Method Article
Evaluating Salidroside as a Therapeutic Agent for Vascular Calcification Using Network Pharmacology and Experimental Rat Models
* These authors contributed equally
In This Article
Summary
This study establishes a rat model of vascular calcification induced by a high-fat diet (HFD) combined with vitamin D3 (VD3). The model was used to evaluate the therapeutic efficacy of salidroside in preventing and treating vascular calcification, providing insights into its potential mechanisms of action through network pharmacology and in vivo experiments.
Abstract
Vascular calcification (VC) is a critical pathological condition associated with significant morbidity and mortality. This study employs a hybrid approach of network pharmacology and molecular biology to delineate the therapeutic mechanisms of salidroside (SAL), an active compound from Rhodiola crenulata, against VC. Through database mining and network analysis, 388 SAL targets intersecting with 2871 VC-associated targets were identified, resulting in 208 common targets. A protein-protein interaction (PPI) network constructed via the String database and topological analysis in Cytoscape 3.9.1 pinpointed 10 key targets, including IL6, TNF, TP53, IL1B, HIF1A, CASP3, and STAT3, among others. The identified genes were concentrated in the lipid and atherosclerosis pathways, indicating that the improvement of VC by SAL may occur through the regulation of abnormal expression of lipid and inflammatory factors. It was also found that SAL inhibits the abnormal expression of inflammatory factors, thereby activating the JAK2/STAT3 pathway to intervene in the progression of VC. The JAK2/STAT3 pathway is a key molecular mechanism by which SAL prevents further deterioration of VC. Functional enrichment analyses revealed the involvement of these targets in inflammatory responses and lipid metabolism, pivotal pathways in VC. In vivo studies in rats demonstrated SAL's efficacy in mitigating dyslipidemia and vascular inflammation, with improved serum lipid profiles and reduced vascular calcium deposition. The mechanistic exploration, grounded in Western blot analysis, demonstrated salidroside's ability to regulate the JAK2/STAT3 signaling pathway, highlighting its potential as a modulator in this critical molecular mechanism and offering a potential therapeutic target for VC. The strength of this research lies in its methodological rigor, integrating computational predictions with in vivo validations. This comprehensive approach establishes a robust framework for exploring the therapeutic mechanisms of natural compounds in combating VC.
Introduction
Vascular calcification (VC) refers to the abnormal deposition of calcium within the vessel walls, which leads to arterial stiffening and decreased elasticity, ultimately impairing vascular function. Traditionally, VC has been divided into two types: intimal calcification, linked to lipid buildup, and medial calcification. The former is closely associated with inflammatory infiltration, triggering an osteogenic transformation in the vascular wall, characterized by the migration, proliferation, and differentiation of vascular smooth muscle cells (VSMCs) into osteoblast-like cells1.
The ability of VSMCs to undergo osteogenic differentiation, influenced by factors such as aging, genetics, and environmental conditions like diabetes and chronic kidney disease, is a major contributor to age-related VC. This osteoblast-like transformation exacerbates arterial calcification and degeneration1.
VC is a multifaceted condition, driven by degenerative changes, metabolic imbalances, and various systemic conditions. Approximately 80% of vascular injuries and 90% of coronary artery disease cases exhibit VC, significantly increasing the risk of severe cardiovascular events1,2. Therefore, there is a pressing need to discover pharmacological treatments that effectively mitigate or reverse this condition.
Currently, treatment strategies for VC involve various pharmacological interventions, though no drugs are specifically designed for this purpose. For patients with mild calcification, statins are often prescribed to stabilize plaques. However, while they may reduce coronary artery stenosis by lowering lipid levels, their effect on calcification is limited2.
Given the complexities of atherosclerosis, many patients exhibit enhanced platelet activation, necessitating the use of antiplatelet drugs like aspirin or clopidogrel to inhibit platelet aggregation and reduce the risk of thrombosis. However, aspirin therapy is only beneficial for individuals with a high coronary artery calcium score and a low risk of bleeding3.
Additionally, research into supplements, such as vitamin K, suggests potential in preventing VC progression4. In severe cases, invasive interventions may be considered, although they are often unsuitable for widespread VC5. For individuals without existing VC, managing risk factors, such as blood pressure, lipid profiles, and lifestyle choices, remains critical6.
Rhodiola crenulata, a perennial herb of the Crassulaceae family, has been traditionally utilized in Chinese medicine. Its principal bioactive constituent, salidroside, commands significant attention due to its notable biological activities. Salidroside is renowned for its ability to inhibit apoptosis, exhibit robust antioxidant properties, and possess anti-inflammatory characteristics7,8. These attributes contribute to its potential to enhance vascular function, delay vascular aging, and safeguard the vascular endothelium. As a potential therapeutic agent for VC, salidroside holds substantial value for research. However, the precise mechanisms by which salidroside ameliorates VC remain to be fully elucidated and warrant further investigation to harness its therapeutic potential in the treatment of VC.
To explore these mechanisms, this study leverages network pharmacology, an innovative methodology that combines pharmacology, bioinformatics, and computer science to analyze biological systems and elucidate drug mechanisms. Compared to traditional single-target drug research, network pharmacology offers a more comprehensive approach by analyzing a drug's effects on multiple biological targets and signaling pathways. As a key tool in modern drug development, it constructs networks of drugs, targets, and pathways to reveal the underlying mechanisms of drug action9,10. Despite its extensive use in exploring therapeutic mechanisms, there has been limited research into the interactive mechanisms between salidroside and VC from the perspectives of bioinformatics and network pharmacology.
This research constructs a molecular network map of salidroside's potential impact on VC by identifying and analyzing key targets through extensive database mining. A protein-protein interaction (PPI) network is generated, and topological analysis is applied to highlight critical nodes in the calcification process.
To confirm the computational predictions, a rat model of VC is developed by administering a high-fat diet with vitamin D3 (VD3). This model replicates the pathological features of human VC. Vascular injury is assessed through histological techniques, serum lipid profiles and inflammation markers are evaluated to investigate the systemic effects of salidroside, and the expression of SAL anti-VC related proteins is measured using Western blotting to exploring the impact of salidroside on experimentally induced VC, this study aims to contribute valuable insights into the potential of this compound as a therapeutic strategy for combating VC.
Protocol
The protocol was approved by the Experimental Animals Committee of Changchun University of Chinese Medicine (Approval No. 2023091). This study adheres to international guidelines, including the European Community Guidelines and the EEC Directive of 1986, ensuring the ethical treatment of animals throughout the study. Male Wistar rats (8-10 weeks, weight 200-220 g) were used for the study. The details of the reagents and equipment used are listed in the Table of Materials.
1. Network pharmacology prediction of potential salidroside-VC targets
NOTE: Network pharmacology utilizes computational methods and large-scale data analysis to investigate the complex interactions between drug molecules and biological targets such as pathways, genes, and proteins within an organism11,12. This approach helps to decipher the biological functions and relationships of the studied entities. The methodology encompasses database utilization, processing of chemical information, acquisition of bioactivity data, retrieval of protein data, analysis of gene expression profiles, construction of interaction networks, and enrichment analysis of pathways11. Figure 1 shows the interaction network of core targets between salidroside and vascular calcification.
- Construction of the "Ingredient" target database
- Use "Salidroside" as the keyword for ingredients to search databases13 (see Supplementary Table 1), such as HERB, TCMSP, PubChem, SwissTargetPrediction, CTD, PharmMapper, SEA, and STITCH.
- Review relevant literature to identify targets associated with salidroside, with the species set to Homo sapiens. After removing duplicates, standardize the target proteins using UniProt (see Supplementary Table 1) and establish a comprehensive Salidroside target database14.
- Construction of the "Disease" target database
- Use "Vascular calcification" as the keyword to search databases15 (See Supplementary Table 1), including GeneCards, OMIM, PharmGkb, and DrugBank, with the species set to Homo sapiens. After de-duplication, create a vascular calcification target database.
- Prediction of potential therapeutic targets
- Input the targets for salidroside and vascular calcification (see Supplementary Table 1) to identify common targets. Generate a Venn diagram to visualize the potential therapeutic targets for salidroside in treating vascular calcification.
- Construction of the "Salidroside-Vascular Calcification" Protein-Protein Interaction (PPI) network
- Compile the potential targets into a Multiple Proteins List and analyze them using STRING (see Supplementary Table 1), with the organism set to Homo sapiens and the interaction score set to medium confidence (>0.4)16. Extract the PPI data in TSV format for further analysis.
NOTE: Given that 85% of rat genes are homologous to human genes, performing similar biological functions, rats were selected as experimental subjects for validating the effects of salidroside on vascular calcification17.
- Compile the potential targets into a Multiple Proteins List and analyze them using STRING (see Supplementary Table 1), with the organism set to Homo sapiens and the interaction score set to medium confidence (>0.4)16. Extract the PPI data in TSV format for further analysis.
- Selection and network construction of key targets
- Import the PPI network data into Cytoscape 3.9.1 (see Supplementary Table 1) for analysis using the CytoNCA plugin to assess parameters such as Betweenness (BC), Closeness (CC), Degree (DC), Eigenvector (EC), Local Average Connectivity (LAC), and Network Centrality (NC). Select key targets with CC=1 to construct a core target action spectrum map for 'SAL-VC'.
- Use the CytoHubba plugin to identify the top 10 hub genes based on Maximal Clique Centrality (MCC), Maximum Neighborhood Component (MNC), and Degree calculations, generating a Hub-genes spectrum map.
- GO and KEGG pathway enrichment analysis
- Perform gene ID conversion using DAVID (see Supplementary Table 1), selecting ENSEMBL_GENE_ID for the gene type-9606 for species information. Analyze the converted gene list using Omicshare (see Supplementary Table 1) for GO functional and KEGG pathway enrichment, with significance set at P value < 0.0518.
NOTE: GO functional analysis includes molecular function (MF), biological process (BP), and cellular component (CC). KEGG pathway analysis involves pathway enrichment and pathway classification enrichment19. The enrichment analysis results are depicted in Figure 2.
- Perform gene ID conversion using DAVID (see Supplementary Table 1), selecting ENSEMBL_GENE_ID for the gene type-9606 for species information. Analyze the converted gene list using Omicshare (see Supplementary Table 1) for GO functional and KEGG pathway enrichment, with significance set at P value < 0.0518.
2. Animal experiment
- Acclimatization
- Acclimate Wistar rats under specific pathogen-free (SPF) conditions with a 12-h light/dark cycle. Ensure they have ad libitum access to food and water to maintain their health prior to the commencement of experiments. Perform adaptive feeding for 1 week.
- Model establishment
- Acclimate the rats to the environmental conditions and randomly assign them into five groups: Control group (Ctrl, ND + Vehicle), Model group (Model, HFD + Vehicle), SAL low-dose group (SAL-L, HFD + SAL 5mg/kg), SAL high-dose group (SAL-H, HFD + SAL 10mg/kg), and Simvastatin (SIM) group (HFD + SIM 5mg/kg).
- Administer a normal diet (ND) to the Ctrl group and a high-fat diet (HFD) to the remaining groups for the entire duration of the experiment. On the first day of HFD administration, inject a single subcutaneous dose of 600,000 IU/kg VD3 to all groups except the Ctrl group20,21. Follow with weekly subcutaneous injections of 100,000 IU/kg VD3 for the following 8 weeks (Figure 3).
- Monitor the health status and survival of the animals daily. Begin experimental interventions in the ninth week.
- Euthanize the animals at the conclusion of the study (following the institutionally approved protocols). Collect serum samples, allow them to stand for 30 min, and centrifuge to isolate the serum (following previously published reports20,21). Dissect vascular tissues (abdominal aorta), and rinse with phosphate-buffered saline (PBS) to remove blood.
- Fix one portion of the tissue in 4% paraformaldehyde for histological examination and store another portion in liquid nitrogen for molecular analysis.
NOTE: Dilute salidroside with warm water before use.
3. Evaluation of vascular tissue injury using HE, VK, EVG staining
NOTE: Fix vascular tissue (abdominal aorta) in 4% paraformaldehyde, dehydrated in ethanol after 48 h, and embedded in paraffin. Cut the embedded paraffin blocks into 5 µm slices for Hematoxylin-Eosin (HE), Elastica van Gieson (EVG), and Von Kossa (VK) staining, and observe the histological morphology under a light microscope. HE staining is used to assess changes in tissue morphology. In vascular tissue, it highlights structural alterations in the vessel wall, including smooth muscle cell proliferation, disorganized cell arrangement, and inflammation. EVG staining visualizes elastic and collagen fibers, which is essential for evaluating elastic fiber damage or remodeling in vascular tissue and helps in understanding the impact of calcification on vascular elasticity. VK staining detects calcium deposits, a key feature in VC, making it crucial for assessing the extent and distribution of calcification in vascular tissue22,23.
- HE staining for vascular tissue damage detection
- Deparaffinization and rehydration
- Deparaffinize sections in two changes of xylene for 8 min each and rehydrate through a graded ethanol series (100%, 95%, 85%, 75%) for 3 min per step. Follow with a 2-min rinse in running tap water.
- Hematoxylin staining
- Stain the sections with hematoxylin for 5-10 min and wash to remove excess stain, followed by a rinse with running tap water.
NOTE: A light stain of 5 min is recommended to avoid overly dark staining, which can affect cytoplasmic color.
- Stain the sections with hematoxylin for 5-10 min and wash to remove excess stain, followed by a rinse with running tap water.
- Differentiation
- Differentiate the sections in a differentiation solution for 30 s, followed by two rinses in tap water for 3 min each.
- Eosin counterstaining
- Place the sections in eosin stain for 1 min. After removing the excess stain, perform rapid dehydration.
- Dehydration, clarification, and mounting
- For rapid dehydration, dip the sections in 75%, 85%, 95%, and 100% ethanol for 3 s each, followed by 100% ethanol for 1 min.
NOTE: Rapid dehydration is recommended as eosin may lose color in water and ethanol gradients.
- For rapid dehydration, dip the sections in 75%, 85%, 95%, and 100% ethanol for 3 s each, followed by 100% ethanol for 1 min.
- Deparaffinization and rehydration
- VK staining for calcium detection
- Deparaffinization and rehydration
- Perform this step following step 3.1.1.
- Silver nitrate reaction
- Blot the sections dry, outline with a fine brush, and stain with Von Kossa (see Table of Materials). Expose them to ultraviolet light for 4 h, then thoroughly rinse with running tap water.
- Counterstaining with hematoxylin
- Stain the sections with hematoxylin for 5 min, rinse in running tap water, differentiate, and rinse again, followed by a running tap water rinse.
- Eosin counterstaining
- Dehydrate the sections through graded ethanol (85% and 95% for 5 min each), then stain with eosin for 5 min.
- Dehydration and mounting
- Dehydrate the sections in ethanol baths (100% Ethanol I, II, III for 5 min each) and clarify in xylene baths (Xylene I and II for 5 min each). Then, mount the sections with neutral balsam.
- Microscopic examination and image capture
- Examine the stained sections under a light microscope and capture images for analysis.
NOTE: Ensure that calcium deposits appear brown-black to dark black, nuclei are blue, and the background is red. Prepare the silver nitrate solution for fresh VK staining immediately before use.
- Examine the stained sections under a light microscope and capture images for analysis.
- Deparaffinization and rehydration
- EVG staining for elastic and collagen fibers
- Deparaffinization and rehydration
- Treat the paraffin sections with heat for 50 min, then immerse them in xylene for 20 min to remove paraffin. Proceed by subjecting the sections to a graded ethanol series (100%, 95%, 85%, 75%) for 5 min each, concluding with a rinse in running tap water for 5 min.
- EVG staining
- Apply the EVG staining solution (see Table of Materials) for 10 min, then rinse briefly in running tap water for 5 s to remove excess stain.
- Next, apply Verhoeff working solution (see Table of Materials) for 5 min, followed by another brief rinse in running tap water for 5 s. Apply Verhoeff's differentiating solution for 5 s until the elastin fibers are clearly visible, and then rinse with running tap water for 5 s.
NOTE: Mix components A, B, and C (provided in the commercially available kit) in a 5:2:2 ratio to prepare the Verhoeff working solution before use. Use the solution within 2 h and replenish as needed during the staining process to prevent the sections from drying out.
- Dehydration and mounting
- Dehydrate the sections through a graded ethanol series (75%, 85%, 95%, and 100%) for 5 s each, followed by a clarification in two changes of xylene for 1 min each. After a brief air-drying period, mount the sections with neutral balsam.
- Microscopic examination and image analysis
- Examine the stained sections under a light microscope to visualize elastic and collagen fibers, and capture images for subsequent analysis.
- Deparaffinization and rehydration
4. Alkaline Phosphatase (ALP) assay
NOTE: Use ALP as a key indicator to evaluate the effectiveness of anti-calcification treatments.
- Reagent preparation
- Dissolve the color-developing substrate in ice-cold 0.05 M pH 9.6 carbonate buffer to a final volume of 2.5 mL.
NOTE: Prepare the buffer by dissolving 1.59 g of sodium carbonate and 2.93 g of sodium bicarbonate in 1,000 mL of double-distilled water.
- Dissolve the color-developing substrate in ice-cold 0.05 M pH 9.6 carbonate buffer to a final volume of 2.5 mL.
- Substrate dilution
- Dilute 10 µL of p-nitrophenol solution (10 mM) with 0.05 M pH 9.6 carbonate buffer to a final volume of 0.2 mL to achieve a final concentration of 0.5 mM.
- Sample preparation
- Homogenize the abdominal aorta in lysis buffer. Centrifuge the homogenate (~12,000 x g for 10 min at 4 °C) and collect the supernatant for ALP activity detection.
NOTE: Ensure the lysis buffer is free of phosphatase inhibitors. Store pending test samples at -80 °C, avoiding repeated freeze-thaw cycles.
- Homogenize the abdominal aorta in lysis buffer. Centrifuge the homogenate (~12,000 x g for 10 min at 4 °C) and collect the supernatant for ALP activity detection.
- Microplate preparation for assay
- Prepare a 96-well plate with blank, standard, and sample wells. Add 50 µL of the standard solution to standard wells and 50 µL of pending test samples to sample wells. Incubate the plate at 37 °C for 10 min.
- Reaction termination and absorbance measurement
- Add 100 µL of stop solution to each well to terminate the reaction. Measure the absorbance at 405 nm and calculate ALP activity based on absorbance values (following the manufacturer's instructions, see Table of Materials).
NOTE: Wells containing the standard or samples with ALP activity will show varying shades of yellow. The color is stable for up to 2 h.
- Add 100 µL of stop solution to each well to terminate the reaction. Measure the absorbance at 405 nm and calculate ALP activity based on absorbance values (following the manufacturer's instructions, see Table of Materials).
5. Calcium content determination
NOTE: Calcium content determination is critical for assessing the extent of mineralization in biological tissues.
- Tissue preparation
- Mince the tissue into small pieces and homogenize in lysis buffer.
- Sample dilution and centrifugation
- Dilute the tissue in lysis buffer at a ratio of 1:10. Homogenize the mixture and centrifuge at 4 °C, 12,000 x g for 5 min. Collect the supernatant for analysis.
NOTE: Prepare calcium standard dilutions (0-1.0 mM) using a 5 mM calcium standard solution (see Table of Materials).
- Dilute the tissue in lysis buffer at a ratio of 1:10. Homogenize the mixture and centrifuge at 4 °C, 12,000 x g for 5 min. Collect the supernatant for analysis.
- Plate setup and incubation
- Add 50 µL of standard or test samples to each well of a 96-well plate. Add 150 µL of assay working solution, mix thoroughly, and incubate the plate in the dark at room temperature for 10 min. Measure the absorbance at 575 nm and construct a standard curve.
- Calculation of calcium content
- Calculate the calcium content using the standard curve, incorporating the dilution factor, sample volume, and the atomic weight of calcium.
6. Enzyme-Linked Immunosorbent Assay (ELISA) for inflammatory cytokines (IL-6, TNF-α, IL-1β)
NOTE: IL-6, IL-1β, and TNF-α are key pro-inflammatory cytokines that indicate the presence and severity of an inflammatory response. Measuring these cytokines is essential for understanding the inflammatory process and evaluating the effectiveness of anti-inflammatory treatments.
- Sample collection
- Collect serum from the rat's abdominal aorta. Allow it to coagulate at room temperature for 2 h. Centrifuge the sample at 3,000 x g for 10 min at 4 °C and collect the supernatant.
- Microplate preparation for the assay
- Prepare a 96-well plate with wells designated for standards and test samples. Add 50 µL of standards (see Table of Materials) at varying concentrations to the appropriate wells.
- Sample preparation
- Add 40 µL of sample diluent to each well designated for test samples. Add 10 µL of the sample to the same wells, resulting in a 5-fold dilution.
- Incubation with enzyme-labeled reagents
- Add 100 µL of enzyme-labeled reagents specific to IL-6, TNF-α, or IL-1β to all wells except the blank. Incubate the plate at 37 °C for 60 min.
- Washing
- Discard the liquid from all wells except the blank. Wash the wells with 1x wash solution (prepared as a 20-fold dilution in distilled water). Repeat this washing step five times and dry the wells.
- Color development and termination
- Add 50 µL of substrate solutions A and B (from the commercially available kit, see Table of Materials) to each well. Mix gently and incubate the plate at 37 °C in the dark for 15 min. Add 50 µL of stop solution to each well to terminate the reaction.
- Absorbance measurement and data analysis
- Set the blank well as zero. Measure the absorbance at 450 nm using a microplate reader. Generate a standard curve based on the OD values and standard concentrations. Calculate the sample concentrations using interpolation and adjust for the dilution factor.
7. Lipid profile assay
NOTE: The Lipid profile assay detects abnormal lipid levels, where elevated or imbalanced lipid levels can accelerate the risk of vascular calcification.
- Total cholesterol and triglycerides (TC and TG)
- Collect serum and prepare a 96-well plate with designated wells for blank, calibration, and pending test samples. Add 2.5 µL of serum, calibration standard, or blank solution to each well.
- Add 250 µL of the working solution to each well. Mix gently and incubate the plate at 37 °C for 10 min. Measure the absorbance at 500 nm using a microplate reader.
- Low-density lipoprotein and high-density lipoprotein (LDL-C and HDL-C)
- Collect serum and prepare a 96-well plate with wells designated for blank, calibration, and pending test samples. Add 2.5 µL of serum, calibration standard, or blank solution to the respective wells.
- Add the appropriate reagent (Reagent One for 180 μL or Reagent Two for 60 μL, see Table of Materials) to each well as specified in the assay kit instructions. Incubate at the temperature (37 °C) and for the time (Reagent One for 5 min or Reagent Two for 10 min) recommended for each reagent.
- Measure the absorbance at the specific wavelength (600 nm) indicated for LDL-C or HDL-C using a microplate reader.
8. Western blotting
NOTE: Western blot (WB) is instrumental in assessing the expression levels of key proteins, allowing for the detection of both total and phosphorylated forms.
- Tissue preparation
- Weigh 0.05 g of tissue and rinse thoroughly with PBS to remove excess debris. Add 500 µL of lysis buffer containing 1x phosphatase and protease inhibitors. Incubate the tissue at 4 °C for 10 min and homogenize it using a tissue grinder.
- Protein extraction
- Allow the homogenized sample to stand for 1 min, then centrifuge at 4 °C and 12,000 x g for 15 min. Collect the supernatant for protein analysis. Determine the protein concentration using the BCA method following the manufacturer's instructions (see Table of Materials). Use a standard curve to calculate the concentration.
NOTE: Pre-cool the centrifuge to 4 °C before use. Measure protein concentration at 562 nm.
- Allow the homogenized sample to stand for 1 min, then centrifuge at 4 °C and 12,000 x g for 15 min. Collect the supernatant for protein analysis. Determine the protein concentration using the BCA method following the manufacturer's instructions (see Table of Materials). Use a standard curve to calculate the concentration.
- Sample preparation
- Mix the protein sample with 5x loading buffer containing β-mercaptoethanol and SDS in a 4:1 ratio. Heat the mixture at 100 °C for 5 min to denature the proteins.
NOTE: If 1x loading buffer is required, dilute the 5x buffer with lysis buffer.
- Mix the protein sample with 5x loading buffer containing β-mercaptoethanol and SDS in a 4:1 ratio. Heat the mixture at 100 °C for 5 min to denature the proteins.
- SDS-PAGE gel preparation
- Prepare a 12% SDS-PAGE gel and place it in the electrophoresis apparatus. Add 1x electrophoresis buffer up to the halfway mark as per the instructions (see Supplementary Table 2).
- Electrophoresis
- Retrieve the protein samples from -20 °C. Load 60 µg of protein per well and run the gel.
- Set the current to 50 mA for 20 min for the stacking gel, then increase to 100 mA for 60 min for the separating gel until the dye front reaches the bottom.
- Membrane transfer
- Activate the PVDF membrane in methanol for 5 min.
- Assemble the transfer sandwich in the following order: filter paper → SDS-PAGE gel → PVDF membrane → filter paper. Transfer proteins at 300 mA for 60 min.
NOTE: Ensure no air bubbles are trapped between the SDS-PAGE gel and PVDF membrane.
- Blocking
- Incubate the PVDF membrane in 5% BSA (prepared in 1x TBST) at room temperature with gentle shaking for 60 min. Wash the membrane with 1x TBST three times for 5 min each.
- Primary antibody incubation
- Incubate the membrane in 5 mL of the appropriate primary antibody (e.g., p-JAK2, JAK2, p-STAT3, STAT3, p-NF-κB p65, NF-κB p65, IκBα) diluted in blocking buffer at room temperature for 2.5 h.
- Secondary antibody incubation
- Wash the membrane with 1x TBST three times for 5 min each after primary antibody incubation. Incubate with the appropriate secondary antibody at room temperature for 1 h. Wash the membrane again with 1x TBST three times for 5 min each.
- Detection
- Visualize protein bands using ECL chemiluminescence detection24 (see Table of Materials).
- Densitometry analysis
- Quantify protein bands using ImageJ software. Follow this workflow in ImageJ: Image → 8-bit → Process → Subtract Background. Analyze → Set Measurements → Analyze → Set Scale. Edit → Invert → Analyze → Measure → Results. File → Save As → IntDen.
- Generate statistical graphs using graphing and statistical analysis software.
NOTE: Ensure results are consistent across replicates to validate findings.
9. Statistical analysis
- Collect and organize data using GraphPad Prism 9.0.
- Calculate and plot error bars using mean ± SD from raw data.
- Perform statistical analysis using one-way ANOVA, followed by Tukey's post-hoc test for multiple comparisons.
- Determine the statistical significance at P < 0.05, with smaller P-values indicating greater differences in the results.
Results
Network pharmacology analysis
Using databases such as HERB, TCMSP, Pubmed, SwissTargetPrediction, CTD, PharmMapper, SEA, and STITCH, 388 potential target genes for salidroside were identified. Additionally, 2871 potential target genes related to VC were retrieved from databases like GeneCards, OMIM, PharmGkb, and DrugBank. Intersection analysis via VENN diagrams revealed 208 overlapping targets, considered key targets for salidroside's intervention in VC (Figure 1A).
PPI network analysis
The STRING platform was used to analyze the interactions among the 208 key targets, from which the top 100 nodes were selected. These nodes were then imported into Cytoscape 3.9.1 to construct a detailed PPI network. Network topology analysis using parameters like EC, BC, NC, LAC, CC, and DC identified 37 core targets. Further refinement focusing on degree, MCC, and MNC pinpointed the top 10 core targets, including IL6, TNF, TP53, IL1B, HIF1A, CASP3, and STAT3 (Figure 1B).
GO functional and KEGG pathway analysis
GO functional analysis of the 208 key targets identified 4808 biological processes, 294 cellular components, and 515 molecular functions. KEGG pathway analysis reported 281 signaling pathways, primarily involving metabolism, genetic and environmental information processing, organismal systems, cellular processes, and human diseases. It was found that most of the identified genes were enriched in the lipid and atherosclerosis pathway, indicating that the key mechanism by which SAL improves VC may involve regulating abnormal changes in lipid and inflammatory factors (Figure 2).
In vivo experiment validation
In comparison to the Ctrl group, the model group rats exhibited obesity, lethargy, and dull fur. Serum lipid profiles showed significant increases in TC, TG, and LDL-C levels and a decrease in HDL-C in the model group (p < 0.05). Conversely, the SAL-L, SAL-H, and SIM groups showed significant improvements in these parameters, with a dose-dependent effect in the SAL groups (p < 0.05) (Figure 4).
HE staining was utilized to identify structural alterations in the rat abdominal aorta, while EVG staining evaluated the condition of the elastic fibers, and VK staining was employed to identify calcium deposits. In the control group, the HE staining delineated the distinct layers of the abdominal aorta tissue, and the EVG staining revealed a neat arrangement of elastic fibers with minimal disruptions. Calcium deposits were not markedly present as per VK staining. Conversely, the model group exhibited vascular tissue with intimal hyperplasia and inflammatory cell infiltration, along with extensive structural disarray in the media layer. The distinction between elastic fibers and smooth muscle cells was indistinct, and the fibers were irregularly arranged, with visible necrotic calcification. Adventitial lymphocyte infiltration was also noted. EVG staining in this group displayed a disordered pattern of elastic fibers, with extensive breaks, and VK staining confirmed substantial calcium deposits.
Following SAL intervention, a noticeable amelioration in the vascular damage was observed by VC. HE staining indicated that the SAL-L and SAL-H groups had well-defined vascular layer structures, with minimal smooth muscle cell degeneration in the media. Post-EVG staining, the elastic fibers in both groups were neatly aligned with few disruptions, with the SAL-H group outperforming the SAL-L group. VK staining showed no significant calcium deposits in either group, suggesting that SAL mitigated the vascular structural alterations induced by VC.
After SIM intervention, the vascular structural changes mirrored those of the SAL-H group. HE staining demonstrated clear abdominal aorta architecture with minimal smooth muscle cell degeneration in the media. EVG staining confirmed the orderly arrangement of elastic fibers with few disruptions, and Von Kossa staining did not detect significant calcium deposits. These findings suggest that the SAL group significantly improved the adverse symptoms associated with VC (Figure 5).
Both calcium ion (Ca2+) and ALP levels were significantly elevated in the model group relative to the Ctrl group(p < 0.05). However, these levels were markedly decreased in the SAL-L and SAL-H groups, with the higher dosage of SAL demonstrating superior efficacy (Figure 6A,B). Additionally, the expression of Bone Morphogenetic Protein-2 (BMP2) was notably enhanced in the model group (p < 0.001), whereas the SAL groups showed a reduction in BMP2 expression to varying extents (p < 0.01) (Figure 6C). Inflammatory factor analysis revealed significant upregulation of TNF-α and IL-6 in the model group (p < 0.05), indicating an inflammatory response due to VC (Figure 6D-F). After SAL treatment, these inflammatory markers decreased significantly (p < 0.05), with better results in the SAL-H group.
Protein expression analysis in vascular tissue showed increased phosphorylation levels of JAK2, STAT3, and NF-κB p65, and decreased expression of IκBα in the model group (p < 0.05). Both SAL-L and SAL-H treatments significantly decreased the phosphorylation levels of JAK2, STAT3, and NF-κB p65, while increasing IκBα expression, suggesting that salidroside mitigates VC progression through the JAK2/STAT3 signaling pathway (Figure 7).
These results highlight the potential therapeutic effects of SAL on VC, supported by network pharmacology and in vivo validation while offering insights into its possible mechanisms of action.

Figure 1: Interaction network of core targets between salidroside and vascular calcification. (A) The flowchart of the interaction network of core targets between salidroside and vascular calcification. (B) Venn diagram showing the overlap between salidroside targets (purple) and vascular calcification targets (green). The intersecting targets are indicated by black text. (C) The potential core target interaction network involved in the effects of salidroside on VC. (1) Top 100 neighboring nodes of the PPI network. Each node represents a target, and each edge represents an interaction between targets. (2) Hub-genes network (37 nodes identified using the CytoNCA plugin). (3) Top 20 neighboring nodes of the PPI network. (4-6) Key targets identified based on Degree, MCC, and MNC criteria using the CytoHubba plugin (10 nodes each). Please click here to view a larger version of this figure.
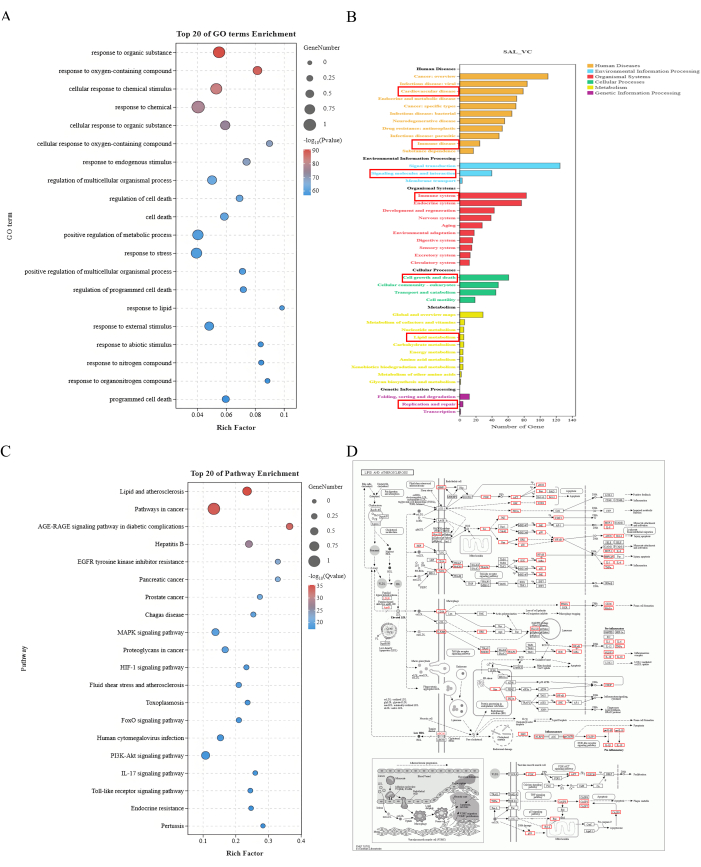
Figure 2: Enrichment analysis results. (A) Top 20 GO term enrichment. The X-axis represents the Rich Factor, and the Y-axis represents the GO terms. The size of the dots indicates the number of genes, and the color represents -log10(P value), with red indicating a smaller P value. (B) KEGG pathway enrichment annotation. The X-axis represents the number of genes, and the Y-axis represents different levels of annotation information. Different colors represent various categories of first-level annotations. (C) Top 20 pathway enrichment. The X-axis represents the Rich Factor, and the Y-axis represents the GO terms. The size of the dots indicates the number of genes, and the color represents -log10(P value), with red indicating a smaller P value. (D) Lipid and atherosclerosis pathway map. Red boxes indicate the potential targets of salidroside intervention in VC that are enriched in the Lipid and atherosclerosis pathway. Please click here to view a larger version of this figure.

Figure 3: Investigation of the effect of salidroside on vascular calcification in rats through in vivo experiments. Flowchart of the experimental design. Wistar rats were used for an 11w experiment. During the first week, the rats were acclimated and then randomly divided into five groups: Ctrl, Model, SAL-L, SAL-H, and SIM for the subsequent experiments. Weeks 1-9 involved feeding the Ctrl group a Normal Diet (ND), while the other groups received a High Fat Diet (HFD) starting from 8w after a single injection of 600,000 IU/kg VD3, followed by weekly injections of 100,000 IU/kg VD3. From weeks 9 to 10, all groups switched to HFD (except the Ctrl group), with the SAL-L and SAL-H groups receiving intraperitoneal injections of salidroside at 5 mg/kg and 10 mg/kg, respectively, while the SIM group received Simvastatin at 5 mg/kg (n = 8). Please click here to view a larger version of this figure.
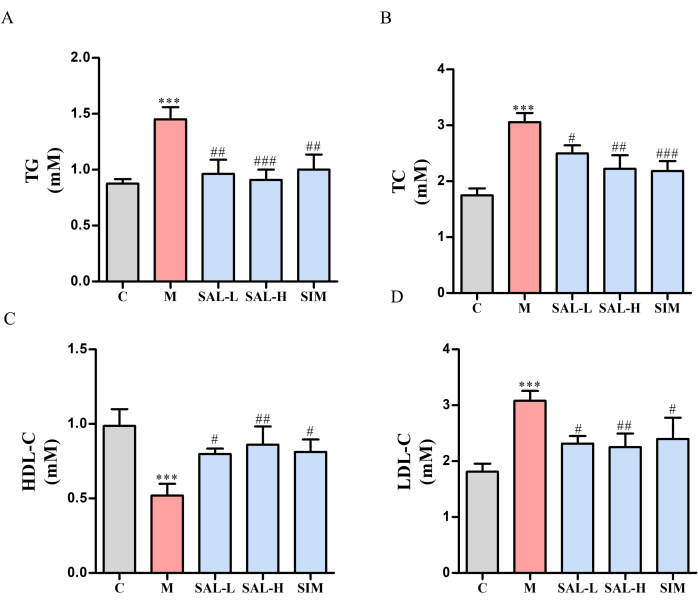
Figure 4: Serum lipid levels in VC rats treated with salidroside. Serum-lipid levels in VC rats were measured using single-reagent GPO-PAP and dual-reagent direct methods. (A) TG levels (single-reagent GPO-PAP method). (B) TC levels (single-reagent GPO-PAP method). (C) HDL-C levels (dual-reagent direct method). (D) LDL-C levels (dual-reagent direct method). Each column represents mean ± SD (n = 8). Compared to the Ctrl group, ***P < 0.001; compared to the Model group, ###P < 0.001, ##P <0.01, #P < 0.05. Please click here to view a larger version of this figure.
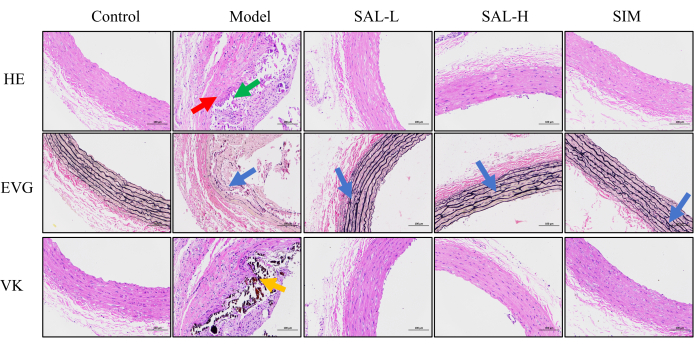
Figure 5: Histological assessment of vascular tissue damage in VC rats. HE staining was used to evaluate tissue damage, with red arrows indicating medial calcification and green arrows indicating lymphocytic infiltration in the adventitia. Nuclei are stained blue, and the cytoplasm is stained red. EVG staining was used to observe damage to elastic fibers in the abdominal aorta, with blue arrows indicating areas of elastic fiber rupture and disorganization. Elastic fibers appear red, and muscle appears pale red. VK staining was used to observe calcium deposition in the abdominal aorta, with orange areas indicating calcium deposits, which appear black (Scale bars: 100 µm, n = 8). Please click here to view a larger version of this figure.
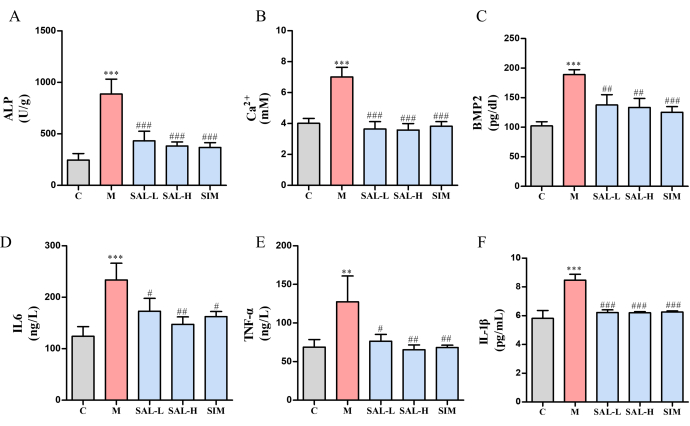
Figure 6: Expression of bone calcification markers and inflammatory cytokines in VC rats treated with salidroside. (A) ALP activity (a marker of vascular calcification). (B) Calcium ion content. (C) BMP2 expression (Bone Morphogenetic Protein). (D) IL-6 expression (pro-inflammatory cytokine). (E) TNF-α expression (pro-inflammatory cytokine). (F) IL-1β expression (pro-inflammatory cytokine). Each column represents mean ± SD (n = 8). Compared to the Ctrl group, ***P < 0.001, **P < 0.01; compared to the Model group, ###P < 0.001, ##P < 0.01, #P < 0.05. Please click here to view a larger version of this figure.
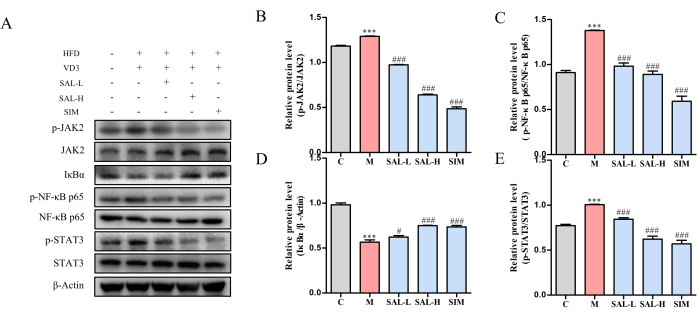
Figure 7: Western blot analysis of key protein expression in VC rats treated with salidroside. (A) Western blot analysis of p-JAK2, JAK2, p-STAT3, STAT3, p-NF-κB p65, NF-κB p65, and IκBα protein expression levels. (B) Relative Protein Expression of P-JAK2/JAK2 in different groups. (C) Relative Protein Expression of p-NF-κB p65, NF-κB p65 in different groups. (D) Relative Protein Expression of IκBα in different groups. (E) Relative Protein Expression of P-STAT3/STAT3 in different groups. Each column represents mean ± SD (n = 8). Compared to the Ctrl group, ***P < 0.001; compared to the Model group, ###P < 0.001, ##P < 0.01, #P < 0.05. Please click here to view a larger version of this figure.

Figure 8: Proposed molecular mechanism of salidroside intervention in VC rats. The proposed molecular mechanism by which salidroside intervenes in VC involves the inhibition of lipid-related factors and pro-inflammatory cytokines (IL-1β, IL-6, TNF-α). Salidroside suppresses the activation of IκBα and phosphorylation of JAK2, thereby inhibiting the NF-κB/STAT3 inflammatory-immune pathway. This ultimately reduces vascular tissue damage associated with VC progression. Please click here to view a larger version of this figure.
Supplementary Table 1: Network pharmacology database information. Please click here to download this File.
Supplementary Table 2: Components of SDS-PAGE (for 1 sample). Please click here to download this File.
Discussion
VC is characterized by degenerative changes in vascular cells and tissues, with pathological mineral deposits within blood vessels leading to stiffening of the vessel walls or the formation of atherosclerotic plaques, which can result in obstructive vascular diseases25. Studies show that about 85% of VC plaques may evolve into thrombosis, which can trigger acute cardiovascular episodes. Additionally, VC is a crucial indicator of potential acute cardiovascular events, strokes, and peripheral vascular diseases26. Current treatment methods primarily focus on anti-coagulation, lipid-lowering drugs, and regulation of vascular tension, but these approaches often have limited efficacy and side effects, particularly in advanced stages of calcification.
Salidroside (SAL), a compound identified as p-hydroxyphenethyl-beta-D-glucopyranoside, has demonstrated significant therapeutic potential in various vascular conditions affecting the nervous, cardiovascular, and immune systems, as well as in chronic kidney diseases27,28. Modern research has confirmed its antioxidant, anti-aging, immunomodulatory, and anti-inflammatory effects29,30,31. Salidroside notably improves endothelial function and enhances blood vessel elasticity by inhibiting vasoconstrictors and promoting vasodilators32. The elasticity of blood vessels directly reflects the changes following calcification, where a loss of elasticity often accompanies the degradation of elastic proteins, damaging the inner and middle layers of blood vessels. This damage often manifests as the activation of inflammatory cells. Numerous studies highlight the close association between VC and inflammation. Chronic inflammation is seen as a key driver of ectopic calcification, where pro-inflammatory cells proliferate and release factors that contribute to VC through multiple pathways33,34,35,36. Salidroside's potential as a multi-target therapeutic agent is particularly relevant given that vascular calcification is a complex pathological process involving inflammation, lipid metabolism, and oxidative stress. The multi-target approach of Salidroside, which inhibits inflammatory pathways while regulating lipid accumulation and oxidative stress, presents a distinct advantage over single-target drugs currently in use. Therefore, in this study, inflammatory factor expression will be examined to further investigate whether salidroside influences VC by reducing inflammation.
The concept of network pharmacology, introduced by Hopkins in 2007, involves using network-based approaches to examine how drugs, diseases, and targets interact across multiple components, targets, and pathways37. In this study, network pharmacology was employed to predict how SAL interacts with VC, pinpointing crucial targets like IL6, STAT3, TNF, TP53, and ALB. The results indicated a concentration of these genes in lipid metabolism and atherosclerosis pathways, suggesting that VC may be linked to lipid accumulation and inflammation in the arteries. To verify these predictions, a rat model of VC induced by a high-fat diet combined with VD3 was used. This model allowed further exploration of the impact of SAL on VC. The findings revealed that VC increased levels of TC, TG, and LDL-C while lowering HDL-C. Moreover, SAL at different doses demonstrated varying effects in regulating these abnormal indicators. Further research results indicated that SAL effectively reduced calcium deposition in the abdominal aorta, corrected abnormal calcium ion levels, and decreased inflammatory cell infiltration, while simultaneously reducing the expression of calcification-related markers such as ALP and BMP2.
The JAK/STAT pathway plays a crucial role in various growth and signaling processes, regulating immune responses and cell differentiation, which makes it integral to inflammatory responses38. STAT3, activated by IL-6, is part of an acute-phase response, with IL-6 inducing JAK phosphorylation, which in turn phosphorylates STAT3. This activation controls gene expression related to cell growth, differentiation, and survival. Persistent phosphorylation of STAT3 has been associated with vascular diseases and can lead to abnormal expression of adhesion molecules, which in early VC stages facilitates the adhesion of monocytes to the vascular intima, further damaging the vascular structure39,40. The experimental results show that SAL inhibits the expression of phosphorylated JAK2, STAT3, and NF-κB p65, while promoting the expression of IκBα, suggesting that the role of SAL in inhibiting VC progression is mediated through the IL-6/JAK2/STAT3 signaling pathway (Figure 8).
In this study, network pharmacology successfully predicted key mechanisms involved in VC progression, identifying the targets and pathways through which SAL intervenes. The physiological and pathological parallels between rat and human systems make rat models particularly valuable for clinical research in the future. Moreover, rats are cost-effective and easy to handle, which makes them suitable for disease research and drug discovery. Despite the advantages of animal models, there are limitations. Rat models, while helpful, cannot fully capture the complexity of VC development in humans. Future studies should involve more diverse models and consider factors such as comorbidities and varying patient backgrounds.
However, this study has several limitations. The experimental period was 10 weeks, during which rats were fed a high-fat diet (HFD) with an initial high dose followed by multiple lower doses of VD3 to induce VC. The high mortality rate observed in the rat model was first noted during preliminary experiments, where it was found that high and prolonged doses of VD3 significantly reduced rat survival rates, ultimately affecting the quality and timeline of the experiment. This underscores the need to optimize the model to improve survival rates and obtain more robust data. While this study confirmed the effects of SAL on VC in rats, the complexity of VC and its prolonged development process means that a single animal study may not fully capture its pathophysiology. Future studies should focus on refining the model to enhance rat survival rates. Additionally, while the in vivo experiments demonstrated that SAL reduces VC by modulating inflammatory factors via the JAK2/STAT3 pathway, further studies should explore upstream and downstream targets in vitro. Finally, clinical studies on SAL are limited, and more research is necessary to evaluate its efficacy and safety in humans. VC is associated with multiple conditions, including chronic kidney disease, cerebrovascular diseases, and coronary atherosclerosis. This study successfully established a VC model that can be further utilized to explore treatments for vascular-related conditions. The experimental design and techniques used here also provide a valuable foundation for drug discovery related to VC.
In summary, this study utilized network pharmacology and molecular biology to investigate salidroside's effect on vascular calcification. The results show that SAL inhibits VC by reducing inflammation, lowering lipid factor expression, and decreasing VC markers via the JAK2/STAT3 pathway, suggesting a promising therapeutic approach for VC treatment.
Disclosures
Ensure that all authors have disclosed any and all conflicts of interest.
Acknowledgements
This work was financially supported by the Jilin Provincial Department of Science and Technology Project (YDZJ202301ZYTS460), and Jilin Provincial Department of Education Project (JJKH20230991KJ).
Materials
| Name | Company | Catalog Number | Comments |
| 30% (29:1) Acrylamide/Bis Solution | Beijing Solarbio Science & Technology Co., Ltd ,China | A1010 | |
| 4% Paraformaldehyde Fix Solution | Beyotime Biotech Inc (Beyotime) , China | P0099 | |
| 5*loading buffer | Beijing Solarbio Science & Technology Co., Ltd ,China | P1040 | |
| Alkaline Phosphatase Assay Kit | Beyotime Biotech Inc (Beyotime) , China | P0321S | |
| AlphaView Software | Proteinsimple Inc.USA | AlphaView SA | |
| BCA Protein Assay Kit | Beyotime Biotech Inc (Beyotime) , China | P0012 | |
| Bluing Solution | Beijing Solarbio Science & Technology Co., Ltd ,China | G1866 | |
| Calcium Colorimetric Assay Kit | Beyotime Biotech Inc (Beyotime) , China | S1063S | |
| Collagen Fiber And Elastic Fiber Staining Kit(EVG-Verh eff Method) | Beijing Solarbio Science & Technology Co., Ltd ,China | G1597 | |
| Dewatering machine | Diapath Biosciences Ltd, Italy | Donatello | |
| Embedding machine | Wuhan Junjie Electronics Co., Ltd,China | JB-P5 | |
| Enzyme-labeled instrument | Biotek Co., Ltd,USA | Epoch | |
| Ethanol absolute | GHTECH Co., Ltd, China | 64-17-5 | |
| Goat Anti-Mouse IgG (H+L) HRP | Bioworld technology, co, Ltd.,China | BS20242-Y | |
| GraphPad Prism Software | GraphPad Software.,USA | GraphPad Prism 9.0 | |
| Hematoxylin-Eosin Stain Kit | Beijing Solarbio Science & Technology Co., Ltd ,China | G1120 | |
| High-density lipoprotein cholesterol assay kit | Nanjing Jiancheng Bioengineering Research Institute Co., Ltd,China | A112 | |
| HRP-labeled Goat Anti-Rabbit IgG(H+L) | Guangzhou saiguo biotech Co.,LTD | A0208 | |
| Image J Software | National Institutes of Health(NIH),USA | Image J | |
| IκB Alpha Polyclonal antibody | Proteintech Group, Inc.A,USA | 10268-1-AP | |
| JAK2 Antibody | Affinity Biosciences Co., Ltd,China | AF6022 | |
| Low-density lipoprotein cholesterol assay kit | Nanjing Jiancheng Bioengineering Research Institute Co., Ltd,China | A113 | |
| NF-κB p65 Antibody | Proteintech Group, Inc.A,USA | 10745-1-AP | |
| Pathological microtome | Leica Biosystems,USA | RM2016 | |
| Phosphatase Inhibitor Cocktail Tables | F. Hoffmann-La Roche, Ltd,Switzerland | 04906845001 | |
| Phospho-JAK2 (Tyr931) Antibody | Affinity Biosciences Co., Ltd,China | AF3024 | |
| Phospho-NF-κB p65(Ser276) Antibody | Affinity Biosciences Co., Ltd,China | AF2006 | |
| Phospho-STAT3(S727) Antibody | Abways Science & Technology Co., Ltd ,China | CY5291 | |
| Protease Inhibitor Cocktail | F. Hoffmann-La Roche, Ltd,Switzerland | 11873580001 | |
| PVDF membrane | F. Hoffmann-La Roche, Ltd,Switzerland | 3010040001 | |
| Rat IL-1β ELISA Kit | Beyotime Biotech Inc (Beyotime) , China | PI303 | |
| Rat IL-6 ELISA Kit | Beyotime Biotech Inc (Beyotime) , China | PI328 | |
| Rat TNF-α ELISA Kit | Beyotime Biotech Inc (Beyotime) , China | PT516 | |
| RIPA Lysis Buffer | Beyotime Biotech Inc (Beyotime) , China | P0013B | |
| Salisoroside | Shanghai yuanye Bio-Technology Co., Ltd,China | S25475 | |
| SDS | Guangzhou saiguo biotech Co.,LTD,China | 3250KG001 | |
| Sodium carbonate | China National Pharmaceutical Group Co., Ltd. , China | 1001921933 | |
| Sodium hydrogen carbonate | China National Pharmaceutical Group Co., Ltd. , China | 10018960 | |
| Sodium thiosulfate | China National Pharmaceutical Group Co., Ltd. , China | 20042518 | |
| STAT3 Antibody | Proteintech Group, Inc.A,USA | 10253-2-AP | |
| TBST (10×) | Beyotime Biotech Inc (Beyotime) , China | ST673 | |
| Total cholesterol assay kit | Nanjing Jiancheng Bioengineering Research Institute Co., Ltd,China | A111 | |
| Triglyceride assay kit | Nanjing Jiancheng Bioengineering Research Institute Co., Ltd,China | A110 | |
| Tris Base | Guangzhou saiguo biotech Co.,LTD | 1115GR500 | |
| Upright optical microscope | Nikon Corporation,Japan | Eclipse E100 | |
| Von Kossa Solution | Wuhan servicebio technology CO.,LTD,China | G1043 | |
| Western Blotting Luminol Reagent | Santa Cruz Biotechnology, Inc. ,USA | SC-2048 | |
| β-Actin antibody | Cell Signaling Technology, Inc.,USA | E4967 |
References
- Sutton, N. R., et al. Molecular mechanisms of vascular health: Insights from vascular aging and calcification. Arterioscler Thromb Vasc Biol. 43 (1), 15-29 (2023).
- Henein, M. Y., Owen, A. Statins moderate coronary stenoses but not coronary calcification: Results from meta-analyses. Int J Cardiol. 153 (1), 31-35 (2011).
- Ajufo, E., et al. Value of coronary artery calcium scanning in association with the net benefit of aspirin in primary prevention of atherosclerotic cardiovascular disease. JAMA Cardiol. 6 (2), 179-187 (2021).
- Vossen, L. M., Kroon, A. A., Schurgers, L. J., De Leeuw, P. W. Pharmacological and nutritional modulation of vascular calcification. Nutrients. 12 (1), 100 (2019).
- Kereiakes, D. J., et al. Principles of intravascular lithotripsy for calcific plaque modification. JACC Cardiovasc Interv. 14 (12), 1275-1292 (2021).
- Demer, L. L., Watson, K. E., Boström, K. Mechanism of calcification in atherosclerosis. Trends Cardiovasc Med. 4 (1), 45-49 (1994).
- Chen, F., et al. Network pharmacology analysis combined with experimental validation to explore the therapeutic mechanism of salidroside on intestine ischemia-reperfusion. Biosci Rep. 43 (8), BSR20230539 (2023).
- Rong, L., et al. Salidroside induces apoptosis and protective autophagy in human gastric cancer ags cells through the pi3k/akt/MTOR pathway. Biomed Pharmacother. 122, 109726 (2020).
- Zhang, P., et al. Network pharmacology: Towards the artificial intelligence-based precision traditional Chinese medicine. Brief Bioinform. 25 (1), bbad518 (2023).
- Jiao, X., et al. A comprehensive application: Molecular docking and network pharmacology for the prediction of bioactive constituents and elucidation of mechanisms of action in component-based Chinese medicine. Comput Biol Chem. 90, 107402 (2021).
- Li, S., Zhang, B. Traditional Chinese medicine network pharmacology: Theory, methodology and application. Chin J Nat Med. 11 (2), 110-120 (2013).
- Wang, X., Hu, Y., Zhou, X., Li, S. Editorial: Network pharmacology and traditional medicine: Setting the new standards by combining in silico and experimental work. Front Pharmacol. 13, 1002537 (2022).
- Huang, Z., Yang, Y., Fan, X., Ma, W. Network pharmacology-based investigation and experimental validation of the mechanism of scutellarin in the treatment of acute myeloid leukemia. Front Pharmacol. 13, 952677 (2022).
- Zhang, R., Zhu, X., Bai, H., Ning, K. Network pharmacology databases for traditional Chinese medicine: Review and assessment. Front Pharmacol. 10, 123 (2019).
- Wang, C., Liu, X., Guo, S. Network pharmacology-based strategy to investigate the effect and mechanism of alpha-solanine against glioma. BMC Complement Med Ther. 23 (1), 371 (2023).
- Li, X., et al. Network pharmacology prediction and molecular docking-based strategy to explore the potential mechanism of Huang Lian jiedu decoction against sepsis. Comput Biol Med. 144, 105389 (2022).
- Holmes, R. S., et al. Recommended nomenclature for five mammalian carboxylesterase gene families: Human, mouse, and rat genes and proteins. Mamm Genome. 21 (9-10), 427-441 (2010).
- Geng, J., Zhou, G., Guo, S., Ma, C., Ma, J. Underlying mechanism of traditional herbal formula Chuang-ling-ye in the treatment of diabetic foot ulcer through network pharmacology and molecular docking. Curr Pharm Des. 30 (6), 448-467 (2024).
- Chen, X., et al. Puerarin inhibits emt induced by oxaliplatin via targeting carbonic anhydrase xii. Front Pharmacol. 13, 969422 (2022).
- Herrmann, J., Babic, M., Tolle, M., Van Der Giet, M., Schuchardt, M. Research models for studying vascular calcification. Int J Mol Sci. 21 (6), 2204 (2020).
- Zhou, H., X, W., Yuan, Y., Qi, X. Comparison of methods for establishing a rat model of atherosclerosis using three doses of vitamin D3 and atherogenic diet. Chin J Arterioscler. 20 (11), 995-998 (2012).
- Zhang, Y., et al. Il-18 mediates vascular calcification induced by high-fat diet in rats with chronic renal failure. Front Cardiovasc Med. 8, 724233 (2021).
- Kazlouskaya, V., et al. The utility of elastic verhoeff-van gieson staining in dermatopathology. J Cutan Pathol. 40 (2), 211-225 (2013).
- Tang, X., et al. Underlying mechanism and active ingredients of tianma gouteng acting on cerebral infarction as determined via network pharmacology analysis combined with experimental validation. Front Pharmacol. 12, 760503 (2021).
- Lee, S. J., Lee, I. K., Jeon, J. H. Vascular calcification-new insights into its mechanism. Int J Mol Sci. 21 (8), 2685 (2020).
- Magdic, J., et al. Intracranial vertebrobasilar calcification in patients with ischemic stroke is a predictor of recurrent stroke, vascular disease, and death: A case-control study. Int J Environ Res Public Health. 17 (6), 2013 (2013).
- Zhou, L., et al. Salidroside-pretreated mesenchymal stem cells contribute to neuroprotection in cerebral ischemic injury in vitro and in vivo. J Mol Histol. 52 (6), 1145-1154 (2021).
- Hutcheson, J. D., Goettsch, C. Cardiovascular calcification heterogeneity in chronic kidney disease. Circ Res. 132 (8), 993-1012 (2023).
- Zhang, X., et al. Salidroside: A review of its recent advances in synthetic pathways and pharmacological properties. Chem Biol Interact. 339, 109268 (2021).
- Zhang, P., Li, Y., Guo, R., Zang, W. Salidroside protects against advanced glycation end products-induced vascular endothelial dysfunction. Med Sci Monit. 24, 2420-2428 (2018).
- Li, Y., et al. Salidroside promotes angiogenesis after cerebral ischemia in mice through shh signaling pathway. Biomed Pharmacother. 174, 116625 (2024).
- Gao, X. F., Shi, H. M., Sun, T., Ao, H. Effects of radix et rhizoma Rhodiolae kirilowii on expressions of von Willebrand factor, hypoxia-inducible factor 1 and vascular endothelial growth factor in myocardium of rats with acute myocardial infarction. Zhong Xi Yi Jie He Xue Bao. 7 (5), 434-440 (2009).
- Li, X., Liu, C., Li, Y., Xiong, W., Zuo, D. Inflammation promotes erythropoietin induced vascular calcification by activating p38 pathway. Bioengineered. 13 (3), 5277-5291 (2022).
- Bessueille, L., Magne, D. Inflammation: A culprit for vascular calcification in atherosclerosis and diabetes. Cell Mol Life Sci. 72 (13), 2475-2489 (2015).
- Li, R., et al. Salidroside prevents tumor necrosis factor-alpha-induced vascular inflammation by blocking mitogen-activated protein kinase and nf-kappa B signaling activation. Exp Ther Med. 18 (5), 4137-4143 (2019).
- Xing, S. S., et al. Salidroside attenuates endothelial cellular senescence via decreasing the expression of inflammatory cytokines and increasing the expression of sirt3. Mech Ageing Dev. 175, 1-6 (2018).
- Hopkins, A. L. Network pharmacology. Nat Biotechnol. 25 (10), 1110-1111 (2007).
- Xin, P., et al. The role of jak/stat signaling pathway and its inhibitors in diseases. Int Immunopharmacol. 80, 106210 (2020).
- Fu, X., et al. Glycosides from buyang huanwu decoction inhibit atherosclerotic inflammation via jak/stat signaling pathway. Phytomedicine. 105, 154385 (2022).
- Macri, F., et al. High phosphate-induced jak-stat signalling sustains vascular smooth muscle cell inflammation and limits calcification. Biomolecules. 14 (1), 107328 (2023).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved