings - 2024 IEEE Conference on Artificial Intelligence, CAI 2024. 1487-1492 (2024).
- Häse, F., Roch, L. M., Kreisbeck, C., Aspuru-Guzik, A. Phoenics: A Bayesian optimizer for chemistry. ACS Cent Sci. 4 (9), 1134-1145 (2018).
- Wadekar, D. et al. Augmenting astrophysical scaling relations with machine learning: Application to reducing the Sunyaev-Zeldovich flux-mass scatter. Proc Natl Acad Sci U S A. 120 (12), e2202074120 (2023).
- Han, N., Tian, Y., Wu, X., Chen, Y. Improving humidity selectivity in formaldehyde gas sensing by a two-sensor array made of Ga-doped ZnO. Sensors and Actuators, B: Chemical. 138 (1), 228-235 (2009).
- Bae, G. et al. Impact of a diverse combination of metal oxide gas sensors on machine learning-based gas recognition in mixed gases. ACS Omega. 6 (36), 23155-23162 (2021).
- Mahboubifar, M., Hemmateenejad, B., Jassbi, A. R. Evaluation of adulteration in distillate samples of Rosa damascena Mill using colorimetric sensor arrays, chemometric tools and dispersive liquid-liquid microextraction-GC-MS. Phytochem Anal. 32 (6), 1027-1038 (2021).
- Cao, Y., Yu, H., Abbott, N. L., Zavala, V. M. Machine learning algorithms for liquid crystal-based sensors. ACS Sens. 3 (11), 2237-2245 (2018).
- Mahata, B., Acharyya, S., Banerji, P., Guha, P. K. Assessment of fish adulteration using SnO2 nanopetal-based gas sensor and machine learning. Food Chem. 438, 138039 (2024).
- Zhang, N. et al. Switchable operating modes enable low power consumption and improved gas sensing efficiency in MoS2/BP heterojunction. Sensors and Actuators B: Chemical. 396, 134620 (2023).
- Amarbayasgalan, T., Pham, V. H., Theera-Umpon, N., Piao, Y., Ryu, K. H. An efficient prediction method for coronary heart disease risk based on two deep neural networks trained on well-ordered training datasets. IEEE Access. 9, 135210-135223 (2021).
- Xu, Q., Jiang, J. Recent development in machine learning of polymer membranes for liquid separation. Mol Syst Des Eng. 7 (8), 856-872 (2022).
- Kimani, S. W. et al. Discovery of a novel DCAF1 ligand using a drug-target interaction prediction model: Generalizing machine learning to new drug targets. J Chem Inf Model. 63 (13), 4070-4078 (2023).
- Xiao, J., Hobson, J., Ghosh, A., Haranczyk, M., Wang, D. Y. Flame retardant properties of metal hydroxide-based polymer composites: A machine learning approach. Composites Communications. 40, 101593 (2023).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved