Method Article
Implantation of Electroencephalogram and Electrocardiogram Telemetry Devices in Neonatal Rabbit Kits
* These authors contributed equally
In This Article
Summary
Mechanisms of sudden unexpected death in epilepsy (SUDEP) are poorly understood and challenging to translate from current models. Transgenic rabbits may offer insights into these mechanisms. We describe a method for long-term, continuous electroencephalography and electrocardiography recordings in transgenic rabbit kits to evaluate serious events that may lead to death.
Abstract
Pathogenic variants in ion channel genes are associated with a high rate of sudden unexpected death in epilepsy (SUDEP). Mechanisms of SUDEP are poorly understood but may involve autonomic dysfunction and cardiac arrhythmias in addition to seizures. Some ion-channel genes are expressed in both the brain and the heart, potentially increasing the risk of SUDEP in patients with ion-channelopathies associated with epilepsy and cardiac arrhythmias. Transgenic rabbits expressing epilepsy variants provide a whole organism to study the complex physiology of SUDEP. Importantly, rabbits more closely replicate human cardiac physiology than do mouse models. However, rabbit models have additional health and anesthesia considerations when undergoing invasive monitoring procedures. We have developed a novel method to surgically implant a telemetry device for long-term simultaneous electroencephalogram (EEG) and electrocardiogram (ECG) monitoring in neonatal rabbit kits. Here, we demonstrate surgical methods to implant a telemetry device in P14 (weight range 175-250 g) kits with detailed attention to surgical approach, appropriate anesthesia and monitoring, and postoperative care, resulting in a low complication rate. This method allows for continuous monitoring of neural and cardiac electrophysiology during critical points in the development of cardiac arrhythmias, seizures, and potential SUDEP in rabbit models of genetic or acquired epilepsies.
Introduction
Sudden unexpected death in epilepsy (SUDEP) is a leading cause of death in patients with epilepsy. Mechanisms of SUDEP are poorly understood but potentially involve autonomic dysfunction, apnea, and cardiac arrhythmias in addition to seizures1,2,3,4,5,6,7. Patients with channelopathy-linked genetic epilepsies have among the highest rates of SUDEP. For example, SUDEP occurs in up to 20% of patients with variants in the voltage-gated sodium channel gene SCN1A8, the gene responsible for Dravet syndrome, a genetic epilepsy with onset in the first year of life. Many epilepsy-linked ion-channel genes are expressed in both the brain and the heart, with laboratory and clinical data suggesting that cardiac arrhythmias may be present in patients with channelopathy-linked genetic epilepsies7, 9,10,11,12, potentially increasing their risk of SUDEP due to a seizure-induced fatal cardiac arrhythmia or simultaneous occurrence of seizures and arrhythmias. Evaluating SUDEP in the laboratory setting poses numerous challenges. From a cardiac viewpoint, cardiac action potentials in mice are very different than in humans13, and human iPSC-cardiac myocyte models14 cannot replicate the complexities of the whole organism. Transgenic rabbit models of genetic epilepsies provide an ideal system to study SUDEP, as rabbit cardiac physiology more closely replicates that of the human13,15, while providing a whole organism to study complex pathophysiology. As SUDEP may occur as early as the first seizure, evaluating these animal models from an early time point is essential to understanding the onset of both seizures and cardiac arrhythmias. Video recording during the neonatal period is challenging, as rabbit kits are often still in the nest. Continuous electroencephalogram (EEG) or electrocardiogram (ECG) recording with a traditional wired system is not possible while kits are with the dam. Intermittent recording is unlikely to capture rare, terminal events associated with SUDEP. We have therefore turned to wireless implantable telemetry monitoring to provide long-term, continuous, simultaneous EEG and ECG recording in rabbit kits.
Keys to success in this protocol are appropriate anesthetic and postoperative support for these vulnerable animals. Rabbits are at a much higher risk of anesthetic death (1.39%-4.8%) compared to dogs and cats (0.17%-0.24%) due to unique anatomical and physiologic characteristics16,17. The main contributors to this increased anesthetic risk include sub-optimal airway management and acute postoperative complications. Multiple factors contribute to the difficulty of intubation in rabbits, including a long, narrow mouth with a broad tongue, an acute angle between the mouth and larynx, dorsal displacement of the epiglottis, increased susceptibility to laryngeal trauma, and increased propensity to laryngospasm18,19,20. After the immediate anesthetic episode, rabbits are at risk of developing life-threatening gastrointestinal stasis syndrome. This is a complex, multi-factorial problem, and anesthesia is postulated to be contributory via direct drug effects inhibiting gastric motility and/or secondary anorexia post-procedurally for any reason (unrelieved pain, nausea, etc.)21.
The unique physiology of rabbit neonates and infants compound the challenges associated with anesthesia and surgery. Rabbits have altricial young born with underdeveloped mechanisms for physiologic homeostasis and special anatomical considerations. Intravenous access and monitoring are difficult as most commercial products are not optimized for the small vascular size, high resting heart rate, and pigmented skin of Dutch-belted and New Zealand White cross rabbit kits. As cardiac output is essentially heart rate dependent in neonates22 and, in general, drug clearance by the renal or hepatic route is decreased compared to adults23, considerations for appropriate drug selection and dosage are critical. The primary cause of anesthetic death in rabbits is thought to be secondary to respiratory depression and apnea. In addition to the airway management problems already discussed for all rabbits, neonates have a depressed respiratory drive in the face of hypoxemia and hypercapnia, making this already challenging aspect of anesthesia more risky24.
In this protocol, we describe a successful method for EEG and ECG telemetry implant (Figure 1) in a neonatal rabbit model of epilepsy with a high surgical and anesthetic survival rate. This information will enable other researchers to tackle challenging neonatal rabbit models to advance research into epilepsy, cardiac arrhythmia, and related neurodevelopmental disorders.
Protocol
All work described was reviewed and approved by the University of Michigan Institutional Animal Care and Use Committee as part of an approved animal use protocol and is in line with relevant federal laws and guidelines, including the USDA Animal Welfare Act and NIH Public Health Service Policy. The University of Michigan is an AAALACi-accredited institution.
1. Animal preparation
- Rough-shave kits (age P14-P19, weight >175 g) 1-2 days prior to the procedure to minimize anesthesia time on the day of surgery using clippers.
- Autoclave or gas sterilize all surgical tools and materials (as possible) in preparation for the procedure.
- Induce anesthesia with ketamine (10 mg/kg IM), buprenorphine HCl (0.01 mg/kg IM) 0.3 mg/mL diluted to 0.03 mg/mL, and sevoflurane and oxygen via mask anesthesia using a non-rebreathing circuit.
- Place a 26 G ¾" intravenous (IV) catheter in the auricular vein (preferred) or cephalic vein and flush with heparinized saline 10 units/mL.
- Shave the abdomen, chest, back, neck, and head as close to the skin as possible using a #40 or #50 blade.
- Apply non-medicated lubricating ophthalmic ointment to prevent corneal ulceration.
- Administer analgesics (carprofen 4 mg/kg SQ - diluted to 25 mg/mL) and peri-operative antibiotic (cefazolin 20 mg/kg IV diluted to 50 mg/mL). Re-administer antibiotics every 90-180 min of surgical time.
2. Surgical preparation (Figure 2)
- Transfer the anesthetized kit to the operating table and place it supine on an infrared heating pad controlled via a rectal thermometer.
- Place the nose and mouth in a custom 3D printed facemask connected with a swivel connector to a non-rebreathing Jackson-Rees circuit (0.5 L bag) and maintain on sevoflurane anesthesia (1.5%-7% to effect) with oxygen flow at 2 L/min.
- Maintain anesthesia with sevoflurane and monitor the depth of anesthesia with a pulse oximeter on either the ear or paw and/or a Doppler on either the femoral artery or directly on the heart.
- Adjust anesthesia throughout the procedure to maintain heart rate (HR) between 180-260, oxygen saturation >85%, and respiratory rate between 10-50 breaths per minute (direct visualization of excursions or movement of the re-breathing bag).
NOTE: Have emergency drugs available (glycopyrrolate, epinephrine, doxapram). - Secure the kit to the face mask by gently taping the front limbs to the mask.
- Position the kit in a slightly right-lateral position by loosely securing the left hind leg to the operating table.
- Prepare the entire abdomen with a warmed surgical scrub alternating betadine and sterile saline.
NOTE: An additional surgical solution can be used to finish the scrub if desired. The surgeon, wearing dedicated scrubs, hair bonnet, and shoe covers, will aseptically scrub and don sterile gown and gloves to perform the procedure under sterile conditions. - Place adhesive surgical towels on either side of the kit and cover it with a large surgical drape. Cut an appropriately sized hole to expose the abdomen and chest.
- Open the implant onto the surgical field and place non-absorbable anchor sutures into each of the implant anchor holes. Leave the suture attached with a 5-6 cm tail (Figure 3A). Place the implant in a bowl of warmed sterile saline.
3. Placement of the implant in the abdomen
- After ensuring adequate anesthesia, make a 3 cm incision through the skin along the linea alba with a scalpel.
- Make a careful incision through the muscle to open the peritoneal cavity.
- Place the implant into the cranial portion of the abdominal cavity and position it to the left of the incision.
- Use a trocar to tunnel the negative ECG wire out of the peritoneal cavity and skin approximately 2 cm to the right of the incision. Tunnel the remaining 3 wires 3-4 cm to the left of the incision to allow the implant to sit comfortably within the peritoneal cavity.
- Secure the implant with the anchor sutures to the ventral wall of the peritoneal cavity, ensuring no bowel entrapment (Figure 3B).
- Close the abdominal wall with an absorbable suture in a continuous pattern.
- Close the skin incision with a non-absorbable suture in an interrupted pattern.
4. Placement of the ECG leads
- Tunnel the negative ECG lead subcutaneously to the right upper chest at the level of the first rib.
- Bluntly dissect a subcutaneous pocket to loosely coil approximately 10 cm of wire.
NOTE: Tight coils under the skin may lead to skin erosion and wire exposure. - Cut the excess wire and create a loop with the exposed wire by tying the end to the insulated wire with a non-absorbable suture.
- Secure the loop to the muscle with 2 non-absorbable sutures.
- Close the peritoneal muscle on the right around the wire with 1-2 absorbable sutures in an interrupted pattern.
- Close the skin at the right upper chest and right abdomen with 2-3 non-absorbable sutures in an interrupted pattern.
- Tunnel the positive ECG lead to the left lower rib and repeat the above steps to secure it to the muscle and close the incision.
- Tunnel the EEG leads subcutaneously to the left lateral side as far as possible in the surgical field.
- Add 1 absorbable suture to the peritoneal muscle on the left around the emerging wires. Close the skin with non-absorbable suture in an interrupted pattern.
- Wrap the exposed EEG wires with sterile aluminum foil.
5. Preparation of the dorsal surface
- A non-sterile assistant will then remove the sterile drape and leg tie.
- Turn the kit into the prone position (Figure 2B) while ensuring the face mask stays securely in place by rotating using the swivel connector between the face mask and circuit. Adjust pulse-ox and/or doppler monitors as needed to ensure continuous anesthetic monitoring.
- Prepare the surgical field with a betadine scrub to include the head, neck and entire back with care to scrub around the region of the exiting wires on the left side.
- The surgeon will then place a sterile adhesive towel under the left side while the aluminum foil packet of wires is held by a non-sterile assistant.
- Sterilely and gently remove the wires from the aluminum packet and place them on the sterile field. Finish draping with sterile towels.
- Cover with a sterile drape and cut a window large enough to expose the entire sterile field.
6. Placement of EEG leads
- Make a 3 cm incision through the scalp at the midline to expose the skull.
- Use a trocar to subcutaneously tunnel the EEG leads from the left side to the skull.
- Clean and scrape the periosteum from the exposed parietal bones using a scalpel.
- Insert a handheld drill into a sterile ultrasound cover. Insert a 1.0 mm drill burr into the drill.
- Drill bilateral burr holes into the parietal bones approximately 0.5 cm anterior to lambda and 0.5 cm lateral to the sagittal suture.
NOTE: Caution with the amount of pressure placed on the dorsal skull as this can occlude the ventral airway, so respiratory monitoring is key at this point in the procedure. A steady or significant drop in heart rate may indicate respiratory occlusion (bradycardia secondary to apnea) and should prompt immediate assessment and action. - Use fine forceps to place a screw into the burr hole. Use the screwdriver to insert approximately halfway (Figure 3C).
- Bluntly dissect a subcutaneous pocket along the back of the neck to loosely coil approximately 10 cm of wire.
- Cut the excess wire. Strip the insulation from the tip and stretch the wire.
- Create a loop at the end of the exposed wire by tying a knot, keeping a small loop. Place the loop over the screw and tighten the screw to the skull, ensuring that the wire is touching the screw. Place the ground wire on the left and the recording wire on the right.
- Assess the telemetry signals on the analysis software for fidelity once all wires are in place. The EEG signal will appear at a low amplitude while the kit is sedated.
- Secure the screws and wires to the skull with dental acrylic and allow it to harden completely.
- Close the skin with a non-absorbable suture on the head and the left flank.
- Inject bupivacaine (maximum dose 2 mg/kg of 5 mg/mL diluted to 2.5 mg/mL) subcutaneously at each incision. Cover each incision with a small amount of skin glue administered using a tuberculin syringe.
7. Anesthesia recovery
- Turn off sevoflurane anesthesia and provide only oxygen for at least 5 min while removing the remaining tape, draping, and anesthetic monitoring.
- Check blood glucose levels using a glucometer and administer warmed subcutaneous fluids at 10% of body weight (kg).
- Once the animal is reactive to a painful stimulus (toe pinch), move to a recovery incubator set at 37-38 °C.
NOTE: Often, the kit's temperature will significantly drop during this transfer. It may be beneficial to return the kit to the biofeedback infrared heating pad or provide additional supplemental heat. - Monitor visually continuously and record rectal temperature, pulse oximeter readings, heart rate, and respiratory rate every 10-15 min.
- Once the animal is consistently ambulatory and alert, remove the intravenous catheter and apply pressure to the site until the bleeding stops.
8. Postoperative care and monitoring
- Return kit to the dam and litter mates. Ensure nesting material and supplemental nutrition (Table of Materials) are available in the cage to aid in thermoregulation and recovery.
- Check on the kit daily for 7-10 days following the surgery, weighing daily and providing supplemental nutrition in the cage.
- For the first 2 days post-recovery (D1 and D2), give additional analgesics every 24 h (carprofen 4 mg/kg SQ - diluted to 5 mg/mL) and subcutaneous fluids (5-7 mL).
- For the first 3 days post-recovery (D1, D2, and D3), check on the kit twice per day, evaluating for evidence of pain, ambulation, incisional appearance, and hydration. Once per day during this time period, take the kit's temperature to ensure no evidence of infection and appropriate thermoregulation.
- Remove sutures if incisions appropriately heal at 7-10 days.
Results
The successful outcome of this project required the development of multiple parameters in the implant procedure and recording protocol. Implant surgery was attempted or performed on 16 rabbit kits, with 14 successfully surviving the procedure. Of those, 12 survived to the experimental endpoint. Reasons for intraoperative or postoperative death are highlighted in Table 1, along with procedure modifications that allowed for future success in achieving the experimental endpoint. The most common operative complication was respiratory depression, leading to hypoxia and bradycardia. The protocol was modified to include multiple monitoring modalities, including 2 pulse oximeters and a Doppler monitor. This allows for backup monitoring if one monitor should fail or become loose during the surgical procedure. Additionally, skin pigments in rabbits can make pulse-oximetry less reliable, necessitating monitoring from multiple points on the body. While induction and recovery are commonly considered the riskiest periods of anesthesia, we observed respiratory depression leading to bradycardia at other points throughout the procedure, highlighting the need for vigilant vital signs monitoring at all times. As an intervention for this complication, additional monitoring and doxapram were added to the protocol after the anesthetic complications were observed during the first two surgical cohorts of animals (animals 1-4). Animals 12 and 13 were in the same cohort and were administered doxapram (2-5 mg/kg, either IV or sublingual) due to poor respiratory drive. Both were successfully resuscitated from respiratory depression and apnea intra-operatively.
Telemetry recordings can be performed immediately once kits are returned to their home cages and often show relatively low amplitude EEG signals until the kit is fully recovered from anesthesia (Figure 4). ECG signal morphology may change slightly during the first few days of recording as scarring forms, and the ECG wire location is further secured (Figure 4B). EEG and ECG signal quality is robust over time without degradation of the signal given adequate excessive wire is left coiled subcutaneously to allow for growth. Telemetry recordings can be customized based on the individual needs of the study, as the implant can monitor various biopotentials, including temperature, acceleration, and signal quality, in addition to EEG and ECG (Figure 5). Data analysis is performed offline with Emka ECGAuto software. With EEG and ECG data collection at 500 Hz, implant batteries last approximately 55 days. This time range can be extended by changing data collection parameters or with intermittent sampling. Software programming can be performed to assist with seizure detection in ECGAuto (Figure 5). Implanted rabbits are monitored for skin erythema, weight, and temperature for the first 7 days following surgery, as this is the highest risk period for infection. Post-operatively, rabbit kits begin to show weight gain 1-3 days after the procedure, suggesting minimal distress from the procedure. Veterinary staff perform thorough examinations 2-3 times in the first postoperative week and will examine at more regular intervals if concerns arise during recovery. In our experience, the doe does not significantly disturb suture sites once the kits are returned to the nest, so wound healing has been excellent. Several kits developed benign seromas at the cranial incision (Table 1), for which they were given an additional 3 days of carprofen, resulting in seroma resolution without further complication. Long-term, rabbits are observed daily for signs of distress by laboratory personnel or husbandry staff. Veterinary staff are readily available to examine and treat rabbits if any concerns arise. Telemeter signal quality is checked daily for the device or lead malfunction until the battery is depleted.
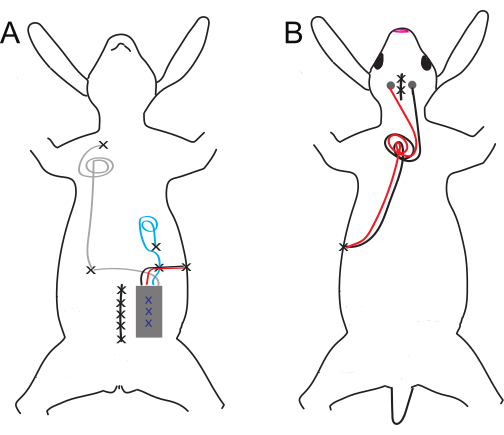
Figure 1: Schematic of ventral and dorsal implant and electrode placement. (A) Ventral and (B) dorsal implants. Suture sites are denoted by black X's, while blue X's denote internal tack sutures of the abdominal implant. Please click here to view a larger version of this figure.
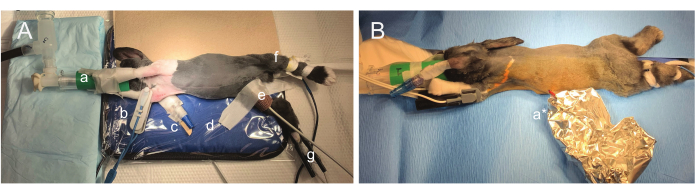
Figure 2: Photographs of surgical positioning. (A) The animal is partially rotated to allow abdominal implant, thoracic ECG lead placement, and partial tunneling of wires to the dorsum in the same surgical position. The nose is comfortably positioned into a custom 3D-printed facemask (a) and loosely taped to the front limbs. SpO2 monitoring can be intermittent, so multiple probes are utilized (right forearm (b) and right hindlimb (g)). An intravenous catheter is secured in the right ear (c). Other anesthetic monitoring shown includes biofeedback infrared temperature monitoring (d) and Doppler monitoring utilizing the right femoral artery (e). Respiration is visually monitored by observation of the animal or the rebreathing bag. (B) After implant and ECG lead placement, the sterile field is broken, and the rabbit is rotated to the prone position. Wires remain sterile inside a foil pouch (a*) as the dorsal surface is prepared for EEG lead placement. Please click here to view a larger version of this figure.

Figure 3: Key steps in the surgical procedure. (A) Three sutures are secured to the implant prior to skin incision. (B) A long tail remains in order to secure the implant to the ventral wall of the peritoneal cavity. (C) Skull screws are held with fine forceps while screwing into the skull. Please click here to view a larger version of this figure.
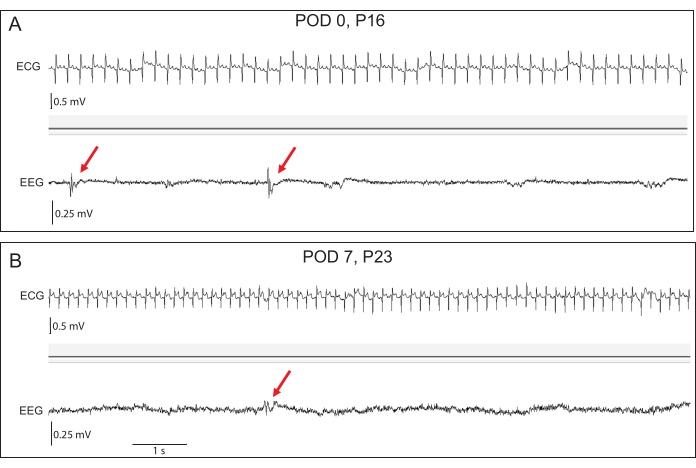
Figure 4: Representative early EEG and ECG recordings. (A) At postoperative day (POD) 0, the baseline EEG amplitudes are low, but interictal epileptiform discharges (red arrows) can be seen in transgenic rabbits. (B) By POD 7, EEG amplitudes have increased. Epileptiform discharges remain apparent. ECG signal fidelity is excellent at both time points. Please click here to view a larger version of this figure.

Figure 5: Representative raw tracings during a tonic-clonic seizure in a P66 transgenic rabbit kit. Pre-processed telemeter data includes (A) EEG, (B) temperature, (C) acceleration, (D) ECG, and (E) signal quality measurements for offline analysis. The signal quality of all biopotentials at postoperative day 52 remains excellent. Seizure onset is indicated by the red arrow. Processed EEG data (a) with baseline adjustment at seizure onset shows signal attenuation during the tonic phase of the seizure. ECG data with a processed baseline (b) is visible even with excessive muscle artifacts during the tonic phase of the seizure. Please click here to view a larger version of this figure.
Table 1: Surgical outcomes. Surgical and postoperative complications led to multiple modifications of the implant placement procedure. Fourteen of 16 rabbits (87.5%) survived the surgical procedure, and 2 rabbits had postoperative complications requiring euthanasia. Animals are displayed in chronological order of surgery date. Please click here to download this Table.
Discussion
The protocol described for anesthetic induction, monitoring, and support balances research needs for surgical approach and ease with gold standards of veterinary care. Prior to the laboratory adopting the described protocol as standard procedure, several other potential refinements were trialed, including dorsal subcutaneous implant placement, the use of an endotracheal tube or laryngeal mask airway, and the use of an esophageal stethoscope attachment for heart rate monitoring. However, all were ultimately abandoned for various reasons. While there is sufficient subcutaneous space for telemetry placement, the inability to close dead space due to the telemetry body and wire exit design, the challenge of positioning and coiling of the wires to allow for animal growth, and the required 2 positional changes intra-operatively to secure the ECG leads significantly extended surgical time and added unnecessary logistical challenges. Additionally, while the use of mask anesthesia is not generally recommended, the lack of appropriately sized LMAs and the inability to secure and maintain an endotracheal tube after placement precluded these as options. The requirement to flip the kit mid-surgery and also the need to access the neck and dorsum of the head limited options to secure the endotracheal tube even after successful placement based on a previously described technique for neonatal rabbit intubation25. We also trialed the use of an esophageal stethoscope probe for heart rate monitoring after the kit was draped in the event of a loss in signal from either the pulse oximeter or Doppler. However, all commercially available esophageal stethoscopes were too large, presumptively causing a vagal response and unsustainable bradycardia every time one was placed.
The anesthetic outcomes were likely greatly enhanced by the election to utilize sevoflurane rather than isoflurane for inhalational anesthesia. Sevoflurane is shown to have less vasodilatory effects in some rabbit tissues26 and is widely regarded in the human literature as enhancing cardiovascular stability27. Of particular importance, given the risks of anesthesia in a setting with sub-optimal airway management, sevoflurane's rapid induction and emergence documented in several species28,29,30 along with the ability to titrate anesthetic dosage resulted in shortened anesthetic episodes and enabled quick changes to the anesthetic plane in a rapidly evolving surgical setting. In addition, inhaled sevoflurane is considered to be significantly less aversive for mask induction, likely contributing to a reduction in breath holding in neonatal rabbits31. Doxapram in rabbits has been demonstrated to be an effective respiratory stimulant in adult rabbits32,33, and we anecdotally appreciated a response in our neonatal rabbit model.
While the success of this protocol is obvious, there are potential avenues for refinement in terms of analgesia and anesthetic monitoring. As there are no studies indicating dosing guidelines for neonatal rabbits, dosages and frequencies were targeted at the lower end of species-specific recommendations. An additional dose of buprenorphine on recovery and trialing rodent-specific anesthetic monitoring platforms could further enhance the survival rate and improve animal welfare in this model. Additional post-recovery dosing of buprenorphine would prolong the effects of multi-modal analgesia while minimizing documented dose-dependent respiratory depressive and gastrointestinal stasis effects in rabbits34,35. All post-surgical animals had moderate to severe elevations in immediate postoperative blood glucose (250-400 mg/dL) using a veterinary handheld glucometer. This could indicate an exaggerated stress response due to peri-acute postoperative pain based on a recent publication linking high blood glucose levels and the severity of gastrointestinal disease in rabbits36. As all animals returned to gaining weight within 3 days or less of the procedure and had normal activity and lack of pain at the incision site within 12-24 h post-operatively, we do not believe that an extended opioid administration is indicated for this model. Rodent-specific anesthetic monitoring devices, which include respiratory monitoring, are becoming increasingly common with products available by various manufacturers37. It stands to reason that if these systems would work with other comparably sized animals with similar high heart rates like the neonatal rabbits, then utilization of one of these systems would increase anesthetic survival. Unfortunately, we did not have access to these products or the ability to try them prior to purchasing them for this study.
Implantable telemetry devices have advanced in recent years to include longer battery life, smaller implants, and the ability to measure multiple biopotentials of interest in rodents, canines, and non-human primates while allowing animals to move freely, reducing stress38,39,40. The use of implantable telemetry devices for simultaneous EEG and ECG recording in rabbits offers advantages over current tethered methods41. With implantable devices, rabbits can move freely in their home cage without tethers or restraints, and kits are able to return to the nest with the doe and litter, reducing stress and promoting animal welfare. Careful postoperative monitoring procedures for signs of infection or stress also allow for early veterinary intervention if complications arise. Implanting kits allow for EEG evaluation during critical periods of brain development, which may be essential in models of genetic epilepsies without altering housing practices. In addition, telemetry data are captured continuously for the life of the implant battery, allowing for the capture of rare SUDEP events. The emergence of transgenic rabbits offers unique opportunities for modeling human disease. In addition, the use of rabbit models may offer significant advantages over other large animal evaluations that are required for FDA drug approvals. Methods such as the one presented will allow for advanced evaluation of cardiac and neural physiology in these models as well as their alteration by therapeutic intervention.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors are grateful for funding by NIH R61NS130070 to LLI.
Materials
| Name | Company | Catalog Number | Comments |
| 1 inch elastic wrap - Coban or Vetwrap | 3M | https://www.3m.com/3M/en_US/p/d/b00003186/ | |
| 4-0 PDS monofilament suture | Ethicon | https://www.jnjmedtech.com/en-US/company/ethicon/all-products | |
| 5-0 Ethilon nylon suture | Ethicon | https://www.jnjmedtech.com/en-US/company/ethicon/all-products | |
| Acquisition computer | Dell | https://www.dell.com/en-us | |
| Adhesive surgical towels | N/A | N/A | |
| Anesthesia circuit - Jackson-Reevs with 0.5 L rebreathing bag | JorVet | J0248GA | |
| Betadine scrub | N/A | N/A | |
| Bupivicaine (0.5%) | N/A | N/A | Diluted to 2.5 mg/mL prior to administration |
| Buprenorphine (0.3 mg/mL) | N/A | N/A | Diluted to 0.03 mg/mL prior to administration |
| Burr - 1.00 mm | Cell Point Scientific | 60-1000 | to drill skull |
| Cafazolin (1 g lypholized) | N/A | N/A | Diluted to 50 mg/mL |
| Carprofen (50 mg/mL) | MWI Veterinary | Diluted to 25 mg/mL prior to administration | |
| Cotton tipped applicators | N/A | N/A | |
| Custom 3-D printed face mask | N/A | https://www.thingiverse.com/thing:923725 | |
| Dental acrylic | N/A | N/A | |
| Diet Gel Criticare | Clear H2O | 72-05-5042 | Nutritional support |
| Dopper Gel - Aquasonic | Patterson | 07-890-5542 | |
| Doppler - Vet-Dop2 | Patterson | 07-888-8986 | |
| Doxapram (20 mg/mL) | MWI Veterinary | N/A | Emergency only |
| Dumont #5 Fine Forceps | Fine Science Tools | 11254-20 | For holding screws |
| Duraprep | 3M | 8630 | Final skin prep |
| ecgAuto data analysis software | emka technologies | N/A | |
| Epinephrine (1:1000) | MWI Veterinary | N/A | Emergency only |
| Gauze | N/A | N/A | |
| Glucometer ipet Pro | MWI Veterinary | 63867 | Monitor if poor recovery |
| Glycopyrrolate (0.2 mg/mL) | MWI Veterinary | N/A | Emergency only |
| Gram scale | N/A | N/A | |
| Hemostats | Fine Science Tools | 13008-12 | Hold wire loops while tying the loop in place |
| Ideal Micro-drill | Cell Point Scientific | 67-1204 | To drill skull |
| Incubator | DRE-veterinary (Infantia - NB1) | N/A | |
| Induction box | VetEquip | 941444 | |
| Infared heating pad - RightTemp Jr | Kent Scientific Corporation | RT-0502 | |
| IOX2 data acquisition software | emka technologies | N/A | |
| IV Catheter - Covidein Monoject 26 G, 3/4 inch PTFE | Patterson | 07-836-8494 | |
| ketamine (100 mg/mL) | MWI Veterinary | N/A | |
| Medical tape | N/A | N/A | |
| Narrow Pattern Forceps - Straight/12 cm | Fine Science Tools | 11002-12 | |
| Neonatal stethescope | Ultrascope | N/A | |
| Olsen-Hegar Needle holder with scissors - 12 cm | Fine Science Tools | 12002-12 | For suturing |
| Ophthalmic ointment Puralube | MWI Veterinary | N/A | Administered to both eyes during anesthesia |
| Opthalmic Lubricant - Paralube Vet | Patterson | 07-888-2572 | |
| Pulse oximeter (AccuWave Portable ) | Patterson | 07-892-9128 | For prep and recovery; reads HR up to 400 |
| Pulse oximeter (SDI - Vet/Ox plus 4700) | Heska | N/A | Intra-operative; no longer producted |
| Receiver | emka technologies | N/A | 1 receiver for every 4 telemetry implants |
| Rectal thermometer | N/A | N/A | |
| Scalpel | Fine Science Tools | 10003-12 | |
| Scissors | Fine Science Tools | 14002-12 | To cut drape |
| Screw driver - 1.0 mm | N/A | N/A | From mini-screwdriver set for electronics |
| Screws 00-96 x 3/32 (2.4 mm) | Protech International | 8L0X3905202F | |
| Sevoflurane | MWI Veterinary | Maintenance anesthesia | |
| Sevoflurane vaporizer and anesthesia machine | N/A | N/A | |
| Skin glue, Gluture | MWI Veterinary | 34207 | Apply sparingly with syringe |
| Small scissors | Fine Science Tools | 14084-08 | |
| Sterile aluminum foil | N/A | N/A | To wrap wires prior to rotating animal |
| Sterile paint brush | N/A | N/A | To apply dental acrylic |
| Sterile Saline | N/A | N/A | |
| Sterile surgical gloves | N/A | N/A | |
| Sterile ultrasound cover | N/A | N/A | To cover the drill |
| Sterile Water | N/A | N/A | For cefazolin reconstitution |
| Surgical blade no. 15 | N/A | N/A | |
| Surgical drape | N/A | N/A | |
| Surgical gown | N/A | N/A | |
| Swivel connector - Jorgensen Labs | Patterson | 07-802-2349 | To connect anesthesia circuit to face mask |
| Telemetry implant | emka technologies | easyTEL+_M1_EETA_B_35 | |
| Trocar | SAI | TRO-10-6 | To tunnel wires |
References
- Bagnall, R. D., Crompton, D. E., Semsarian, C. Genetic Basis of Sudden Unexpected Death in Epilepsy. Front Neurol. 8, 348 (2017).
- Surges, R., et al. Pathologic cardiac repolarization in pharmacoresistant epilepsy and its potential role in sudden unexpected death in epilepsy: a case-control study. Epilepsia. 51 (2), 233-242 (2010).
- Surges, R., Thijs, R. D., Tan, H. L., Sander, J. W. Sudden unexpected death in epilepsy: risk factors and potential pathomechanisms. Nat Rev Neurol. 5 (9), 492-504 (2009).
- Shorvon, S., Tomson, T. Sudden unexpected death in epilepsy. Lancet. 378 (9808), 2028-2038 (2011).
- Schuele, S. U., et al. Video-electrographic and clinical features in patients with ictal asystole. Neurology. 69 (5), 434-441 (2007).
- Massey, C. A., Sowers, L. P., Dlouhy, B. J., Richerson, G. B. Mechanisms of sudden unexpected death in epilepsy: the pathway to prevention. Nat Rev Neurol. 10 (5), 271-282 (2014).
- Sahly, A. N., Shevell, M., Sadleir, L. G., Myers, K. A. SUDEP risk and autonomic dysfunction in genetic epilepsies. Auton Neurosci. 237, 102907 (2022).
- Cooper, M. S., et al. Mortality in Dravet syndrome. Epilepsy Res. 128, 43-47 (2016).
- Negishi, Y., et al. SCN8A-related developmental and epileptic encephalopathy with ictal asystole requiring cardiac pacemaker implantation. Brain Dev. 43 (7), 804-808 (2021).
- Meisler, M. H., et al. SCN8A encephalopathy: Research progress and prospects. Epilepsia. 57 (7), 1027-1035 (2016).
- Watanabe, H., et al. Sodium channel β1 subunit mutations associated with Brugada syndrome and cardiac conduction disease in humans. J Clin Invest. 118 (6), 2260-2268 (2008).
- Goldman, A. M., Glasscock, E., Yoo, J., Chen, T. T., Klassen, T. L., Noebels, J. L. Arrhythmia in heart and brain: KCNQ1 mutations link epilepsy and sudden unexplained death. Sci Transl Med. 1 (2), 2ra6 (2009).
- Nerbonne, J. M. Mouse models of arrhythmogenic cardiovascular disease: challenges and opportunities. Curr Opin Pharmacol. 15, 107-114 (2014).
- Frasier, C. R., et al. Channelopathy as a SUDEP Biomarker in Dravet Syndrome patient-derived cardiac myocytes. Stem Cell Rep. 11 (3), 626-634 (2018).
- Camacho, P., Fan, H., Liu, Z., He, J. -. Q. Small mammalian animal models of heart disease. Am J Cardiovasc. 6 (3), 70-80 (2016).
- Brodbelt, D. Perioperative mortality in small animal anaesthesia. Vet J. 182 (2), 152-161 (2009).
- Lee, H. W., Machin, H., Adami, C. Peri-anaesthetic mortality and nonfatal gastrointestinal complications in pet rabbits: a retrospective study on 210 cases. Vet Anaes Anal. 45 (4), 520-528 (2018).
- Comolli, J., et al. Comparison of endoscopic endotracheal intubation and the v-gel supraglottic airway device for spontaneously ventilating New Zealand white rabbits undergoing ovariohysterectomy. Vet Rec. 187 (10), e84-e84 (2020).
- Grint, N. J., Sayers, I. R., Cecchi, R., Harley, R., Day, M. J. Postanaesthetic tracheal strictures in three rabbits. Lab Anim. 40 (3), 301-308 (2006).
- Phaneuf, L. R., Barker, S., Groleau, M. A., Turner, P. V. Tracheal injury after endotracheal intubation and anesthesia in rabbits. J Am Assoc Lab Anim Sci. 45 (6), 67-72 (2006).
- Quesenberry, K. E., Orcutt, C. J., Mans, C., Carpenter, J. W. Gastrointestinal Diseases of Rabbits. Ferrets, Rabbits, and Rodents. , (2020).
- Desai, A., Macrae, D. . Cardiovascular Physiology in Infants, Children, and Adolescents. Pediatric and Congenital Cardiology, Cardiac Surgery and Intensive Care. , (2020).
- Bansal, N., Momin, S., Bansal, R., Venkata, S. K. R. G., Ruser, L., Yusuf, K. Pharmacokinetics of drugs: newborn perspective. Pediatr Med. 7, 19 (2024).
- Trachsel, D., Erb, T. O., Hammer, J., von Ungern-Sternberg, B. S. Developmental respiratory physiology. Paediat Anaesth. 32 (2), 108-117 (2022).
- Benito, S., Hadley, S., Camprubí-Camprubí, M., Sanchez-de-Toledo, J. Blind endotracheal intubation in neonatal rabbits. J Vis Exp. 168, e61874 (2021).
- Okamoto, S., Matsuura, N., Ichinohe, T. Effects of volatile anesthetics on oral tissue blood flow in rabbits: A comparison among isoflurane, sevoflurane, and desflurane. J Oral Maxillofac Surg. 73 (9), 1714.e1-1714.e8 (2015).
- Elshalakany, N. A., Salah, A. M. Comparative study: evaluation of the effect of sevoflurane versus isoflurane in general anesthesia for pediatric patients undergoing cardiac catheterization. Egypt J Anaesth. 38 (1), 409-414 (2022).
- Anjana, R. R., Parikh, P. V., Mahla, J. K., Kelawala, D. N., Patel, K. P., Ashwath, S. N. Comparative evaluation of isoflurane and sevoflurane in avian patients. Vet World. 14 (5), 1067-1073 (2021).
- Johnson, R. A., Striler, E., Sawyer, D. C., Brunson, D. B. Comparison of isoflurane with sevoflurane for anesthesia induction and recovery in adult dogs. Am J Vet Res. 59 (4), 478-481 (1998).
- Campbell, C., Nahrwold, M. L., Miller, D. D. Clinical comparison of sevoflurane and isoflurane when administered with nitrous oxide for surgical procedures of intermediate duration. Can J Anaesth. 42 (10), 884-890 (1995).
- TerRiet, M. F., et al. Which is most pungent: isoflurane, sevoflurane, or desflurane. Br J Anaesth. 85 (2), 305-307 (2000).
- Khanna, V. K., Pleuvry, B. J. A study of naloxone and doxapram as agents for the reversal of neuroleptanalgesic respiratory depression in the conscious rabbit. Br J Anaesth. 50 (9), 905-912 (1978).
- Flecknell, P. A., Liles, J. H., Wootton, R. Reversal of fentanyl/fluanisone neuroleptanalgesia in the rabbit using mixed agonist/antagonist opioids. Lab Anim. 23 (2), 147-155 (1989).
- Shafford, H. L., Schadt, J. C. Respiratory and cardiovascular effects of buprenorphine in conscious rabbits. Vet Anaesth Analg. 35 (4), 326-332 (2008).
- Feldman, E. R., Singh, B., Mishkin, N. G., Lachenauer, E. R., Martin-Flores, M., Daugherity, E. K. Effects of cisapride, buprenorphine, and their combination on gastrointestinal transit in New Zealand white rabbits. J Am Assoc Lab Anim Sci. 60 (2), 221-228 (2021).
- Harcourt-Brown, F. M., Harcourt-Brown, S. F. Clinical value of blood glucose measurement in pet rabbits. Vet Rec. 170 (26), 674-674 (2012).
- Rivera, D. A., Buglione, A. E., Ray, S. E., Schaffer, C. B. MousePZT: A simple, reliable, low-cost device for vital sign monitoring and respiratory gating in mice under anesthesia. PLoS One. 19 (3), e0299047 (2024).
- Nicou, C. M., Passaglia, C. L. Characterization of intraocular pressure variability in conscious rats. Exp Eye Res. 239, 109757 (2024).
- Sadko, K. J., Leishman, D. J., Bailie, M. B., Lauver, D. A. A simple accurate method for concentration-QTc analysis in preclinical animal models. J Pharmacol Toxicol Methods. 128, 107528 (2024).
- Vuong, J. S., Garrett, J. J., Connolly, M. J., York, A. R., Gross, R. E., Devergnas, A. Head mounted telemetry system for seizures monitoring and sleep scoring on non-human primate. J Neurosci Methods. 346, 108915 (2020).
- Bosinski, C., Wagner, K., Zhou, X., Liu, L., Auerbach, D. S. Multi-system monitoring for identification of seizures, arrhythmias and apnea in conscious restrained rabbits. J Vis Exp. (169), e62256 (2021).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved