Method Article
Cranial Neural Crest Cells Three-Dimensional In Vitro Differentiation Protocol for Multiplexed Assay
In This Article
Summary
We present a three-dimensional (3D) in vitro differentiation protocol generating neurospheres of reproducible size to produce cranial neural crest cells from mouse embryonic stem cells. We show that this methodology reduces variability compared to previous protocols and how it can be used for multiplexed assay to study cranial neural crest cell development.
Abstract
With their remarkable capacity to generate both ectodermal and mesenchymal derivatives, cranial neural crest cells (CNCC) have attracted a lot of interest in studying the mechanisms regulating cell fate decisions and plasticity. Originating in the dorsal neuroepithelium, this cell population is transient and relatively rare in the developing embryo - making functional tests, genomic screens, and biochemistry assays challenging to perform in vivo. To overcome these limitations, several methods have been developed to model CNCC development in vitro. Neurosphere (NS) based culturing methods provide a complex microenvironment that recapitulates the developing anterior neuroepithelium in 3D. These systems allow the growth of many NS in the same plate to generate a large amount of CNCC, but the produced NS present a high variability in shape, size, and number of CNCC formed - making quantitative assays difficult to perform. This protocol outlines a reproducible method for generating NS from mouse embryonic stem cells (mESC) in a 96-well format. NS generated in 96-well plates produce cranial neural crest cells (CNCC), which can be further cultured. By controlling the number of starting cells, this approach reduces variability in the size and shape between NS and increases reproducibility across experiments. Finally, this culture system is adaptable to several applications and offers a higher degree of flexibility, making it highly customizable and suitable for multiplexing experimental conditions.
Introduction
Cranial neural crest cells (CNCC) are a stem-like cell population that arises in the anteriormost part of the developing embryo, at the border between the neural plate and the surface ectoderm1. CNCC then undergo an epithelial-to-mesenchymal transition (EMT), delaminate from the neuroepithelium, and migrate dorsoventrally towards various locations in the embryo where they differentiate into a wide variety of cell types2. Studying this cell population is of great interest as it possesses a remarkable plasticity3 and the unique ability to differentiate into both ectodermal and mesenchymal derivatives, such as craniofacial bones and cartilages4. Although CNCC are relatively accessible in the embryo, they are a transient population with a low number of cells, making systemic mechanistic studies difficult to conduct in vivo. CNCC cell lines have been isolated and characterized in the last few years to overcome these limitations. In particular, the O9-1 CNCC cell line is a great tool for studying migratory and post-migratory neural crest development5,6; however, this cell line does not allow the study of the early events prior to migration leading to neural crest induction and specification. In this regard, there have been significant developments in the development of in vitro differentiation protocols to differentiate CNCC in a dish via the use of 3D structures resembling the developing neuroepithelium called neurospheres (NS)7,8- obtained after differentiation of embryonic stem cell (ESC) colonies. These 3D protocols robustly produce high numbers of CNCC, allowing the conduct of biochemical and genomic mechanistic studies9,10. NS are cultured on low attachment plates in N2B27 supplemented medium, together with Fibroblast Growth Factor (FGF) and Epidermal Growth Factor (EGF)10,11 to stimulate cell proliferation. These protocols are carried out in Petri dishes, cultivating numerous NS in the same plate. Within the growing NS, cells aggregate and continue to divide - reaching a diameter of 100-200 µm upon maturity. At maturity (about day 5), NS attach to the substrate and differentiate into CNCC resembling their in vivo counterparts9,12. These CNCC then undergo EMT and delaminate onto the plate surface. Morphological differences can be observed depending on NS size, as larger spheres will appear darker in the core due to lower availability of nutrients and oxygen, leading to cells undergoing apoptosis13. While this type of procedure generates a large number of CNCC at the endpoint of differentiation, it presents several limitations, making the study of the various molecular dynamics occurring during the differentiation process nearly impossible. First, the use of ESC colonies - which vary in size - makes it difficult to control the starting cell number for each experiment. This results in the generation of NS of various shapes and diameters that develop differently by activating specific signaling pathways, leading to altered cell differentiation and, thus not forming a uniform sample at a given time point. Second, culturing multiple NS in the same plate often leads to them fusing together14 and potentially releasing signaling molecules that influence their neighbors' microenvironment and, thus, their development. Altogether, these procedures generate a lot of variability between samples and experiments.
Here, we present a strategy to overcome these difficulties that generate single NS - capable of producing CNCC - by aggregating mouse ESC (mESC) in non-TC treated U-bottomed 96-well plates. Starting from mESC allows studying the specification process and early stages of CNCC development compared to starting from already established neural crest cell lines. This protocol begins with the disaggregation of mESC colonies to obtain a single cell suspension, followed by the seeding of a specific number of mESC in each well of a non-TC treated U-bottomed 96-well plate. The cells are left to aggregate for two days and subsequently moved to a non-TC treated flat-bottomed 96-well plate, in which NS will be able to attach to the plate bottom. By controlling the starting cell number and the microenvironment of each NS during the differentiation process, this protocol reduces sample variability, which increases experimental reproducibility. We believe this will be a convenient platform for designing multiplexed experiments, such as testing the effect of different culture conditions or performing gene perturbation screens.
Protocol
1. Generation of a single-cell suspension from mouse ESC colonies
NOTE: This protocol is adapted to the use of CK35 mESC (an mESC line competent for germ line transmission, to have then the option to develop in vivo models15) grown on inactivated feeders in a gelatin-coated TC-treated 6-well plate. One well of a TC-treated 6-well plate should yield approximately 1.5 × 106 mESC, which is sufficient for the rest of the protocol. This can be scaled up if necessary. Adjust the initial steps in accordance with the chosen ESC strain and maintenance culture method, as well as the proper culture medium. This protocol is to be performed under sterile conditions. See the Table of Materials for details related to all the materials, reagents, and equipment used in this protocol.
- Start from mESC at a 70%-80% confluence grown in mESC culture medium. See Table 1 for the mESC culture medium composition used in this study.
NOTE: Do not let mESC grow over 80% confluence, as they will start to differentiate, and this will affect the aggregation process. Colonies must be compact and show a healthy morphology (no cracks or waves, distinct nuclear-cytoplasm contrast). - Prepare CNCC differentiation medium. See Table 1 for CNCC differentiation medium composition.
NOTE: Once growth factors are added, the medium can be stored for up to 3 weeks at 4 °C. Ensure the medium is protected from light. - Prepare a fresh collagenase solution at a concentration of 2 mg/mL in the DMEM-Knockout medium.
NOTE: Use of collagenase ensures only the colonies are detached and not the feeders, as their presence in the following steps will interfere with NS aggregation. Filter collagenase solution before use with a 0.22 µm filter. - Aspirate the ESC medium from mESC.
- Gently add 1 mL of PBS to the side of the well. Rock the plate gently to ensure even washing.
- Remove PBS and replace with 2 mL of collagenase solution. Incubate at 37 °C for 30-45 min.
- Check the plate under a light microscope at 10x magnification after the first 20 min and then every 5 min.
- When the colonies show rolled-up edges, tap vigorously on the plate side, and the colonies will detach.
- With a 5 mL serological pipette, collect the colonies and transfer them to a 15 mL conical tube.
NOTE: Check the plate under a light microscope for leftover colonies. These can be collected with a PBS wash. - Centrifugate the colonies at 16 × g for 3 min at room temperature (RT).
- Aspirate as much medium as possible from the conical tube, being careful not to disturb the colonies at the bottom of the tube.
- Add 1 mL of 0.05% trypsin solution and incubate the tube at 37 °C for 5 min.
- Dissociate colonies in the tube by pipetting vigorously up and down, first with a p1000 and then with a p200 micropipette.
NOTE: This ensures the obtention of a single-cell suspension. - Add 2 mL of mESC culture medium to block the trypsin, and centrifugate at 160 × g for 3 min at RT. Remove the supernatant and add 1 mL of CNCC differentiation medium.
- Count cells using an automated cell counting device or a standardized system under the microscope, following manufacturer instructions.
- After counting, dilute with sufficient CNCC differentiation medium to obtain a concentration of 3000 live cells per 50 µL.
- Using a p200 micropipette, seed 50 µL of the cell suspension in each well of a non-TC treated U-bottom 96-well plate, then top up the well until 200 µL with CNCC differentiation medium.
NOTE: While filling the plate, resuspend from time to time the single cell suspension with a p1000 micropipette to obtain a homogeneous concentration. - Incubate overnight in an incubator at 37 °C, 5% CO2.
2. Transferring into a flat-bottom 96-well plate for CNCC differentiation
- The next day (day 1), observe the plate under a light microscope. Ensure one small cell cluster with clear borders is visible at the bottom of each well. Put the plate back in the incubator overnight.
NOTE: There may be some cells around the main aggregate, some dead. This will not interfere with NS aggregation. - On day 2, slowly remove 100 µL of medium from each well.
- When aspirating medium, be careful not to remove the NS. To avoid doing so, place the pipette tip close to the surface and far from the bottom.
- Cut the tip of a p200 micropipette at around 3-4 mm from the tip. Use this to aspirate the NS with the remaining medium.
NOTE: To facilitate picking up the NS, gently pipette up and down a couple of times before aspirating. - Transfer NS and the remaining medium into a non-TC treated flat-bottom 96-well plate and verify the transfer under a light microscope, then top each new well with 100 µL of prewarmed CNCC differentiation medium. Leave NS in the incubator at 37 °C, 5% CO2, until day 4.
- On day 4, remove 100 µL of medium from each well and replace it with 100 µL of prewarmed CNCC differentiation medium.
NOTE: To avoid disturbing NS attachment at the bottom of the plate, slowly aspirate and replace medium. - On days 5 and 6, check the NS attachment under the light microscope. Ensure lighter cells - delaminating from the NS -start surrounding the main body of the NS.
- On day 7, change the medium as described in 2.5. Change the medium with the same procedure every 2 days until the endpoint of the study.
3. CNCC passaging and maintenance
NOTE: CNCC passaging can be performed as soon as there is a sufficient quantity of cells visible around NS. This can be as early as day 7, as earlier time points do not provide a sufficient amount of CNCC.
- Prepare CNCC Maintenance medium. See Table 1 for CNCC Maintenance medium composition.
- Before adding it to the medium, filter BSA after solubilization through a 0.22 μm filter. Once growth factors are added, store the medium for up to 3 weeks at 4 °C. Ensure the medium is protected from light.
- Prepare fibronectin at 7.5 µg/mL in PBS. Mix vigorously.
- Coat the wells of a non-TC treated 96-well plate by adding 100 μL of fibronectin solution per well. Let coat under the hood for 30 min at RT.
NOTE: If CNCC are meant for immunofluorescence staining, place a sterile glass coverslip at the bottom of the well before coating. Ensure that the slide stays at the bottom of the well and does not float to avoid coating the opposite side to the one that will be used. Follow the instructions in section 5 for CNCC fixation and mounting. - In the meantime, in the non-TC treated flat-bottom 96-well plate, aspirate as much CNCC differentiation medium as possible from the wells and substitute with 50 µL accutase. Incubate at 37 °C for 5 min.
- After incubation, add 100 µL of CNCC maintenance medium per well to quench the accutase. Remove fibronectin from the wells of the receiving non-TC treated flat-bottom 96-well plate. Filter the detached post-migratory CNCC by passing them through a 40 µm filter on top of the well of the receiving non-TC treated flat-bottom 96-well plate.
NOTE: This will filter out cell clumps or remaining NS that have not been previously removed. CNCC grown in the same condition can be pooled in one 50 mL tube to be then seeded in the same well of a TC-treated 6-well plate. In this case, coat the well with 1 mL of fibronectin and use 1 mL of CNCC maintenance medium to quench the accutase. - Let post-migratory CNCC attach for 15-30 min at 37 °C. Discard medium and replace with 100 µL of CNCC maintenance medium. Change medium every 2 days.
NOTE: Gently add medium as fast flow induces differentiation of CNCC into neural derivatives. If working in a 6-well format, use 1 mL of CNCC maintenance medium.
4. NS fixation and mounting for immunofluorescence
- At the desired time point, transfer NS from the non-TC treated flat-bottom 96-well plate into a DNA low binding 2 mL tube by cutting the tip of a p200 micropipette and gently pipet up and down to pick up the NS.
NOTE: At later time points (day 7 onwards), NS will be big enough to be seen by the eye but also will be harder to detach. - Let NS settle in the tube for 3 min at RT, remove as much medium as possible, and rinse with 1 mL of cold PBS.
NOTE: If transferring small NS from early time points (before days 3-4), spin at 16 × g for 3 min to ensure NS settle to the bottom. - Remove PBS and, in a chemical hood, replace with 2 mL of 4% PFA in PBS. Incubate for 20 min at RT.
- Invert the tube slowly after adding 4% PFA solution and let it rest during fixation on the side. The goal is to spread NS slightly so they do not stick together in this step.
- Remove 4% PFA solution (in the chemical hood) and wash NS with 1 mL of cold PBS/0.5% Tween20. Let it settle at RT for 3 min. Repeat this 3 times in total.
NOTE: NS will settle at the bottom. - Remove PBS/0.5% Tween20 and add 2 mL of PBS/0.1% Triton X-100. Invert the tube and let it rest on the side. Incubate for 1 h at RT.
- Wash NS 3 times in cold PBS/0.5% Tween20, as indicated in step 4.4.
- Remove PBS/0.5% Tween20 and block with 2% BSA in PBS at 4 °C for a minimum of 1 h, preferably overnight.
NOTE: Samples can be stored in 2% BSA/PBS for up to 1 week at 4 °C. Protect from light. - Prepare primary antibody solution by adding the correct dilution of primary antibody to 2% BSA/PBS in a final volume of 500 µL. For the primary antibody mix used in this study, see Table 2.
- Remove as much of the blocking solution as possible and transfer the NS to a 0.5 mL tube by cutting the tip of a p200 micropipette.
- Add the primary antibody solution and pipette up and down slowly to resuspend the NS. Incubate overnight on a rotator at 4 °C.
- Wash NS 3 times in cold PBS for 5 min at RT.
- Prepare the secondary antibody solution by diluting the secondary antibodies of choice in 2% BSA/PBS and adding 1/1000 DAPI. For the secondary antibody mix used in this study, see Table 2.
NOTE: Keep the tubes protected from light. - Replace PBS with 500 µL of secondary antibody solution and wrap the tube with aluminum foil to protect it from light. Incubate for 1 h on the rotator at RT.
- Wash NS 3 times in cold PBS, as indicated in step 4.11.
- Remove as much PBS as possible without aspirating the NS, substitute with 50 µL of clearing agent, and follow manufacturer instructions.
- Incubate overnight at RT, protected from light.
- Prepare mounting chambers.
- On a microscope slide, place three layers of double-sided tape. Use transparent, fibreless tape to avoid residues in the mounting chamber.
- Using a razor, cut a 3 mm × 8 mm window in the double tape.
NOTE: Chamber dimensions depend on the sample time point. This example is suited for hosting 50 µL of mounting medium, which is optimal for 10-20 single NS at later time points (days 7-9).
- Under a stereoscope, carefully transfer the NS in the clearing agent into the mounting chamber by cutting a low adhesion p200 micropipette tip. Use a magnification between 2x and 4x to provide a field of view of the whole chamber and enough magnification to identify the NS. If the stereoscope is outfitted for fluorescent imaging, working with a blue fluorescence filter will make identifying the NS easier, as the DAPI staining will make the otherwise transparent NS stand out.
NOTE: Verify that the medium forms a slight convex meniscus above the chamber. This will ensure there are no bubbles in the chamber. - Place a coverslip on the surface and lightly press on its sides to make it adhere. Perform this step under the stereoscope and verify that the NS are not pushed outside the chamber.
- Store at 4 °C protected from light until imaging.
5. CNCC fixation and mounting for immunofluorescence
- Remove CNCC maintenance medium from the wells and wash them with PBS. Add PBS gently by pipetting on the side of the well.
- Proceed with fixation, permeabilization, and staining as described in steps 4.3-4.14.
- Mount coverslips on a microscopy glass slide by adding mounting medium onto the slide, grabbing and rotating the coverslip with the use of tweezers so that post-migratory CNCC face the glass slide, and placing it on the drop.
- Optional: Seal the coverslip with nail polish or other commonly employed sealing systems.
- Store at 4 °C protected from light until imaging.
Results
Following the protocol, mESC colonies were dissociated, and 3000 cells were seeded in non-TC treated U-bottom 96-well plates. On day 2, aggregated NS were transferred into non-TC treated flat-bottom 96-well plates to allow them to attach. A simplified visualization of the NS aggregation protocol is provided in Figure 1A. NS were cultured until day 9 and then processed for immunofluorescence staining. Cells that migrated from the NS onto the plate were transferred to coverslips for imaging and cultured until day 13 in the CNCC maintenance medium. In parallel, the protocol for generating NS in a Petri dish was carried out as a reference and point of comparison. The overall goal of the experiment was to reduce the NS-to-NS variability observed in the protocol carried out in the Petri dish.
When cultured individually in non-TC treated 96-well plates, NS showed reduced phenotypical variability. All NS start attaching at day 5, while the proportion of attached NS was very variable and, overall, less important at day 7 when cultured in a Petri dish (Figure 1B,C). In addition, when cultured in a Petri dish, NS fusion is consistently observed, as can be observed on day 4 (Figure 1B). At day 9, extensive cell delamination from NS can be observed in both non-TC treated 96-well plates and Petri dish culture conditions (Figure 1B,C). NS were collected on day 4 and day 7 to compare size variability between protocols. NS cultured in non-TC treated 96-well plate show a significant reduction in size variability at both day 4 and day 7. In comparison, when cultured in Petri dish NS display an important size variability (Figure 2A). In the non-TC treated 96-well plate, day 4 NS diameter ranges between 139 µm and 295 µm, and between 383 µm and 552 µm on day 7, while in the Petri dish, day 4 NS diameter ranges between 126 µm and 505 µm, and from 106 µm to 868 µm at day 7. NS cultured in non-TC treated 96-well plate were collected every day from day 2 to day 9, and their growth was measured. Cell number increases exponentially, from an average of 1082 cells on day 2 and an average of 48352 cells on day 9 (Figure 2B), while NS diameter size follows a linear growth, with an average 136 µm diameter at day 2 and an average 570 µm diameter at day 9 (Figure 2C).
After culture in the non-TC treated 96-well plate, NS and delaminated cells were stained for CNCC markers AP2α, PAX7, and SOX916, and TWIST1, an EMT marker16. A schematic visualization of sample collection, staining, and mounting protocol is shown in Figure 3. A representative image of the mounting chamber for NS immunofluorescence analysis is shown in Figure 4A. This analysis aimed to verify NS cultured in non-TC treated 96-well plate generate CNCC that undergo EMT and that delaminating cells present a CNCC identity. Both NS and post-migratory CNCC showed extensive expression of AP2α, SOX9, and TWIST1 at days 9 and 13, while PAX7 - a pre-migratory CNCC marker16- is only present in NS and not in post-migratory CNCC (Figure 4C). Together, these results confirm that the non-TC treated 96-well plate NS culture protocol generates CNCC that undergo EMT and migrate on the plate surface. Day 9 NS and day 13 post-migratory CNCC were also stained for neuronal marker TUJ117 (Figure 4B) to visualize neuronal derivatives in the developing NS.
Lastly, RT-qPCR gene expression analysis was performed on mESC and day 13 post-migratory CNCC (Figure 4D). RNA was extracted using Trizol, and then RT-qPCR was performed using manufacturer instructions. In post-migratory CNCC, expression of neural crest markers Ap2α, Sox9, and Sox1018 is increased by 5.42-fold, 51.07-fold, and 46.75-fold compared to mESC, respectively. Neural progenitor markers Eya2, Ascl1, and Neurog219 expression was also increased on day 13 post-migratory CNCC by 7.78-fold, 43.92-fold, and 13.83-fold compared to mESC, respectively. Mesenchymal/osteoblastic markers Mef2c, Alx4, and Ets120showed increased expression in post-migratory CNCC by 168.44-fold, 156.47-fold, and 41.23-fold compared to mESC, respectively (primer sequences in Table 3). Together this indicates the post-migratory CNCC generated in this protocol are not biased towards a neuronal or an ectomesenchyme fate.
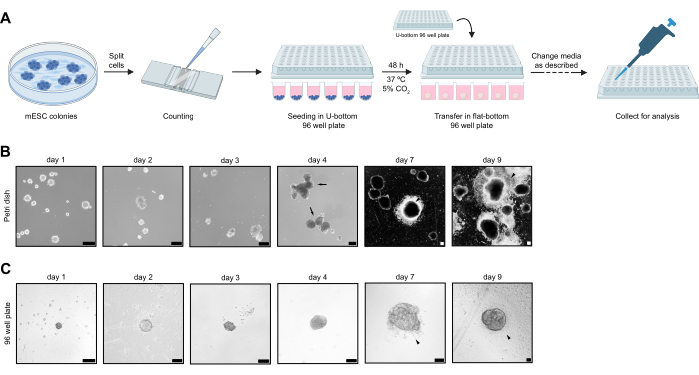
Figure 1: NS aggregation workflow and phenotypical comparison with Petri dish-grown NS. (A) Workflow procedure of mESC seeding and NS culture. (B) Representative images of NS differentiation carried out in a Petri dish. NS of different sizes can be observed from early time points. Arrows indicate NS fusion. White arrowheads show cells delaminating from NS. (C) Representative images during time course NS differentiation performed in non-TC treated 96-well plates. Black arrowheads show cells delaminating from NS. Days are indicated at the top. Scale bars represent 100 µm. Please click here to view a larger version of this figure.
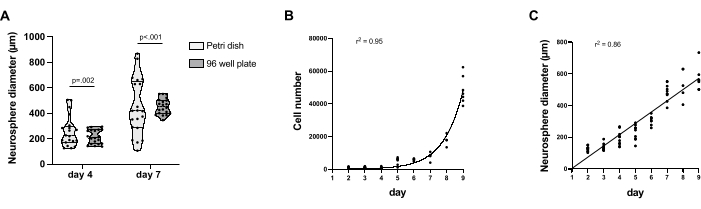
Figure 2: Aggregated NS growth characterization and comparison with Petri dish grown NS. (A) Violin plot showing NS size variation in Petri dish and non-TC treated 96-well plate culture protocol. 20 NS were collected for each protocol and time point. F-tests for standard deviation variability were performed, and results are shown in the bars above the graph. (B) Time course quantification of NS cell number during differentiation in non-TC treated 96-well plates. (C) Quantification of NS diameter size variation during differentiation in non-TC treated 96-well plate. Cell count was performed using CellProfiler 4.2.5. Graph generation and regression analysis were performed using Graphpad Prism 9. For each time point, 5 to 10 NS were quantified. Please click here to view a larger version of this figure.

Figure 3: NS collection and immunofluorescence analysis workflow. Schematic representing NS collection, preparation for immunofluorescence staining, and imaging with indicative time spans for every step indicated at the top. Please click here to view a larger version of this figure.

Figure 4: NS grown in non-TC treated 96-well plate generate CNCC. (A) Representative picture of the imaging slide (1) and the scalpel utilized for cutting the chamber (2). (B) Immunofluorescence images of NS at day 9 (left) and post-migratory CNCC at day 13 (right) showing expression of neuron marker TUJ1. White arrows indicate cell bodies. The dashed square indicates zoom regions shown in the middle panels. Scale bar represents 100 µm. (C) Immunofluorescence images of NS at day 9 (top) and post-migratory CNCC at day 13 (bottom) showing expression of CNCC specification marker AP2α, CNCC markers PAX7 and SOX9, and EMT marker TWIST1. Scale bar represents 100 µm. (D) Column bar graphs comparing relative gene expression in mESC (in black) and post-migratory CNCC (in grey). Expression of CNCC (Ap2α, Sox9 and Sox10), neuronal (Eya2, Ascl1, Neurog2) and ectomesenchyme (Mef2c, Alx4, Ets1), marker genes is compared to the average expression of housekeeping genes ActinB, Ywhaz, and Tbp. Error bars represent the standard errors calculated from three technical replicates (asterisk = p<0.05, double asterisk = p<0.01). Graphs were generated using GraphPad Prism 9. Please click here to view a larger version of this figure.
Table 1: Culture media compositions. Please click here to download this Table.
Table 2: Primary and secondary antibodies utilized in the study. Please click here to download this Table.
Table 3: Primers sequences. Please click here to download this Table.
Discussion
In vitro 3D differentiation models allow analyzing complex cell interactions that could be difficult - or could not - be observed in 2D cell culture. Several models have been developed to study CNCC development in vitro. These are generally directly derived from ESC colonies7,21 or tissue explants22,23. Although these systems have proven efficient for generating neural crest cells, such methods show significant sensibility to the culturing method and microenvironment, which leads to differences in both cell composition and cell properties inside NS24. Furthermore, these methods do not account for the initial heterogeneity of ESC colony size and cell number.
To address these limitations, this paper outlines a method for generating single NS through mESC aggregation in non-TC treated 96-well plates. This protocol allows for standardization of the starting conditions by generating a single-cell suspension from mESC colonies and controlling the initial cell number in the experiment. This leads to a reduction in NS diameter size variability and cell number during differentiation. Moreover, generating a single NS per well abrogates NS fusion and prevents neighboring NS from influencing the microenvironment by releasing extracellular factors that cannot be controlled. Using a 96-well plate format presents additional advantages such as (i) assessing the influence of the starting culture condition, such as cell states or cell number; (ii) testing a high number of culture conditions in a standardized environment; (iii) performing live-imaging experiments; and (iv) designing high throughput genetic perturbation screen using siRNA-mediated gene silencing. Of note, this protocol can be performed with both mouse and human ESC lines9,25. Together, these will allow one to investigate further the molecular mechanisms controlling CNCC plasticity and cell fate decisions.
A limitation of this protocol is the reduced number of NS produced, which results in fewer CNCC for the following culture. While CNCC can later be amplified10, this limits the usage of this protocol for procedures that require a large number of cells. A second potential drawback is that NS manipulation requires more dexterity, and this can lead to losing samples - especially in the earlier time points - and thus further reducing the sample size. This can be overcome by increasing the number of plates used.
A critical aspect of the protocol is the generation of a single-cell suspension from mESC. It is fundamental that any feeder cell used to culture mESC is removed prior to aggregation, as they interfere with aggregation and lead to unexpected phenotypes and misleading results.
In summary, this method offers a reproducible, scalable and highly modulable way to produce NS to use in parallel with in vivo studies.
Disclosures
The authors declare no conflict of interest.
Acknowledgements
We thank Dr. Remi Xavier Coux for advice on primer design and expertise in cell culture. This work was supported by the European Research Council (ERC Starting Grant 101039995 - REGENECREST) and the Fondation pour la Recherche Médicale (Amorçage - AJE202205015403).
Materials
| Name | Company | Catalog Number | Comments |
| 0.22 μm syringe filters | ClearLine | 146560 | |
| 15 mL High-Clarity Polypropylene Conical Tube | Falcon | 352096 | |
| 200 µL ClearLine Plus Low Binding Filter Tips | Dutscher | 713263 | |
| 40 µm filters | Falcon | 352340 | |
| 5 mL Serological pipette | Starstedt | 86.1253.001 | |
| 50 mL High-Clarity Polypropylene Conical Tube | Falcon | 352070 | |
| Accutase | Merck-Sigma | A6964 | |
| Alexa Fluor 488 donkey anti rabbit IgG (H+L) | Thermofisher Scientific | A21206 | |
| Alexa Fluor 594 donkey anti mouse IgG (H+L) | Thermofisher Scientific | A21203 | |
| Alexa Fluor 647 donkey anti goat IgG (H+L) | Thermofisher Scientific | A31571 | |
| Antibiotic-antimycotic solution | Merck-Sigma | A5955 | |
| B27 PLUS supplement | Thermofisher Scientific | 17504044 | |
| Bovine serum albumin (BSA) | Merck-Sigma | A9418 | |
| Chloroform | Carlo Erba | 438601 | |
| Collagenase Type IV | Thermofisher Scientific, Gibco | 17104019 | |
| Costar 6 well clear TC-treated multiple well plates | Corning | 3516 | |
| Cover glasses, round | VWR | 630-2113 | |
| DMEM KnockOut | Thermofisher Scientific | 10829018 | |
| DMEM/F12+Glutamax | Thermofisher Scientific | 10565018 | |
| DMEM high glucose | Merck-Sigma | D0822 | |
| DNA LoBind Tubes, 2 mL | Eppendorf | 30108078 | |
| DNase/RNase-Free Distilled Water | Thermofisher Scientific | 10977-035 | |
| Dulbecco’s Phosphate Buffered Saline (PBS) | Thermofisher Scientific | 14190144 | |
| Eppendorf Safe-Lock Tubes, 0.5 mL | Eppendorf | 30121023 | |
| Eppendorf Safe-Lock Tubes, 2 mL | Eppendorf | 30120094 | |
| ESGRO mLIF Medium Supplement | Merck-Sigma | ESG1107 | |
| Ethanol 70% | Carlo Erba | 528170 | |
| Fetal Bovine Serum | Merck-Sigma | F7524 | |
| Fibronectin | Merck-Sigma | F085-2MG | |
| Fluoromount-G | Invitrogen | 00-4958-02 | |
| Gelatin solution | Merck-Sigma | ES-006-B | |
| GlutaMAX | Thermofisher Scientific | 35050061 | |
| Human EGF | Peprotech | AF-100-15-500UG | |
| Human FGF-basic | Peprotech | 100-18B | |
| Human SOX9 Antibody | R&Dsystems | AF3075 | |
| Insulin from bovine pancreas | Merck-Sigma | I6634 | |
| iScript cDNA Synthesis Kit | Biorad | 1708891 | |
| Mouse Anti-Human AP-2 alpha Monoclonal Antibody, Unconjugated | DSHB | 3B5 | |
| Mouse Anti-Human PAX7 Monoclonal Antibody, Unconjugated | DSHB | PAX7 | |
| N2 supplement | Thermofisher Scientific | 17502048 | |
| Neurobasal Medium | Thermofisher Scientific | 21103049 | |
| Non-Tissue culture treated plate, 96 well, Flat bottom | Falcon | 351172 | |
| Non-Tissue culture treated plate, 96 well, U-bottom | Falcon | 351177 | |
| Paraformaldehyde 16% solution, em grade | Electron Microscopy Sciences | 15710 | |
| Propan-2-ol | Carlo Erba | 415154 | |
| Purified anti-Tubulin β 3 (TUJ1) Antibody | Biolegend | MMS-435P | |
| RapiClear 1.47 | Sunjin Lab | RC147001 | |
| RapiClear 1.52 | Sunjin Lab | RC152001 | |
| Scotch Double Sided 12.7 mm × 22.8 m | Clear fibreless double sided tape | ||
| SensiFAST SYBR No-ROX Kit | Meridian Bioscience | BIO-98020 | |
| Sterile Disposable Surgical Scalpels | Swann-Morton | 05XX | |
| Superfrost Plus Adhesion Microscope Slides | Epredia | J1800AMNZ | |
| Triton X-100 | Thermofisher Scientific | A16046.AP | |
| TRIzol Reagent | FisherScientific | 15596026 | |
| Trypsine-EDTA (0.05%) | Thermofisher Scientific | 25300054 | |
| Tween-20 | Fisher Scientific | 10113103 | |
| TWIST1 Rabbit mAb (IF Formulated) | Cell signaling technology | E7E2G | |
| β-mercaptoethanol | Thermofisher Scientific | 31350010 |
References
- Rothstein, M., Bhattacharya, D., Simoes-Costa, M. The molecular basis of neural crest axial identity. Dev Biol. 444, S170-S180 (2018).
- Smeriglio, P., Zalc, A. Cranial neural crest cells contribution to craniofacial bone development and regeneration. Curr Osteoporos Rep. 21 (5), 624-630 (2023).
- Zalc, A., et al. Reactivation of the pluripotency program precedes formation of the cranial neural crest. Science. 371 (6529), eabb4776 (2021).
- Perera, S. N., Kerosuo, L. On the road again: Establishment and maintenance of stemness in the neural crest from embryo to adulthood. Stem Cells. 39 (1), 7-25 (2021).
- Nguyen, B. H., Ishii, M., Maxson, R. E., Wang, J. Culturing and manipulation of O9-1 neural crest cells. J Vis Exp. (140), e58346 (2018).
- Ishii, M., Arias, A. C., Liu, L., Chen, Y. B., Bronner, M. E., Maxson, R. E. A stable cranial neural crest cell line from mouse. Stem Cells Dev. 21 (17), 3069-3080 (2012).
- Rada-Iglesias, A., Bajpai, R., Prescott, S., Brugmann, S. A., Swigut, T., Wysocka, J. Epigenomic annotation of enhancers predicts transcriptional regulators of human neural crest. Cell Stem Cell. 11 (5), 633-648 (2012).
- Cederquist, G. Y., et al. Specification of positional identity in forebrain organoids. Nat Biotechnol. 37 (4), 436-444 (2011).
- Rada-Iglesias, A., Bajpai, R., Swigut, T., Brugmann, S. A., Flynn, R. A., Wysocka, J. A unique chromatin signature uncovers early developmental enhancers in humans. Nature. 470 (7333), 279-283 (2011).
- Prescott, S. L., et al. Enhancer divergence and cis-regulatory evolution in the human and chimp neural crest. Cell. 163 (1), 68-83 (2015).
- Chaddah, R., Arntfield, M., Runciman, S., Clarke, L., van der Kooy, D. Clonal neural stem cells from human embryonic stem cell colonies. J Neurosci. 32 (23), 7771-7781 (2012).
- Bajpai, R., et al. CHD7 cooperates with PBAF to control multipotent neural crest formation. Nature. 463 (7283), 958-962 (2010).
- Sipahi, R., Zupanc, G. K. Stochastic cellular automata model of neurosphere growth: Roles of proliferative potential, contact inhibition, cell death, and phagocytosis. J Theor Biol. 445, 151-165 (2018).
- Mori, H., et al. Effect of neurosphere size on the growth rate of human neural stem/progenitor cells. J Neurosci Res. 84 (8), 1682-1691 (2006).
- Kress, C., Vandormael-Pournin, S., Baldacci, P., Cohen-Tannoudji, M., Babinet, C. Nonpermissiveness for mouse embryonic stem (ES) cell derivation circumvented by a single backcross to 129/Sv strain: establishment of ES cell lines bearing the Omd conditional lethal mutation. Mamm Genome. 9 (12), 998-1001 (1998).
- Simões-Costa, M., Bronner, M. E. Establishing neural crest identity: a gene regulatory recipe. Development. 142 (2), 242-257 (2015).
- Memberg, S. P., Hall, A. K. Dividing neuron precursors express neuron-specific tubulin. J Neurobiol. 27 (1), 26-43 (1995).
- Kim, C. N., Shin, D., Wang, A., Nowakowski, T. J. Spatiotemporal molecular dynamics of the developing human thalamus. Science. 382, eadf9941 (2023).
- Grimaldi, A., Comai, G., Mella, S., Tajbakhsh, S. Identification of bipotent progenitors that give rise to myogenic and connective tissues in mouse. ELife. 11, e70235 (2022).
- To, K., et al. A multiomic atlas of human early skeletal development. Nature. 635 (8039), 657-667 (2024).
- Bajpai, R., et al. Molecular stages of rapid and uniform neuralization of human embryonic stem cells. Cell Death Differ. 16 (6), 807-825 (2009).
- Kerosuo, L., Nie, S., Bajpai, R., Bronner, M. E. Crestospheres, Long-term maintenance of multipotent, premigratory neural crest stem cells. Stem Cell Reports. 5 (4), 499-507 (2015).
- Abe, S., Yamaguchi, S., Sato, Y., Harada, K. Sphere-derived multipotent progenitor cells obtained from human oral mucosa are enriched in neural crest cells. Stem Cells Transl Med. 5 (1), 117-128 (2016).
- Jensen, J. B., Parmar, M. Strengths and limitations of the neurosphere culture system. Mol Neurobiol. 34 (3), 153-161 (2006).
- Ziegler, L., Grigoryan, S., Yang, I. H., Thakor, N. V., Goldstein, R. S. Efficient generation of Schwann cells from human embryonic stem cell-derived neurospheres. Stem Cell Rev Rep. 7 (2), 394-403 (2011).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved