Method Article
Chicken Embryo as an In Vivo Model to Revive Viable but Non-Culturable Pathogens
In This Article
Summary
This method showcases the chicken embryo as a simple and cost-effective in vivo model to revive the bacterial pathogen L. monocytogenes from a viable but non-culturable (VBNC) state and with potential further uses in the understanding of bacterial dormancy mechanisms.
Abstract
The chicken embryo has emerged as a popular in vivo model with increasing application in biomedical research due to its simplicity, affordability, and adaptability in the study of various biological phenomena. This model has been used to investigate microbial pathogenicity and is becoming a useful tool to study bacterial dormancy. The viable but non-culturable (VBNC) state is a dormant state in which bacteria become metabolically quiescent and resistant to cultivation to preserve their viability in harsh environments. Under favorable conditions, VBNC bacteria can wake up back into a metabolically active and culturable state. Bacterial pathogens that switch to a VBNC state, such as the foodborne listeriosis-causing Listeria monocytogenes, are a public health concern, as they elude detection by conventional growth-dependent methods and can recover their virulence upon revival. This urges a better understanding of the conditions and mechanisms driving the revival of VBNC pathogens. The method presented here showcases the chicken embryo as an efficient in vivo model to revive VBNC L. monocytogenes back into a culturable status. Where in vitro revival attempts, largely based on nutritional replenishing, were unproductive, this protocol succeeds in promoting the reactivation of cell wall-deficient VBNC forms of L. monocytogenes generated by starvation in mineral water. Importantly, the results obtained underline the requirement of the embryo for the revival of VBNC L. monocytogenes, indicating an important role of embryo-associated factors in this process. Other potential uses for this method include the screening and identification of bacterial factors implicated in the mechanisms of VBNC state revival. This model can thus provide insight into the molecular workings of bacterial dormancy, whose knowledge is critical to reduce the public health risks entailed by undetectable pathogens.
Introduction
In the search for alternative in vivo research models, with reduced associated costs and logistical and ethical considerations, the chicken embryo emerged and quickly became one of the most applicable, manageable, and reproducible in vivo vertebrate model systems1,2. Compared to other animal models, such as rodents and rabbits, fertilized chicken eggs are inexpensive to obtain and require no complex housing logistics for their development. Moreover, the chicken egg size enables the handling of numerous embryos in parallel, supporting a robust number of experimental test conditions/groups and replicates. The short embryogenesis duration (21 days) and the simplicity of accessing and observing the embryo and associated structures at any point of the developmental period make this a useful model in a variety of fields, such as developmental biology (e.g., heart and brain formation)3,4 and pharmacology (testing drug activity, delivery, and toxicity)1,5,6. In addition, the immature immune system of the chicken embryo makes it a suitable system for immune-based studies and cancer research-related approaches7. Importantly, because of the embryonic nature of this model, which only acquires a mature nociceptive system by developmental day 13-148, research applications conducted within this timeframe are not constrained by legal and ethical concerns7.
The chicken embryo model has also been widely used to study the pathogenicity of microbes causing disease in humans and other mammals. Indeed, numerous studies have explored and validated this model to investigate the virulence of protozoan (e.g., Neospora caninum, Eimeria tenella, Cryptosporidium spp.)9,10,11, fungal (e.g., Candida albicans, Aspergillus fumigatus)12,13, and bacterial species (e.g., Enterococcus spp., Salmonella enterica, Francisella spp., Campylobacter jejuni, Clostridium perfringens, Listeria monocytogenes, Neisseria gonorrhoeae, Staphylococcus aureus)10,14,15,16,17,18,19,20,21,22, as well as to test the therapeutic effect of antimicrobial compounds17,23.
Microorganisms, like the ones mentioned above, are often exposed to stressful stimuli in their environment(s) and have, therefore, evolved stress-coping strategies to endure potentially harmful/lethal situations. Some bacterial species can produce highly resilient and metabolically dormant structures called endospores, which preserve cellular and genetic integrity under severe environmental constraints. If favorable environmental conditions are gathered, endospores can regenerate into viable active cells by germination24. Non-sporulating bacteria, however, may enter into an alternative metabolically dormant state called viable but non-culturable (VBNC), whose main phenotypic trait is the loss of culturability in routine growth media25. Given that a large part of the > 100 bacterial species reported to enter a VBNC state are pathogenic to humans and other animals26, and that VBNC pathogens may revive back into a metabolically active and virulent state25,26, the failure of conventional growth-based methods to detect VBNC pathogens is a concerning public health issue. The environmental cues and molecular and physiological mechanisms driving this revival process are not yet well understood and may vary with the microbial species and the VBNC state-inducing stress(es).
Researchers have taken advantage of the particularities of the chicken embryo model to investigate the in vivo revival capacity of bacterial pathogens in a VBNC state. Human-derived C. jejuni isolates, driven into a VBNC state by nutritional deprivation in water, recovered their culturability and virulence in human cells after passage in embryonated chicken eggs27. Similarly, successful attempts to revert the VBNC state were also reported for other pathogens, such as Edwardsiella tarda28, Legionella pneumophila29, and L. monocytogenes30.
We have recently reported that when L. monocytogenes is driven into a VBNC state by starvation in mineral water, it shifts from a rod-shaped to a coccoid cell. We revealed that this morphological transformation is caused by cumulative damage to the cell wall, leading to its complete shedding by the bacterium, which then becomes a cell wall-deficient spherical cell form31. Our unsuccessful attempts to revive these wall-less VBNC L. monocytogenes forms in vitro, using nutrient replenishing approaches, led us to investigate their potential rescue in vivo. Given its promising use with L. monocytogenes30, we selected the chicken embryo model for this task. The results confirmed the capacity of the embryonated chicken egg system to promote the restoration of cell wall-deficient VBNC L. monocytogenes back to an active culturable state31.
Here, we provide a detailed protocol enabling the in vivo revival of L. monocytogenes from a dormant VBNC state through exposure to the embryonated chicken egg environment. We describe the preparation and monitoring of chicken eggs and VBNC bacteria, the inoculation of eggs, the processing of the embryonated and non-embryonated eggs, and the scoring of the culturable bacterial burden to assess VBNC cell revival efficiency. The results reaffirm the chicken embryo as a simple, cost-effective, and suitable model to understand the mechanisms governing different aspects of microbial life, such as bacterial dormancy. This in vivo system can be further explored to investigate the contribution of individual bacterial genes in the resurrection process.
Protocol
This protocol follows the applicable institutional French (Decree no. 2013-118) and European (Directive 2010/63/EU) guidelines for animal use in research. Furthermore, under French law (Decree no. 2020-274), this protocol is not concerned with ethical restrictions because all experimentation with chicken embryos is performed and completed before the last third period of embryogenesis (i.e., before day 14).
1. Preparation of VBNC bacteria
- Streak bacterial strains from glycerol stocks (stored at −80 °C) onto brain heart infusion (BHI) agar media using inoculation loops. Incubate overnight at 37 °C to obtain isolated colonies.
NOTE: If required, supplement the agar media with antibiotic(s) for which the strain(s) possess(es) resistance (either naturally or by genetic modification). - For each strain, prepare 2-3 tubes with 5 mL of BHI broth and use an inoculation loop to inoculate with 2-3 colonies. Incubate the cultures overnight at 37 °C with agitation (200 rpm) to grow bacteria until the stationary phase. Each of these cultures will be used to prepare independent biological replicates of VBNC bacterial suspensions.
- Dilute each culture 1:10 in BHI broth and measure the optical density in a spectrophotometer at a wavelength of 600 nm (OD600). The OD600 value of optimally grown stationary phase cultures of Listeria monocytogenes ranges between 2 and 4. This equates to a bacterial concentration of 2-4 x 109 colony-forming units (CFU)/mL.
- Pellet 1 mL of bacterial culture in a 1.5 mL microtube by centrifugation at 6,000 x g for 2 min. Aspirate the supernatant using a vacuum pump. Resuspend the cell pellet in 1 mL of autoclaved and filtered mineral water.
- Wash the bacteria thoroughly by repeating step 1.4 three times. This ensures the complete removal of culture medium nutrients that would otherwise delay the formation of VBNC bacteria.
- Prepare bacterial suspensions at a starting concentration of 106 CFU/mL by adding 30 µL of washed bacteria to T25 cell culture flasks containing 30 mL of mineral water.
- Mix the suspensions using a serological pipette. Store them at room temperature, under static (flask in upright position) and dim lighting conditions.
2. Monitoring the formation of VBNC bacteria
- Determine the culturable population in the bacterial suspensions on the 1st day (immediately after preparation) and then on a weekly basis, as described below.
- Prepare 10-fold serial dilutions of the VBNC bacterial suspension in mineral water (from 10-1 to 10-3 dilutions). Plate 100 µL of undiluted suspension and of each dilution, in duplicate, on BHI agar and incubate overnight at 37 °C.
NOTE: When the culturability approaches < 1 CFU/mL, a larger volume (0.5-2 mL) of the undiluted suspension should be plated to confirm the presence/absence of culturable bacteria. - Calculate the concentration of culturable bacteria as follows:
concentration of culturable bacteria (CFU/mL) = (average number of colonies x sample dilution factor) / plated sample volume (in mL).
- Prepare 10-fold serial dilutions of the VBNC bacterial suspension in mineral water (from 10-1 to 10-3 dilutions). Plate 100 µL of undiluted suspension and of each dilution, in duplicate, on BHI agar and incubate overnight at 37 °C.
- Determine the viable population in the bacterial suspensions on the 1st day (immediately after preparation) and then on a weekly basis, as described below.
- Incubate a sample of the bacterial suspension (i.e., at a concentration of about 106 cells/mL) with the viability dye 5(6)-carboxyfluorescein diacetate (CFDA) at a final concentration of 30 µM for 30 min in the dark. Prepare also a sample without CFDA (unstained control) and a sample treated at 95 °C for 30 min before incubation with CFDA (non-viable/dead cell control).
- Analyze the samples in a flow cytometer equipped with a 488 nm excitation laser and a 520 nm emission detector for CFDA fluorescence. For statistical robustness, acquire about 1 x 105 bacterial events per sample (this translates to an acquired sample volume of 10-100 µL) at a flow rate of at least 30 µL/min.
- Use the unstained sample to find and gate the bacteria-associated events in a logarithmic-scaled forward scatter (FSC) versus side scatter (SSC) plot. Plot the gated bacterial events in a logarithmic-scaled CFDA fluorescence histogram and use the unstained and non-viable/dead cell samples to distinguish the viable (CFDA-positive) from the non-viable (CFDA-negative) populations.
- Calculate the concentration of viable bacteria as follows:
concentration of viable bacteria (cells/mL) = average number of CFDA-positive events in acquired sample / acquired sample volume (in mL)
NOTE: Depending on the cytometer model, cell quantification may require the use of flow cytometry counting beads.
3. Incubation of eggs
- Upon reception from the supplier, allow the fertilized eggs to be accommodated at room temperature until the next day.
- In the meantime, fill the water reservoir of the egg incubator with deionized water and turn the power on. Set the temperature to 37.7 °C and the maximum relative humidity to 47%.
NOTE: In our hands, eggs are incubated at a relative humidity range of 38%-42%, with no visible impact on the expected embryogenesis rate and success. - Transfer the eggs into the incubator to initiate embryogenesis. Position them on the incubator tray(s) with the air pocket facing up (i.e., pointy end facing down).
- Incubate the eggs for 6 days. After 4 days, distinguish live embryonated eggs from dead or non-embryonated eggs by candling.
- Place the egg under/over a strong light source (candler) to illuminate the interior and visually discern its content. After 3 or 4 days of incubation, live embryonated eggs display a network of blood vessels expanding downwards from the air pocket. This network appears collapsed and disorganized in dead embryonated eggs and is completely absent in eggs that failed to start embryogenesis. If visible, a live embryo may even display twitching movement (Figure 1).
4. Preparation of eggs for inoculation
- On the day of inoculation, candle the eggs to determine the available number of viable embryonated and non-embryonated eggs. Identify the non-embryonated eggs (about 10%-20% of the total number) and any eggs with dead embryos, as described in step 3.4. Discard the latter according to the institutional guidelines for biological waste disposal.
- Mark the injection spot on the eggshell at 2-5 mm above the air pocket border (Figure 2A). Disinfect this area by rubbing with tissue paper soaked with 70% (v/v) ethanol.
- Using an 18G needle, carefully make a puncture dent on the shell at the injection spot without piercing through the shell membrane (Figure 2B). Disinfect the injection spot with a tissue paper soaked with 70% (v/v) ethanol.
5. Inoculation of eggs
- Plug a 25G (0.5 mm x 16 mm) needle into a 1 mL syringe and fill it with VBNC bacterial suspension. If necessary, remove air bubbles stuck to the inner wall of the syringe by flicking it with a finger.
- Perform inoculation with mineral water alone or a suspension of L. monocytogenes (5 x 103 CFU/mL) grown overnight in BHI broth as control conditions for sterility and growth derived from culturable bacteria, respectively.
- Introduce the needle through the punctured shell at the injection spot and insert it all the way through, at a perpendicular angle, until the base of the needle touches the shell surface (Figure 2C). This positions the needle tip to deliver the syringe content into the allantoic cavity (in embryonated eggs) or the albumen (in non-embryonated eggs).
- Carefully and slowly inject 100 µL of the inoculum. Keep the angle of the needle as still as possible when inside an embryonated egg to avoid causing lethal injuries to the embryo.
- Carefully and slowly remove the needle and cover the injection spot with a round sticker to seal the inoculation entry site. For convenience and clarity, use stickers with a different color for each condition/group of eggs.
- Return the inoculated eggs to the incubator for an additional 2 days. Ensure that eggs with developing embryos are not out of the incubator (or at a temperature below 37.7 °C) for more than 30 min. Perform the inoculation in batches, if necessary.
6. Assessing the presence of culturable cells in the inoculation samples
- Dispense 100 µL of VBNC bacterial suspension into multiple wells of a 96-well microplate. Calculate the Number of wells ≥ 2 x the number of inoculated (embryonated + non-embryonated) eggs.
- If using the control conditions mentioned in step 5.2, dispense 100 µL of each inoculum (i.e., mineral water or culturable bacteria) into at least three wells in the same 96-well microplate.
- Using a multichannel pipette, add 100 µL of BHI medium to each of the wells prepared in the previous two steps.
- Incubate the plate at 37 °C under static conditions to promote bacterial growth in wells containing culturable bacteria. Depending on the presence of culturable bacteria and their regrowth rate in these conditions, one or more incubation days may be necessary to fully reveal wells containing culturable bacteria.
- For each inoculum, visually inspect and count the number of wells scoring positive and negative for bacterial growth.
7. Monitoring embryo viability
- The day after the inoculation, candle the embryonated eggs to check for embryo lethality.
- Put aside eggs containing dead embryos and discard them according to the institutional guidelines for infected biological waste disposal.
8. Processing of embryonated eggs
- Recover the embryonated eggs from the incubator and candle to check for embryo lethality. Discard dead embryos and do not consider them in the final results.
- Remove the sticker from the egg and disinfect the top end of the shell (covering the air pocket) with tissue paper soaked with 70% (v/v) ethanol.
- Using a clean pair of dissection scissors, cut the shell open from the injection spot to expose the air pocket. Using a clean pair of dissection tweezers, tear open the inner shell membrane that separates the air pocket from the rest of the egg.
- Carefully empty the egg contents into a sterile Petri dish. Using a pair of tweezers, isolate and transfer the embryo onto a new Petri dish. Wash the embryo with sterile PBS, if necessary.
- Transfer the embryo into a 15 mL centrifuge tube containing 4 mL of sterile PBS. Dissociate the embryo using a homogenizer (speed: 10,000 rpm). Clean the tip of the dispersion tool between each homogenization by passing it sequentially in sterile PBS, 70% (v/v) ethanol, and again sterile PBS.
- Plate 500 µL of the embryo homogenate on BHI agar. Incubate the plates at 37 °C overnight (or longer, if necessary).
NOTE: Optionally, serial dilutions of the homogenate can be plated in case elevated CFU numbers (i.e., > 300) are expected in the undiluted homogenate. - For each inoculation group, count the number of eggs scoring positive and negative for L. monocytogenes growth on agar plates.
NOTE: Despite the aseptic conditions under which the embryo extraction and homogenization are performed, the use of a non-selective agar medium for plating embryo homogenates entails a risk of contaminant growth. The use of a genus/species-specific growth medium or supplementation with a selective antibiotic (for which only the tested bacterial strain is resistant) can reduce or prevent such contamination.
9. Processing of non-embryonated eggs
- Recover the non-embryonated eggs from the incubator. Repeat steps 8.2 and 8.3 to gain access to the egg albumen.
- Using a micropipette, collect 500 µL of albumen and spread directly on BHI agar. Incubate the plates at 37 °C overnight or longer, if necessary.
- For each inoculation group, count the number of eggs scoring positive for L. monocytogenes growth on agar plates.
Results
To test the potential of the chicken embryo model to revive cell wall-deficient VBNC forms of L. monocytogenes generated by starvation in mineral water, it was important to articulate the timing of the preparation of the VBNC bacterial inoculum (≥ 28 days) with that of the embryonated eggs (6 days). Replicate suspensions of L. monocytogenes were thus set up at a concentration of 1 x 106 CFU/mL in mineral water 28 days before the planned egg inoculation day. This starting bacterial concentration was shown to result in a desired residual culturability of <1 CFU/mL after 28 days31. A final verification of the bacterial cell culturability and viability levels of the suspensions resulted in the selection of a VBNC L. monocytogenes inoculum containing 1 x 106 viable cells and 0.5 culturable cells per mL. For experimentation, 100 µL of this inoculum (i.e., 1 x 105 viable cells and < 0.1 CFU) were delivered into groups of embryonated and non-embryonated eggs. Control groups were prepared in parallel by injecting embryonated and non-embryonated eggs with the same dose volume of mineral water or of a 5 x 103 CFU/mL suspension of L. monocytogenes grown overnight in BHI broth.
At 2 days post-inoculation, eggs were processed to assess the presence of culturable L. monocytogenes. As expected, no bacterial growth was recovered from the sterility control group eggs injected with mineral water, while 100% of eggs from the control group treated with culturable L. monocytogenes scored positive for growth on agar media (Table 1). Importantly, all embryos recovered from eggs inoculated with VBNC bacteria gave rise to L. monocytogenes growth on the plate, contrasting with its complete absence on every plate spread with the content of a non-embryonated egg injected with the same VBNC bacteria (Table 1). This result underlines the requirement of the chicken embryo for the awakening process of VBNC L. monocytogenes, as previously reported30.
To determine the likelihood that the L. monocytogenes growth retrieved from embryonated eggs injected with VBNC bacteria comes from a true VBNC cell recovery, not from the regrowth of lingering culturable cells, it is necessary to compare the frequency of inoculum doses producing bacterial growth before and after egg passage. To obtain the former, multiple 100 µL doses of the VBNC bacterial inoculum were mixed with BHI, a nutrient-rich medium that does not support VBNC L. monocytogenes revival32, in a 96-well plate and incubated at 37 °C. A frequency of 9.5% of inoculated BHI wells positive for bacterial growth (8 in 84) differed significantly (Fisher's exact test, p = 1.6 × 10−17) from the 100% frequency of eggs showing recovered culturable bacteria (Table 1). This substantial and statistically significant difference strongly indicates that the L. monocytogenes growth recovered from embryonated eggs was largely due to the reactivation of VBNC bacteria. In addition, unlike its vegetative forms, VBNC L. monocytogenes were unable to revive in non-embryonated eggs, supporting that VBNC cell revival in embryonated eggs is not due to the residual culturable bacteria in the inoculum.
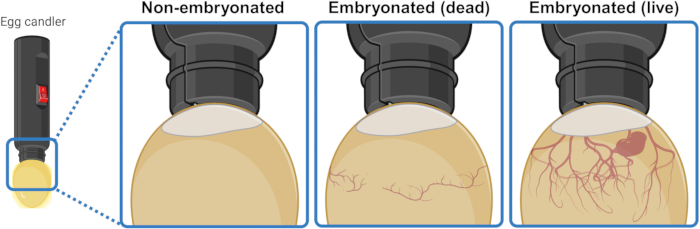
Figure 1: Distinguishing non-embryonated, dead embryonated, and live embryonated chicken eggs by candling. The embryogenesis status is determined by illuminating the egg with a light source (egg candler) from close range. Eggs containing a developing live embryo (visible or not) display a strong network of blood vessels expanding downwards from the air pocket. This network appears collapsed and disorganized in eggs with dead embryos and is completely absent in eggs that failed to start embryogenesis. Created in BioRender.com. Please click here to view a larger version of this figure.

Figure 2: Preparation of the egg for inoculation. (A) The injection site is marked at a position 2-5 mm above the air pocket border, as seen by candling. (B) The shell at the injection site is puncture-dented with the help of the tip of an 18G needle without piercing the outer shell membrane. (C) The inoculum is delivered through a 25G (0.5 mm x 16 mm) needle into the allantoic fluid in embryonated eggs or the albumen in non-embryonated eggs. Created in BioRender.com. Please click here to view a larger version of this figure.
| Inoculum | Culturability before egg passagea | Culturability after egg passageb | |||
| Embryonated eggs | p-valuec | Non-embryonated eggs | p-valued | ||
| Mineral water | 0/3 (0%) | 0/10 (0%) | >0.999 | 0/10 (0%) | >0.999 |
| VBNC Lm | 8/84 (9.52%) | 24/24 (100%) | 1.63E-17 | 0/18 (0%) | 0.344 |
| Culturable Lm | 3/3 (100%) | 8/8 (100%) | >0.999 | 2/2 (100%) | >0.999 |
| aNumber of BHI wells with bacterial growth/Number of BHI wells inoculated. | |||||
| bNumber of eggs with bacterial growth/Number of eggs inoculated. | |||||
| cComparison of culturability before and after passage in embryonated eggs (two-sided Fisher's exact test). | |||||
| dComparison of culturability before and after passage in non-embryonated eggs (two-sided Fisher's exact test). | |||||
Table 1: VBNC L. monocytogenes revert back to a culturable state after passage in embryonated chicken eggs. Embryonated and non-embryonated chicken eggs (6 days) were inoculated with 100 µL of mineral water only or with mineral water suspensions of VBNC (1 x 104 cells, 28 days) or culturable (500 cells) L. monocytogenes. After 2 days, embryos (or albumen in non-embryonated eggs) were recovered and plated on BHI agar to assess the presence of culturable L. monocytogenes. The frequency of culturable cells present in an inoculation dose before egg passage was determined by mixing 100 µL of each suspension with BHI broth in multiple wells of a 96-well microplate and scoring the number of wells with bacterial growth after incubation at 37 °C. Statistical significance was calculated using a two-tailed Fisher's exact test, with p-values ≥ 0.05 considered non-significant. This table was reproduced from31.
Discussion
The public health and economic risks associated with viable but non-culturable (VBNC) forms of bacterial pathogens are a consequence of their capacity to evade detection by conventional microbial growth-based methods as well as of their tolerance/insensitivity to most antimicrobials used in clinical and food industry settings26,33. It becomes, therefore, urgent to find research tools and models to better understand the mechanisms driving the transition, maintenance, and revival of bacterial cells in a VBNC state.
In many aspects, the chicken embryo is an advantageous alternative to more conventional mammalian models of research (e.g., mouse, rat, rabbit). It is technically more practical to work with an oviparous organism, as its embryonic development is fast and occurs externally and independently of its progenitor. Importantly, the financial and logistical burdens associated with the production, housing, and maintenance of chicken eggs and embryos are markedly lower. In addition, the ease to obtain large numbers enables parallel testing of multiple experimental conditions and/or replicates and the generation of comprehensive and statistically robust data. Although non-mammalian models, such as fish or nematodes, can provide similar advantages, the chicken embryo, as a warm-blooded vertebrate, is evolutionarily closer to humans and thus a better research model in areas such as immunology, disease modeling, development, and toxicology. Embryonic models are also simpler to work with from an ethical standpoint, particularly during the first two-thirds of development, when a mature nociceptive system is not yet established8. This short time window is convenient for applications requiring quick turnaround times (e.g., drug testing1), but can pose restrictions for assays lasting into later embryogenesis stages or even post-hatching life (e.g., developmental and behavioral studies). The lack of an adaptive immune system also renders the chicken embryo a useful research tool in areas such as host-pathogen interactions and cancer biology7.
The chicken embryo has been extensively used in the study of microbial virulence9,10,11,12,13,14,15,16,17,18, but sparingly as a model to revive VBNC pathogens27,28,29,30. Here, we have provided a detailed methodological description of how this in vivo system can be successfully used for the rescue of VBNC L. monocytogenes.
A critical point to consider in this method is the issue of the residual presence of culturable cells and their potential to overpower true VBNC cell revival. The limitations of the VBNC cell production method to address this matter (VBNC cell purity levels are coupled with starting bacterial concentration) raise the need to employ alternative approaches to eliminate contaminating culturable cells altogether or reduce them to a negligible level. Such approaches may include, for instance, neutralization/killing by antimicrobials targeting exclusively culturable cells. Alternatively, physical removal of the culturable cell population can be achieved based on unique morphophysiological characteristics (e.g., cell shape, surface or cytoplasmic structures or molecules) by flow cytometry. This approach has the added advantage of allowing the isolation/purification of VBNC cell subpopulations with different ages (i.e., formed at different time points), which can also be tested in ovo for their revival capacity.
Other points of this method requiring attention include the careful handling of embryonated eggs, particularly during and immediately after the injection step (to avoid unintended needle-associated trauma or lethality), and striving to reduce the manipulation time outside of the optimal incubation temperature. In addition, despite the aseptic conditions under which the retrieval, processing, and plating of embryos should be performed, unwanted microbial growth on agar plates may still occur. This can be solved by the use of agar media with a formulation (basal composition and/or supplements) selective for the bacterial species of interest or by using a strain possessing resistance to a given antibiotic.
This work showcases the chicken embryo as a simple yet powerful and cost-effective in vivo model to investigate the mechanistic aspects of bacterial dormancy. In this context, it can potentially be used to identify bacterial factors implicated in the VBNC state awakening process through the screening of individual bacterial mutants or on a genome-wide scale by employing a mixed library (e.g., transposon mutant library).
Disclosures
The authors declare no conflicts of interest.
Acknowledgements
This work was supported by a grant from the Micalis Institute (AAP Micalis FAMe 2023). F.C. was supported by postdoctoral grant funding from Agence Nationale de Recherche (THOR: ANR-20-CE15-0008; PERMALI: ANR-20-CE35-0001).
Materials
| Name | Company | Catalog Number | Comments |
| 5(6)-carboxyfluorescein diacetate (CFDA) | Sigma-Aldrich | 21879 | Prepare stock solution at 30 mM in DMSO. Aliquot and store at −20 °C. |
| Benchtop centrifuge, 24 tubes 1.5–2 mL | Eppendorf | ||
| Biosafety cabinet, class II | |||
| Brain heart infusion (BHI) agar | BD | 248130 | |
| Brain heart infusion (BHI) broth | BD | 237500 | |
| Cell culture flasks, 25 cm2 (T25), vented cap | |||
| Centrifuge tubes, 15 mL | Sarstedt | ||
| Deionised water | To fill water reservoir of egg incubator | ||
| Dissection scissors, pointed ends | |||
| Dissection tweezers, curved + serrated ends | |||
| Egg candler | FIEM (Guanzate, IT) | IM35 | |
| Egg incubator (MG 244 Mercurius SH) | FIEM (Guanzate, IT) | SH244AD | Capacity for 144 chicken eggs (rocking trays) + 100 (hatching tray) |
| Eggs, fertilized | PFIE (INRAE Val-de-Loire, FR) | From white Leghorn chickens, raised in specific pathogen-free conditions | |
| Ethyl alcohol, 70% (v/v) | |||
| Flow cytometer (CytoFLEX S) | Beckman Coulter | V0-B2-Y4-R0 | |
| Heating block (95 °C) | |||
| Incubator (37 °C) | For growth of bacteria on agar plates | ||
| Incubator w/ shaking (37 °C, 200 rpm) | INFORS-HT | For growth of liquid bacterial cultures | |
| Inoculation loops | |||
| Microcentrifuge tubes, 1.5 mL | Eppendorf | ||
| Microtiter plates w/ lid, 96-well, transparent | Greiner | To prepare serial dilutions and analyze samples in flow cytometer | |
| Mineral water | Autoclaved (121 °C, 20 min) and sterile-filtered (0.2 µm) | ||
| Needle, hypodermic, 18G | Terumo | AN1838R1 | Dimensions: 1.2 × 38 mm |
| Needle, hypodermic, 25G | Terumo | AN2516R1 | Dimensions: 0.5 × 16 mm |
| Petri dishes, 10 cm ø | Greiner | For preparation of BHI agar plates and processing of chicken embryos | |
| Phosphate buffered saline (PBS), 1X | Gibco | 14190 | |
| Plate spreaders | |||
| Serological pipettes, disposable, 5 + 10 + 25 mL | |||
| Spectrophotometer (UV-Vis) + cuvettes 1 mL | |||
| Stickers, round, colored (Tough-Spots) | Dutscher | Dimensions: 9.5 mm ø | |
| Syringe, Luer slip, 1 mL | Terumo | ||
| Tissue homogenizer | IKA | ULTRA-TURRAX T25 disperser + S25N 8G dispersion tool | |
| Vacuum pump |
References
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved