Method Article
A Preclinical Human-Derived Autologous Gastric Cancer Organoid/Immune Cell Co-Culture Model to Predict the Efficacy of Targeted Therapies
* These authors contributed equally
In This Article
Summary
The goals of the protocol are to use this approach to 1) understand the role of the immunosuppressive gastric tumor microenvironment and 2) predict the efficacy of patient response, thus increasing the survival rate of patients.
Abstract
Tumors expressing programmed cell death-ligand 1 (PD-L1) interact with programmed cell death protein 1 (PD-1) on CD8+ cytotoxic T lymphocytes (CTLs) to evade immune surveillance leading to the inhibition of CTL proliferation, survival, and effector function, and subsequently cancer persistence. Approximately 40% of gastric cancers express PD-L1, yet the response rate to immunotherapy is only 30%. We present the use of human-derived autologous gastric cancer organoid/immune cell co-culture as a preclinical model that may predict the efficacy of targeted therapies to improve the outcome of cancer patients. Although cancer organoid co-cultures with immune cells have been reported, this co-culture approach uses tumor antigen to pulse the antigen-presenting dendritic cells. Dendritic cells (DCs) are then cultured with the patient's CD8+ T cells to expand the cytolytic activity and proliferation of these T lymphocytes before co-culture. In addition, the differentiation and immunosuppressive function of myeloid-derived suppressor cells (MDSCs) in culture are investigated within this co-culture system. This organoid approach may be of broad interest and appropriate to predict the efficacy of therapy and patient outcome in other cancers, including pancreatic cancer.
Introduction
Gastric cancer is the fifth most common cancer worldwide 1. The effective diagnosis and treatment of Helicobacter pylori (H. pylori) have resulted in a low incidence of gastric cancer in the United States 2. However, the 5-year survival rate for patients diagnosed with this malignancy is only 29%, making gastric cancer an important medical challenge3. The purpose of the methods presented here is to develop an approach to predict immunotherapy responses in individual patients accurately. Solid tumors consist of cancer cells and various types of stromal, endothelial, and hematopoietic cells, including macrophages, myeloid-derived suppressor cells (MDSCs), and lymphocytes (reviewed in 4,5). Interactions between cancer stem cells and the tumor microenvironment (TME) substantially impact tumor characteristics and the response of the patient to treatment. This approach strives to allow investigators to acquire knowledge for preclinical drug development and biomarker discovery for the personalized treatment of gastric cancer.
The method presented here uses human-derived autologous organoid/immune cell co-cultures generated from gastric cancer patients to understand the immunosuppressive role of the MDSCs. Presented is a preclinical model that may predict the efficacy of targeted therapies to improve the survival of patients. Cancer organoid co-cultures with immune cells have been extensively reported in the pancreatic cancer field6,7,8,9,10. However, such co-cultures have not been reported to study gastric cancer. Overall, this method demonstrates the co-culturing of autologous human-derived immune cells within the same matrix environment as the cancer organoids, thus allowing the immune cells to be in contact with the target organoids.
The study by Tiriac et al.10 reported that patient-derived pancreatic cancer organoids, which exhibited heterogeneous responses to standard-of-care chemotherapeutics, could be grouped into organoid-based gene expression signatures of chemosensitivity that could predict improved patient responses to chemotherapy. The investigators proposed that combined molecular and therapeutic profiling of pancreatic cancer organoids may predict clinical response10. Co-clinical trial data from Yao et al.11 also showed that rectal cancer-derived organoids represent similar pathophysiology and genetic changes similar to the patient tumor tissues in response to chemoradiation. Thus, it is fundamental for organoid cultures to be used in the context of the patient's immune cells and tumor immune phenotype when using these cultures as predictive models for therapy.
Tumors expressing PD-L1 that interact with PD-1 inhibit CD8+ cytotoxic T lymphocyte proliferation, survival, and effector function 12,13,14. While approximately 40% of gastric cancers express PD-L1, only 30% of these patients respond to immunotherapy15,16,17. Anti-PD1 antibodies are used in clinical trials for gastric cancer treatment18,19,20. However, there are currently no preclinical models that allow testing of therapeutic efficacy for each patient. Optimizing the organoid culture such that the patient's immune cells are included in the system would potentially allow for the individualized identification of the efficacy of immunotherapy.
Protocol
Approval was obtained for the collection of human-biopsied tissues from patient tumors (1912208231R001, University of Arizona Human Subjects Protection Program; IRB protocol number: 1099985869R001 , University of Arizona Human Subjects Protection Program TARGHETS).
1. Establishing patient-derived gastric organoids from biopsies
- Collect 1-2 mm of human biopsied tissues from the tumor region of gastic cancer patients undergoing esophageal gastro-duodenoscopy in 1x phosphate-buffered saline (PBS) (Table 1).
NOTE: All procedures henceforth should be conducted in an aseptic environment using sterile materials and reagents. - Mince the biopsied tissues using scalpel blades on a Petri dish. Transfer the minced tissues to a 15 ml conical tube and add 5-10 mL of 1x PBS.
- Centrifuge at 300 × g for 5 min at room temperature. Discard the supernatant. Add 1-2 mL of prewarmed digestion buffer (Table 1) to the pelleted tissues.
- Incubate at 37 °C for 15-30 min, depending on the size of the minced tissues. Monitor the status of digestion by looking under the microscope for small cell clusters every 5-10 min.
- Stop the digestion by diluting 5-fold with cold Advanced Dulbecco's Modified Eagle Medium/Ham's F-12 medium (Advanced DMEM/F-12). If clumps of undigested material are visible, filter the tissues through 30 µm filters. Collect the flow-through.
- Centrifuge the flow-through at 300 × g for 5 min at room temperature to pellet the cells. Discard the supernatant.
- Resuspend the pelleted cells (10,000-100,000) in an appropriate volume (2-4 mL) of thawed basement membrane matrix (see the Table of Materials) on ice. Seed as 30-50 µL cell-basement membrane matrix drops in 24-well culture plates.
- Incubate the plates at 37 °C for 15 min to allow the cell-basement membrane matrix drops to solidify. Overlay the cell-basement membrane matrix drops with prewarmed gastric organoid culture medium (Table 1).
- Maintain organoid cultures at 37 °C in 5% CO2. Replace the culture medium with fresh medium every 3-4 days, depending on the organoid growth. Passage the organoids once every 7-10 days in a 1:3 ratio.
2. Establishing patient-derived gastric organoids from surgical specimens
- Collect human gastric cancer tissues from resected specimens of gastric cancer patients during surgical procedures in 1x Dulbecco's phosphate-buffered saline (DPBS) supplemented with antibiotics (Table 1).
NOTE: All procedures hereafter should be processed in a biosafety cabinet, maintaining an aseptic environment and using sterile materials and reagents. - Mince the resected tissues using surgical scalpel blades on a Petri dish. Wash the minced tissues with 5-10 mL of 1x DPBS containing antibiotics.
- Transfer the tissue to a 50 mL conical tube. Add 10 mL of prewarmed ethylenediamine tetraacetic acid (EDTA) stripping buffer to the minced tissues. Monitor the status of digestion every 5-10 min. Incubate the tube in a 37 °C shaker for 10 min.
- Let the tissues settle at the bottom of the tube, remove the EDTA buffer without disturbing the tissues, and add 10 mL of fresh EDTA buffer. Incubate the tube in a 37 °C shaker for 5 min.
- Let the tissues settle at the bottom of the tube. Remove the EDTA buffer and wash the tissues twice (follow step 2.4) with 10 mL of advanced DMEM/F-12 supplemented with antibiotics (no centrifuge) (Table 1).
- Add 5-10 mL of prewarmed digestion buffer (Table 1) to the tissues, depending on the tissue size. Incubate the tube at 37 °C for 15-30 min with mild agitation, depending on the size and texture of the tissues. Check for the appearance of cell clusters under a microscope every 10 min.
- Stop the digestion by diluting two-fold with cold Advanced DMEM/F-12 plus antibiotics. Filter the undigested tissues through 40 µm filters and collect the flow-through.
- Centrifuge the flow-through at 400 × g for 5 min at 4 °C to pellet the cells. Discard the supernatant, and wash the cells with cold 1x DPBS plus antibiotics at 400 × g for 5 min at 4 °C. Carefully discard the supernatant, and store the cells on ice.
- Resuspend the pelleted cells in an appropriate volume of the basement membrane matrix on ice. Seed 30-50 µL cell-basement membrane matrix drops in 24- or 12-well cell culture-treated plates.
- Incubate the plates at 37 °C for 15 min to allow the cell-basement membrane matrix drops to solidify as a dome. Overlay the cell-basement membrane matrix dome with prewarmed gastric organoid culture medium (Table 1).
- Maintain the organoid cultures at 37 °C in 5% CO2. Replace the culture medium with fresh medium every 3-4 days, depending on the organoid growth. Passage the organoids once every 7-10 days in a 1:2 or 1:3 ratio, based on the organoid density.
3. Maintenance and expansion of organoid cultures
NOTE : All procedures should be conducted in an aseptic environment using sterile materials and reagents.
- Maintenance and expansion
- Maintain the organoid cultures for 7-10 days until they are 70-80% confluent. Harvest the organoids in ice-cold DMEM. Transfer the organoids to 5 mL round-bottom polystyrene tubes.
- Centrifuge the organoids at 400 × g for 5 min at 4 °C to pellet cells. Carefully remove the supernatant and resuspend the pellet in 1 mL of prewarmed cell dissociation reagent solution. Incubate the organoids at 37 °C for 6 min.
- Gently pass the organoids through a 26 G needle 4 times. Add 2 mL of DMEM to stop the action of the cell dissociation reagent.
- Centrifuge the organoids at 400 × g for 5 min at 4 °C to pellet the cells. Carefully discard the supernatant and store the cells on ice.
- Resuspend the pelleted cells in an appropriate volume of basement membrane matrix on ice. Seed 30-50 µL of the cell-basement membrane matrix drops in 24- or 12-well cell culture treated plates.
- Incubate the plates at 37 °C for 15 min to allow the cell-basement membrane matrix drops to solidify as a dome. Overlay the cell- cell-basement membrane matrix dome with prewarmed gastric organoid culture medium (see Table 1).
- Maintain the organoid cultures at 37 °C in 5% CO2. Replace the culture medium with fresh medium every 3-4 days, depending on the organoid growth. Repeat passaging of the organoids once every 7-10 days in a 1:2 or 1:3 ratio, based on the organoid density.
- Expansion of organoid-derived cell lines
- Harvest the organoids when they are 70-80% confluent. Feed the cells adherent to the plastic plates with gastric organoid culture medium and maintain the culture by feeding them every 3-4 days depending on the cell growth.
- Passage the cells when they reach 80-90% confluence.
- Remove the culture medium and gently wash the plate with prewarmed DPBS. Add 1 mL of prewarmed cell dissociation reagent.
- Incubate the cells for 5 min at 37 °C. Harvest the cells in 2 mL of DMEM.
- Centrifuge the cells at 400 × g for 5 min at 4 °C. Remove the supernatant and resuspend the pellet in gastric organoid culture medium or human gastric epithelial cell culture medium (Table 1).
- Plate the cells from 3.2.2.3 either in basement membrane matrix-coated or gelatin-coated plates. Maintain the organoid cultures at 37 °C in 5% CO2. Replace the medium with fresh medium every alternate day depending on the cell growth. Repeat the passaging of the cells once every 7-10 days in a 1:2 or 1:3 ratio, based on the cell density.
- To coat the plates with basement membrane matrix, dilute the matrix in ice-cold cell culture grade water in a 1:10 ratio. Uniformly coat the plate with 1 mL of ice-cold diluted matrix solution using a cell spreader, and incubate the coated plate at 37 °C for 2 h. Remove the remaining coating solution and let the plate dry without the lid inside the biosafety hood for 30 min, right before plating the cells.
- To coat the plates with gelatin, uniformly coat the plate with 1 mL of an ice-cold gelatin-based coating solution. Incubate the coated plate at room temperature for 5 min. Remove the remaining gelatin solution and plate the resuspended cells.
4. Culturing immune cells from peripheral blood mononuclear cells (PBMCs)
NOTE: All procedures should be conducted in an aseptic environment using sterile materials and reagents.
- PBMC isolation
- Dilute whole blood with an equal volume of 1x PBS.
- Depending on the total volume of the diluted blood sample, dispense an appropriate volume of density gradient medium (see the Table of Materials) into a 15 mL tube.
NOTE: The recommended ratio is 3 mL of the density gradient medium to 4 mL of the diluted blood sample. - Carefully layer the diluted blood sample onto the density gradient medium. Centrifuge at 400 × g for 30 min-1 h without brake.
- After centrifugation, carefully transfer the mononuclear cells at the interface into a new 15 mL conical tube. Dilute the mononuclear cells with 3 volumes of 1x PBS.
- Centrifuge at 400 × g for 10 min. Discard the supernatant. Repeat once.
- Resuspend the pelleted cells in an appropriate medium for downstream applications or cryopreserve as frozen stocks.
- If needed, determine the yield and cell viability of PBMCs using the trypan blue dye exclusion assay.
- Culture of human dendritic cells
- Resuspend the isolated PBMCs in PBMC medium (see the Table of Materials and Table 1) and plate them in a 24-well tissue culture plate for 1-2 h at 37 °C in 5% CO2.
- Firmly tap the culture plate and discard the spent culture medium containing non-adherent cells.
- Add the DC culture medium (Table 1) to the adherent cells. Maintain the cultures for 3 days at 37 °C in 5% CO2. Replace the supernatant medium with fresh culture medium on alternate days.
- On day 3, replace the exhausted medium with fresh DC maturation medium (Table 1). Maintain the cultures for 24 h at 37 °C in 5% CO2.
- Culture of human cytotoxic T lymphocytes (CTLs)
- Transfer 1 mL of PBMCs to a 5 mL polystyrene round-bottom tube.
- Add 50 µL of Enrichment Cocktail (see the Table of Materials) to the PBMCs. Incubate for 10 min at room temperature.
- Add 150 µL of Magnetic Particles (Table of Materials) to the sample. Incubate for 5 min at room temperature.
- Increase the sample volume to 2.5 mL with the cell separation buffer (Table of Materials). Place the polystyrene round-bottom tube into a cell separation magnet (Table of Materials) for 5 min to enable cell separation.
- Pour the enriched cell suspension into a new 15 mL conical tube. Centrifuge at 300 × g for 5 min. Discard the supernatant.
- Resuspend the pelleted cells and culture in CTL culture medium (Table 1) for 24 h at 37 °C in 5% CO2.
- Culture of human myeloid-derived suppressor cells (MDSCs)
- Culture PBMCs in MDSC culture medium (Table 1) for 7 days at 37 °C in 5% CO2 to enrich for MDSCs. Replace the supernatant medium with fresh culture medium on alternate days.
- Co-culture of DCs and CTLs
- Collect conditioned medium from the organoid cultures.
- Gently remove 50% of the medium from the matured DC culture and replace it with organoid-derived conditioned medium.
- Incubate the DCs with the organoid-derived conditioned medium for 2 h at 37 °C in 5% CO2.
- Harvest the loosely adherent DCs and centrifuge them at 300 × g for 5 min at 4 °C. Remove the supernatant, resuspend the pellet in RPMI co-culture medium, and add this cell suspension back into the same well.
- Harvest the CTLs from the same patient line and transfer them to the matured DCs. Continue DC-CTL co-culture (Table 1) for 72 h at 37 °C in 5% CO2.
5. Establishing organoid/immune cell co-cultures
- Isolate CTLs from co-cultures of DCs and CTLs using a kit (see the Table of Materials) as described previously in section 4.3.
- Incubate the CTLs with 5 µM carboxyfluorescein diacetate succinimidyl ester (CFSE) at 37 °C for 20 min.
NOTE: CFSE is a blue laser excitable dye used for flow cytometric monitoring of cell divisions. - Harvest the organoids and mix them with CFSE-labeled CTLs. Keeping the CTL/organoid ratio at 50,000 CTLs per 200 organoids, resuspend the organoids in an appropriate volume of thawed basement membrane matrix on ice. Seed as 25 µL cell-basement membrane matrix drops in 24-well tissue culture plates.
NOTE: For experimental conditions requiring MDSCs, mix the MDSCs with the organoids and CFSE-labeled CTLs before seeding. The MDSC/CTL ratio should be 4:1. - Incubate the plates at 37 °C for 15 min to allow the cell-basement membrane matrix drops to solidify.
- Overlay the cell-basement membrane matrix drops with prewarmed organoid culture medium.
- Maintain the organoid/immune cell co-cultures at 37 °C in 5% CO2 until analysis.
Results
When completed, gastric organoids appear as spheres within the well, typically within 2-4 days post embedding (Figure 1). Figure 1A demonstrates a thriving gastric organoid culture that exhibits a regular membrane. Tumor organoids will often exhibit a divergent morphology that is unique to the patient sample. Unsuccessful cultures will appear dense or not exhibit any growth from the initial digestion of tissue (Figure 1B). Cultures that are robust and actively growing will be successfully passaged and expanded as detailed in the protocol. We have often observed the migration and attachment of a subpopulation of cells to the base of the culture plate (Figure 1C). Following the protocol, these attached cells may be passaged and expanded onto gelatin-coated cell culture plates (Figure 1D).
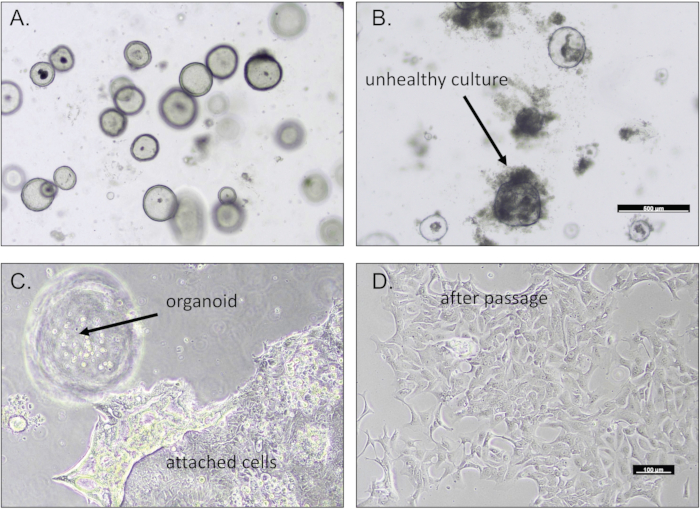
Figure 1: Gastric organoids derived from biopsies. Representative images of (A) a robust gastric organoid culture and (B) an unhealthy culture of dying organoids. (C) Gastric cells often migrate and attach to the base of the culture dish. (D) These gastric cells may be passaged. Scale bars = 100 µm. Please click here to view a larger version of this figure.
This protocol describes a method to culture DCs, MDSCs, and CTLs from patient PBMCs. Figure 2 represents the morphology of the immune cell cultures derived from PBMCs. DCs in culture exhibited an irregular shape with extensive and elongated dendritic processes (Figure 2A). MDSCs appear as typically large mononuclear cells with basophilic, granular cytoplasm (Figure 2B). The morphology of CTLs in culture is shown in Figure 2C. Granulocytic MDSCs may be further characterized by flow cytometry using a gating strategy identifying HLA-DR/CD14-, CD33/CD11b/CD15+ cells.
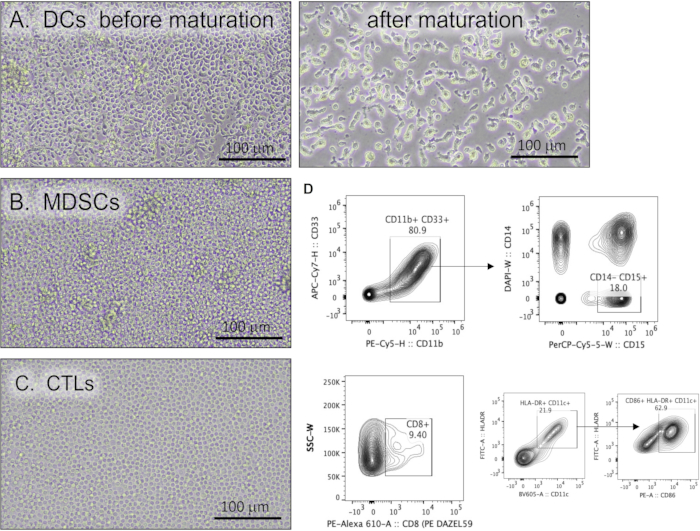
Figure 2: Human immune cells cultured from PBMCs. Representative light micrographs of (A) dendritic cells before and after the maturation protocol, (B) myeloid-derived suppressor cells, and (C) cytotoxic T lymphocytes. Scale bars = 100 µm. (D) Representative flow cytometric contour plots demonstrating immune cell markers. Abbreviations: PBMCs = peripheral blood mononuclear cells; DCs = dendritic cells; MDSCs = myeloid-derived suppressor cells; CTLs = cytotoxic T lymphocytes; APC = allophycocyanin; CD = cluster of differentiation; PE = phycoerythrin; H = height of peak; W = width of peak; PerCP = Peridinin-chlorophyll-protein; DAPI = 4′,6-diamidino-2-phenylindole; FITC = fluorescein isothiocyanate. Please click here to view a larger version of this figure.
Immunofluorescence of the organoid/immune cell co-culture demonstrates the presence of CTLs (CD8+, green) with tumor organoids (PD-L1+, red) (Figure 3A-D). A three-dimensionally rendered image is shown in Video 1. Time-lapse microscopy demonstrates the migration of MDSCs and CTLs towards the gastric organoids (Video 2) and organoid death in cultures treated with a checkpoint inhibitor (Video 3).

Figure 3: Gastric cancer organoid/immune cell co-cultures. Immunofluorescence staining of a representative organoid/immune cell co-culture showing the expression of (A) CD8+ (green), (B) PD-L1 (red), and (C) Hoechst (blue)-stained cells. A merged image is shown in (D). (E) Immunofluorescence staining of a representative organoid/immune cell co-culture showing the expression of MDSCs (CD11b, green) and E cadherin (red). Scale bars = 50 µm. Abbreviations: CD = cluster of differentiation; PD-L1 = programmed cell death-ligand 1; MDSCs = myeloid-derived suppressor cells; Hoechst = Hoechst 33258. Please click here to view a larger version of this figure.
Video 1: A three-dimensional, rendered image of gastric cancer organoid/immune cell co-culture containing PD-L1+ tumor cells (red) and CD8+ lymphocytes (green). Please click here to download this Video.
Video 2: Time-lapse microscopy of organoid/immune cell co-culture resistant to checkpoint inhibitor treatment. Please click here to download this Video.
Video 3: Time-lapse microscopy of organoid/immune cell co-culture sensitive to checkpoint inhibitor treatment. Please click here to download this Video.
Table 1: Composition of media and solutions. Please click here to download this Table.
Discussion
We present the use of human-derived autologous gastric cancer organoid/immune cell co-culture that may be used as a preclinical model to predict the efficacy of targeted therapies to ultimately improve treatment outcome and patient prognosis. Although cancer organoid co-cultures with immune cells have been reported, this is the first report of such a co-culture system for the study of gastric cancer. Numerous other organoid-based patient profiling efforts are well-developed at multiple institutions, including co-culture models. There are three major co-culture systems, to our knowledge, that have been developed, which have the following features: 1) Pancreatic cancer organoids co-cultured with immune cells outside of the basement membrane matrix dome6. Immune/tumor cell adhesion would be important in a system studying the interaction between PD-L1 and PD-1. 2) Autologous non-small-cell lung and colorectal cancer organoid/peripheral blood lymphocyte co-cultures21.
All the experiments performed in this study were conducted using anti-CD28-coated plates to activate T cells, and co-cultures were performed in the presence of interleukin (IL)-2 to maintain T cell proliferation. Although all in vitro cultures have limitations and do not entirely represent physiological conditions, CD8+ T cells in these cultures are activated using the patient's tumor antigen and dendritic antigen-presenting cells to activate the T cells. This approach may be considered closer to what occurs within the TME. 3) The air-liquid interface (ALI) method to propagate patient-derived organoids22. The investigators state that organoids were passaged every 14-30 days, and in some cases, the medium was supplemented with recombinant human IL-2. First, cytotoxic CD8+ T lymphocyte maintenance and expansion requires more than simply adding IL-2 to the media ex vivo. Second, for long-term culture of CD8+ T cells, an initial CD3/CD28 followed by maintenance in IL-2 for 2-3 weeks is required. This may be considered an artificial approach to T cell activation and not relevant within the TME.
The current limitations of tumor tissue-derived organoid cultures warranted the refinement of these cultures with immune cells. For example, the likelihood of tumor invasion is increased significantly in patients exhibiting a dense stromal compartment, such as that observed in invasive gastric cancer23. Thus, it may be difficult for the isolation and organoid culture of immune cells from native patient tumor tissue with a dense stromal compartment, especially in patients with poor prognosis. Importantly, published RNA sequencing data have shown that although there is a phenotypical similarity between the organoids and the patient's tumor tissue, the immune compartment is essentially missing24. Thus, a limitation of the current organoid and cell line cultures is the lack of the immune component found within the patient's TME.
Critical steps in the protocol include the generation of a robust organoid culture and immune cells. The most common problem observed with the patient-derived organoid cultures is bacterial or fungal contamination. Thus, it is also critical to include antifungal agents and antibiotics when washing the tissue before digestion. There are also limitations of the culture that will be addressed in future experiments. First, the heterogeneity of the organoid cultures may be considered a limitation when investigating the specific cell population that immunotherapies and chemotherapies are targeting. It is often difficult to determine from these cultures whether a single organoid is clonal and derived from a single cell. A future approach may be to use a single-cell analytical approach to complement in vitro experiments that test targeted therapies. Second, the heterogeneity of the cells within the culture may not reflect the patient's exact tumor microenvironment. The complexity of these cultures can be increased by incorporating additional fundamental cells, including macrophages and cancer-associated fibroblasts. However, organoid/immune cell co-culture has been used here to investigate a focused research question regarding the fundamental tumor and immune cell interactions relevant to the role of MDSCs as immunosuppressive cells. Third, samples are collected from the primary tumor site. The tissue is collected based on the decision of the pathologist. Future research will be focused on organoids derived from metastatic sites, which may allow us to decipher the differences in the cancer organoids based on the location from where the tissue was collected. Overall, this culture system may be of broad interest and appropriate to predict the efficacy of therapy and patient outcome in other gastrointestinal cancers, including the colon and the pancreas.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by NIH (NIAID) 5U19AI11649105 (PIs: Weiss and Wells, Project Leader 1: Zavros) and NIH (NIDDK) 2 R01 DK083402-06A1 (PI: Zavros) grant. This project was supported in part by PHS Grant P30 DK078392 (Integrative Morphology Core) of the Digestive Diseases Research Core Center in Cincinnati and 5P30CA023074 UNIVERSITY OF ARIZONA CANCER CENTER – CANCER CENTER SUPPORT GRANT (PI: Sweasy). We would like to acknowledge the assistance of Chet Closson (Live Microscopy Core, University of Cincinnati) and past members of the Zavros laboratory, Drs. Nina Steele and Loryn Holokai, for their contribution to the development of the organoid culture system. We sincerely thank the patients who consented to donate tissue and blood for the development of the gastric organoid/immune cell co-cultures. Without their willingness to participate in the study, this work would not be possible.
Materials
| Name | Company | Catalog Number | Comments |
| 12 well plate | Midwest Scientific | 92012 | |
| 15 mL Falcon tube | Fisher scientific | 12-565-269 | |
| 24 well plate | Midwest Scientific | 92024 | |
| 30 μm filters | Miltenyi Biotec | 130-041-407 | |
| 40 μm filters (Fisher Scientific) | Fisher scientific | 352340 | |
| 5 mL round bottom polystyrene tubes | Fisher scientific | 14956-3C | |
| 50 mL Falcon tube | Fisher scientific | 12-565-271 | |
| Advanced DMEM/F12 | Thermo Fisher Scientific | 12634010 | |
| AIMV | Thermo Fisher Scientific | 12055091 | Basal medium for PBMCs and DCs |
| Amphotericin B/ Gentamicin | Thermo Fisher Scientific | R-01510 | |
| B-27 supplement | Thermo Fisher Scientific | 12587010 | |
| β-mercaptoethanol | Thermo Fisher Scientific | 800-120 | |
| Bone morphogenetic protein inhibitor (Noggin) | Peprotech | 250-38 | |
| Bovine Serum Albumin (BSA) | Sigma Aldrich | A7906 | |
| Cabozantinib | Selleckchem | S1119 | |
| Carboxyfluorescein diacetate succinimidyl ester (CFSE) | Biolegend | 423801 | |
| Collagenase A | Sigma Aldrich | C9891 | |
| Dulbecco’s Phosphate Buffered Saline (DPBS) | Fisher scientific | 14190-144 | cell separation buffer |
| EasySep Buffer | Stem Cell Technologies | 20144 | Contains Enrichment Cocktail and Magnetic Particles used in CTL culture |
| EasySep Human CD8+ T Cell Enrichment Kit | Stem Cell Technologies | 19053 | cell separation magnet |
| EasySep Magnet | Stem Cell Technologies | SN12580 | |
| EDTA | Sigma Aldrich | E6758 | |
| Epidermal Growth Factor (EGF) | Peprotech | 315-09 | |
| Farma Series 3 Water Jacketed Incubator | Thermo Fisher Scientific | 4120 | |
| Fetal Calf Serum (FCS) | Atlanta Biologicals | SI2450H | |
| Fibroblast growth factor 10 (FGF-10) | Peprotech | 100-26 | density gradient medium |
| Ficoll-Paque | GE Healthcare | 171440-02 | |
| Gastrin 1 | Tocris | 30061 | |
| Gelatin | Cell Biologics | 6950 | |
| GM-CSF | Thermo Fisher Scientific | PHC6025 | |
| Hank's Balanced Salt Solution (HBSS) | Thermo Fisher Scientific | 14175095 | |
| HEPES (2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid) | Fisher scientific | BP299-100 | |
| Human Epithelial Cell Basal Medium | Cell Biologics | H6621 | |
| human serum AB | Gemini Bioscience | 21985023 | |
| Hyaluronidase Type IV-S | Sigma Aldrich | H3884 | |
| Insulin-Transferrin-Selenium | Thermo Fisher Scientific | 41400045 | |
| Interleukin 1β (IL-1β) | Thermo Fisher Scientific | RIL1BI | |
| Interleukin 6 (IL-6) | Thermo Fisher Scientific | RIL6I | |
| Interleukin 7 (IL-7) | Thermo Fisher Scientific | RP-8645 | |
| Kanamycin | Thermo Fisher Scientific | 11815024 | |
| L-glutamine | Fisher scientific | 350-50-061 | basement membrane matrix |
| Matrigel (Corning Life Sciences, Corning, NY) | Fisher scientific | CB40230C | |
| N-2 supplement | Thermo Fisher Scientific | 17502048 | |
| N-acetyl-L-cysteine | Sigma Aldrich | A7250 | |
| Nicotinamide (Nicotinamide) | Sigma Aldrich | N0636 | |
| PD-L1 inhibitor | Selleckchem | A2002 | |
| Penicillin/Streptomycin | Thermo Fisher Scientific | SV3000 | |
| Petridish | Fisher scientific | 07-202-030 | |
| Potassium chloride (KCl) | Fisher scientific | 18-605-517 | |
| Potassium dihydrogenphosphate (KH2PO4) | Fisher scientific | NC0229895 | |
| prostaglandin E2 (PGE2) | Sigma Aldrich | P0409 | |
| RPMI 1640 | Thermo Fisher Scientific | 11875119 | |
| Sodium chloride (NaCl) | Fisher scientific | 18-606-419 | |
| Sodium hydrogen phosphate (Na2HPO4) | Fisher scientific | NC0229893 | cell dissociation reagent |
| StemPro Accutase solution | Thermo Fisher Scientific | A1110501 | |
| Transforming growth factor beta 1 (TGF-β1) | Thermo Fisher Scientific | 7754-BH-005/CF | |
| Tumor necrosis factor α (TNF-α) | Thermo Fisher Scientific | PHC3015 | |
| Vascular endothelial growth factor (VEGF) | Thermo Fisher Scientific | RVGEFI | |
| Y-27632 ROCK inhibitor | Sigma Aldrich | Y0350 |
References
- Ferlay, J., et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer. 136 (5), 359-386 (2015).
- Piazuelo, M. B., Epplein, M., Correa, P. Gastric cancer: an infectious disease. Infectious Disease Clinics of North America. 24 (4), 853-869 (2010).
- Siegel, R., Naishadham, D., Jemal, A. Cancer statistics. CA: A Cancer Journal for Clinicians. 63 (1), 11-30 (2013).
- Quante, M., Wang, T. C. Inflammation and stem cells in gastrointestinal carcinogenesis. Physiology. 23, 350-359 (2008).
- Quante, M., Varga, J., Wang, T. C., Greten, F. R. The gastrointestinal tumor microenvironment. Gastroenterology. 145 (1), 63-78 (2013).
- Tsai, S., et al. Development of primary human pancreatic cancer organoids, matched stromal and immune cells and 3D tumor microenvironment models. BMC Cancer. 18 (1), 335 (2018).
- Boj, S. F., et al. Organoid models of human and mouse ductal pancreatic cancer. Cell. 160 (1-2), 324-338 (2015).
- Huang, L., et al. Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nature Medicine. 21 (11), 1364-1371 (2015).
- Tiriac, H., et al. Successful creation of pancreatic cancer organoids by means of EUS-guided fine-needle biopsy sampling for personalized cancer treatment. Gastrointestinal Endoscopy. 87 (6), 1474-1480 (2018).
- Tiriac, H., et al. Organoid profiling identifies common responders to chemotherapy in pancreatic cancer. Cancer Discovery. 8 (9), 1112-1129 (2018).
- Yao, Y., et al. Patient-derived organoids predict chemoradiation responses of locally advanced rectal cancer. Cell Stem Cell. 26 (1), 17-26 (2020).
- Ahmadzadeh, M., et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 114 (8), 1537-1544 (2009).
- Chen, Z., et al. Intratumoral CD8(+) cytotoxic lymphocyte is a favorable prognostic marker in node-negative breast cancer. PLoS One. 9 (4), 95475 (2014).
- Reissfelder, C., et al. Tumor-specific cytotoxic T lymphocyte activity determines colorectal cancer patient prognosis. Journal of Clinical Investigation. 125 (2), 739-751 (2015).
- Muro, K., et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with metastatic gastric cancer: a JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Annals of Oncology. 30 (1), 19-33 (2019).
- Subhash, V. V., Yeo, M. S., Tan, W. L., Yong, W. P. Strategies and advancements in harnessing the immune system for gastric cancer immunotherapy. Journal of Immunology Research. 2015, 308574 (2015).
- Muro, K., et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncology. 17 (6), 717-726 (2016).
- Abdel-Rahman, O. PD-L1 expression and outcome of advanced melanoma patients treated with anti-PD-1/PD-L1 agents: a meta-analysis. Immunotherapy. 8 (9), 1081-1089 (2016).
- Abdel-Rahman, O. Correlation between PD-L1 expression and outcome of NSCLC patients treated with anti-PD-1/PD-L1 agents: A meta-analysis. Critical Reviews in Oncology/Hematology. 101, 75-85 (2016).
- Abdel-Rahman, O. Immune checkpoints aberrations and gastric cancer; assessment of prognostic value and evaluation of therapeutic potentials. Critical Reviews in Oncology/Hematology. 97, 65-71 (2016).
- Dijkstra, K. K., et al. Generation of tumor-reactive T cells by co-culture of peripheral blood lymphocytes and tumor organoids. Cell. 174 (6), 1586-1598 (2018).
- Neal, J. T., et al. Organoid modeling of the tumor immune microenvironment. Cell. 175 (7), 1972-1988 (2018).
- Peng, C., Liu, J., Yang, G., Li, Y. The tumor-stromal ratio as a strong prognosticator for advanced gastric cancer patients: proposal of a new TSNM staging system. Journal of Gastroenterology. 53 (5), 606-617 (2018).
- Steele, N. G., et al. An organoid-based preclinical model of human gastric cancer. Cellular and Molecular Gastroenterology and Hepatology. 7 (1), 161-184 (2019).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved