Method Article
Isolation of Small Extracellular Vesicles Derived from Mesenchymal Stem Cells from Large-Volume Samples with GMP Compliance for Clinical Trials
* These authors contributed equally
In This Article
Summary
Small extracellular vesicles derived from mesenchymal stem cells (MSC-sEVs) have been underscored as a cell-free treatment modality with minimal adverse effects. This study provides a protocol combining hemodialysis with ultracentrifugation, significantly reducing the time spent on the entire process and ensuring compliance with good manufacturing practice (GMP) standards.
Abstract
Small extracellular vesicles (sEV) derived from mesenchymal stem cells (MSC-sEVs) have been underscored as a cell-free treatment modality with minimal adverse effects. In contrast, traditional extraction methods such as ultracentrifugation and size-exclusion chromatography are limited by their time intensity, cost, and scalability. To overcome these limitations, we propose a method integrating a hemodialyzer and ultracentrifugation. This approach utilizes a hemodialysis device with a 100 kDa molecular weight cut-off (MWCO) membrane, which selectively concentrates sEVs while filtering out a plethora of proteins, thereby enhancing the yield and purity of sEVs. This initial purification step is followed by ultracentrifugation to further refine the sEV preparation. The integration of these two technologies not only significantly reduced the time spent on the entire process but also ensured compliance with good manufacturing practice (GMP) standards. The method here demonstrates high efficiency in isolating sEVs from a large volume of samples, offering a significant advancement over traditional methods. This protocol holds promise for accelerating the translation of EV-based therapies into clinical practice by providing a scalable, cost-effective, and GMP-compliant solution.
Introduction
Small extracellular vesicles derived from mesenchymal stem cells (MSC-sEVs) are heterogeneous vesicles enriched with multiple components such as mRNA, micro-RNA, cytokines, lipids, and metabolites1. In recent years, many studies have underscored the immense therapeutic potential of MSC-sEVs as a cell-free treatment modality with minimal adverse effects2, showing promise in addressing a spectrum of conditions, including aging, tissue degeneration, cancer, and inflammatory disorder3,4,5,6. Nevertheless, a critical challenge persists in large-scale extraction of sEV, with traditional methods proving to be either laboriously time-intensive or economically unfeasible. Furthermore, ensuring reproducibility is paramount for the clinical application and translation of EV-based therapies7. Researchers are in dire need of a purification method that is not only simple and efficient but also compliant with good manufacturing practice (GMP) standards8.
The conventional purification methods, including ultracentrifugation, ultrafiltration, size-exclusion chromatography, immunoaffinity, and polymer precipitation, have been extensively applied in previous research9. In general, traditional methods for sEV isolation exhibit limitations such as low yield rate, compromised purity, and challenges in meeting stringent aseptic standards. Furthermore, previous research has reported the potential of promising techniques like microfluidic systems10,11, label-free magnetic isolation12, and covalent chemistry isolation13 for achieving outstanding performance. However, the requirement for specialized equipment makes these advanced techniques challenging for the majority of research teams to adopt. In summary, the efficient method to isolate GMP grade sEVs from a large volume of samples remains a critical obstacle, limiting the progress of numerous teams in both research and clinical applications.
Ultracentrifugation is the most widely adopted method for sEV isolation and is recognized as the gold-standard method14,15. It is a technique that leverages differences in density and size to isolate sEVs. Isolated sEVs are commonly rinsed with phosphate-buffered saline (PBS) to eliminate residual contaminants. Then, an appropriate volume of PBS is generally used to resuspend the rinsed sEVs and different expected concentrations of sEVs can be harvested by controlling the volume of PBS. Furthermore, it is reported that the purity of plasma sEVs obtained by ultracentrifugation appears to be better than that of plasma sEVs isolated by size exclusion chromatography (SEC), and the sEVs obtained by ultracentrifugation have lower non-vesicular extracellular particles (NVEPs) impurities. This also makes ultracentrifugation the most widely used and difficult to replace in many treatments that require high concentrations of sEVs. However, in addition to quality and purity, efficiency is also a factor that cannot be ignored in large-volume sEV extraction. So far, a single round of ultracentrifugation can support a sample volume of up to approximately 600 mL, which determines that it is difficult to meet the demand for large-scale extraction by just ultracentrifugation16.
A hemodialysis device consists of a membrane-based module that houses thousands of hollow fibers. Blood circulates through these fibers within an enclosed cylindrical chamber17. The constituents of the blood can selectively pass through these membranes based on their molecular size and ionic concentration. In the clinic, it is widely used as an artificial kidney to remove waste products and excess fluids from the blood of patients18,19,20. In other words, the hemodialyzer also has the potential to concentrate large-volume samples, relying on a process similar to tangential flow filtration (TFF). In the recent guideline issued by the International Society for Extracellular Vesicles (ISEV), sEV concentrates are considered suitable for large-volume samples, such as cell culture medium. After decades of development, hemodialyzers have been widely adopted in hospitals, supported by an abundance of mature consumables and a pool of skilled operators, which makes it easier to keep the sample sterile.
This study presents a sEV purification method based on a hemodialyzer and ultracentrifuge compatible with GMPs. Here, we choose dialyzers of 100 kDa molecular weight cut-off (MWCO), which has been demonstrated to effectively capture sEVs and filter out numerous proteins22. Ultracentrifugation also provides a step for further purification. The work demonstrates that the hemodialyzer is equally suitable for the concentration of sEVs. This protocol allows researchers to isolate sEVs from large-volume samples efficiently. We have registered the clinical trial in Chinese Clinical Trial Registry (ChiCTR, NO. ChiCTR2200059018), which is still in progress and has not been completed yet. Although clinical data is not readily available for publication at this moment, a reliable, large-scale, efficient, and compliant method for producing sEVs as reported in this protocol is a prerequisite for conducting pre-clinical and clinical trials.
Protocol
The protocol was approved and conducted in accordance with the Human Research Ethics Committee of the Southwest Hospital.
1. Removing cell debris from the culture medium
NOTE: The procedures below should be operated in a GMP-compliant environment, especially when the samples may be directly exposed to the environment.
- Given the requirement of microbiota-free, ensure that the filter and rubber tube are autoclaved for effective sterilization. Ensure all other consumables that might encounter the culture medium are sterile and in sealed packaging. Perform the process of removing cell debris in a laminar flow cabinet.
- Assemble the 0.45 µm and 0.22 µm filtration membrane to the filter apparatus. Connect a peristaltic pump (see Table of Materials) and the filter apparatus with the rubber tube. Position the 0.45 µm membrane above the 0.22 µm membrane; otherwise, it may decrease filtration efficiency (Figure 1). Pay attention to distinguishing between the microfiltration membrane's front and back sides.
- Start the peristaltic pump for the filtration. Powered by the pump, the mesenchymal stem cell basal medium (MSCBM) with 5% supplement (see Table of Materials) passes through the filter from one end, and the filtered culture medium can be obtained at the other end of the filter, as shown in (Figure 1). Gather the filtered culture medium in a drainage bag with dual channels. Maintain the integrity of the filtration system by optimizing the peristaltic pump speed and replacing the filtration membrane as needed.
- After filtration is complete, close the inlet valve on the drainage bag and further seal it with parafilm (see Table of Materials). Continue to the next step or temporarily store the filtered cell culture medium at 4 °C.
2. Concentrating the filtered culture medium with a hemodialyzer
NOTE: Refrain from using Dulbecco's Modified Eagle Medium (DMEM) containing phenol red in cell culture, as the blood-leak detector in the hemodialyzer will be activated or shut down the blood-leak detector in the hemodialyzer setting.
- Prepare the consumables.
- Prepare bloodlines, 100 kDa MWCO high-flux polysulfone membranes, blood dialyzer, infusion set, physiological saline solution, infusion bag, iodine solution, and cotton swabs before concentration (see Table of Materials).
- Power on the system and self-test.
- Check the connection of the hemodialyzer power cable and switch on the main power.
- Wait for the machine to complete the entire self-diagnostic procedure.
- Install the bloodlines and dialyzer.
- Verify the expiration dates of the disposable bloodlines and the blood dialyzer, ensuring the integrity of their outer packaging and checking for any damage to the lines.
- Install the tubing in the same direction as blood flow in the extracorporeal circuit, sequentially closing the branch valves to form a closed-loop circulation.
- Start the rinse procedure.
- Use an infusion set to connect the physiological saline solution (without heparin) from a tube before the pump to the dialysis circuit. Connect the venous collection site to the waste fluid bag.
NOTE: The physiological saline should not contain heparin or any other anticoagulants. - Connect the dialysate supply/return lines to the dialyzer.
- Start the hemodialyzer and set the flow rate to 80-100 mL/min. Expel all the air from the dialysis circuit and the dialyzer to ensure the entire tubing system is filled with fluid.
- Adjust the liquid plane in the arterial and venous chambers to approximately 2/3 full. The saline sequentially flows through the arterial line, the dialyzer, the venous line, and the waste fluid bag.
- Use an infusion set to connect the physiological saline solution (without heparin) from a tube before the pump to the dialysis circuit. Connect the venous collection site to the waste fluid bag.
- Start the ultrafiltration concentration.
- After finishing the rinse procedure, hang the drainage bag containing the filtered culture medium on the infusion stand, disinfect the output channel with iodine-soaked cotton swabs, and connect the output channel of the drainage bag to a pre-pump port using an infusion set.
- Join the arterial port and the venous port of the bloodlines. Establish an isolated ultrafiltration (ISO-UF, ultrafiltration without dialysate flow) dialysis circuit (Figure 1).
- Adjust the ultrafiltration target volume on the dialysis machine to correspond with the total volume of fluid present in the drainage bag. Set the dialysis time of the ultrafiltration to the minimum operational time of the system. After the parameters are set successfully, initiate the ultrafiltration.
NOTE: Due to the calculation of the hemodialysis system, the dialysis time will not be too short but will be maintained at a more moderate rate.
- Obtain the concentrated fluid.
- After achieving the target dehydration volume, shut the arterial clamp and connect the venous port to a sterile infusion bag.
- Open the pre-pump port of the disposable blood lines equipped with an air filter. Activate the blood pump at a speed of approximately 50 mL/min, allowing the fluid within the bloodlines to circulate once again from the arterial line to the venous line. Retract all the concentrated fluid, approximately 158 mL, from the circuit back into the sterile infusion bag.
- In the late stage of concentration, collect the wasted fluid from the dialysate return line. Apply subsequent processes identical to those of the concentrated culture medium to the wasted fluid sample to monitor any accidental loss of sEVs that may have occurred during concentration.
- Close all valves and remove the infusion bag, dialyzer, and all lines. Turn off the power supply and disinfect the machine by wiping it down.
3. Separating the sEVs with ultracentrifuge
- Transfer the concentrated culture medium into ultracentrifuge tubes and then balance them.
- Centrifuge in a clinical-grade ultracentrifuge (see Table of Materials) at 110,000 × g for 70 min, then wash the pellet with sterile saline solution and proceed with a second centrifugation at 110,000 × g for another 70 min.
- Resuspend the pelleted sEVs in an appropriate amount of sterile saline solution.
- Transfer the sEVs to a sterile tube for further testing or store them at -80 °C for future use.
4. Characterization of obtained sEVs
- Nanoparticle tracking analysis (NTA)
- Dilute the obtained sEVs (1 µL) with PBS (1 mL) (see Table of Materials). Adjust the number of sEVs visible in the current field of view to be between 50 and 200.
- Carefully inject the diluted sample into the sample well, avoiding the introduction of air bubbles.
- Perform the measurement when the number of particles in the field of view is as high and stable as possible for all positions. The analysis parameters are as follows: Max Area: 1000, Min Area: 10, Min Brightness: 30, Trace length: 15, Temperature: 25 °C, Sensitivity: 75.
- Measure each sample at least three times.
- Western blotting analysis
- Dilute sEVs solution in PBS. Use a Bicinchoninic Acid (BCA) Protein Assay Kit (see Table of Materials) to measure the protein concentration.
- Boil the sEVs in the sample buffer at 100 °C for 5 min.
- Prepare a 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel (see Table of Materials). Assemble the gel electrophoresis apparatus. Load equal amounts of protein of each sample into the SDS-PAGE gel wells. Set the voltage of electrophoresis to 80 V for concentration and 100 V for separation.
- Activate the polyvinylidene difluoride (PVDF) membrane (see Table of Materials) with methanol for 1 min, then soak the PVDF membrane in transfer buffer before proceeding with transfer at 100 V for 1 h.
- Immerse the PVDF membrane in TBST buffer (20 mM Tris, 150 mM NaCl, and 0.1% Tween 20) containing 5% bovine serum albumin (BSA; see Table of Materials) and then block for 30 min.
- Incubate the PVDF membrane with the primary antibody overnight at 4 °C on a shaker. The primary antibodies used are as follows: human anti-CD63 (1:1000 dilution), human anti-CD9 (1:1000 dilution), and human anti-HSP70 (1:1000 dilution) (see Table of Materials).
- Next day, wash the PVDF membrane with TBST solution five times for 5 min each and then incubate with HRP-conjugated secondary antibody (1:10000 dilution) (see Table of Materials) at room temperature (RT) for 1 h.
- After washing with TBST five times, use a chemiluminescence detection reagent for imaging on a chemiluminescence system.
- Transmission electron microscopy
- Add a 20 µL PBS-diluted sample drop to a wax paper. Then, place a copper grid (see Table of Materials) onto the drop so the sample drop can cover the copper grid and let it stand at RT for 20 min.
- Absorb excess liquid with filter paper. Then, fix the samples in a 20 µL solution of 2% paraformaldehyde, 2% glutaraldehyde, and 0.05 M phosphate solution for 2 min.
- Rinse the copper grid three times with double-distilled water, then stain with 20 µL of 2% phosphotungstic acid (PTA) for 1 min at RT.
- Absorb redundant liquid with filter paper. Dry the grids overnight before being analyzed by transmission electron microscopy (see Table of Materials).
NOTE: The recommended settings for TEM are as follows: Exposure: 1.0 s, HT Voltage 100.00 kV, Beam Current: 50 µA, Spot Size: 1, Mode: TEM. These settings might change following the manufacturer's instructions (see Table of Materials).
Results
Morphological characterization of sEVs
In the final stage of concentration, the wasted fluid was also collected as described. The concentrated medium and wasted fluid were ultracentrifuged, respectively. We collected the precipitations for transmission electron microscopy (TEM) analysis. As anticipated, a significant number of cup-shaped nanovesicles were observed in the concentrated medium group (Figure 2A,B). However, typical sEV particles were not detected in the wasted fluid group. These findings provide evidence that the protocol is capable of separating sEVs without experiencing significant loss during the crucial concentration step.
Characterization of protein markers for sEVs
Furthermore, Western blot analysis was employed to characterize the protein markers (CD9, CD81, HSP70) of the isolated sEVs. Clear protein bands were observed in the concentrated medium group, while no corresponding bands were detected in wasted fluid samples (Figure 2C). Together, these results validate the presence of sEV components.
Size characteristics and yield of extracellular vesicles
To further characterize the quantity and size, NTA was applied to the samples obtained by traditional ultracentrifugation (traditional UC) and our protocol. Since both methods utilized ultracentrifugation, sEVs isolated from the MSC culture medium by traditional UC was used as a control, which mainly included centrifugation at 300 × g for 15 min, then 2,000 × g for 15 min and an ultracentrifuge at 110,000 × g for 70 min at 4 °C, and then pellets were washed in saline and second ultracentrifugation was performed for 70 min at 110, 000 × g. The result demonstrated that the size range of sEVs obtained by this protocol was from 48.1 nm to 166.2 nm with a mean size of 103.6 nm, the size range of sEVs obtained by traditional UC was from 51.8 nm to 186.7 nm with a mean size of 112.6 nm (Figure 3A,B). We further introduced the dilution ratio to NTA results to measure the total particles harvested by two protocols. The result also indicated that, although loss could not be avoided, we harvested a considerable amount of sEVs. Next, two isolation protocols' time-consuming pattern diagrams are presented here (Figure 3C). Then, we quantified the time consumption of the protocols. The traditional UC method required about 2030 min, and this protocol required 260 min (Figure 3D). The yield was higher using this protocol (Figure 3E), showing the efficiency of the protocol for large-volume samples.
Quality control sterility tests
The tests of sterility are critical for ensuring that the products are safe. Detections of bacteria, fungi, viruses, and endotoxin were applied to the sEVs we isolated. The results were sorted in here (Table 1). And the results indicated that the sEVs obtained by our protocol were well protected from the contaminants.

Figure 1: Schematic illustration of the protocol for MSC-sEVs concentration and isolation from MSC culture supernatant. Please click here to view a larger version of this figure.
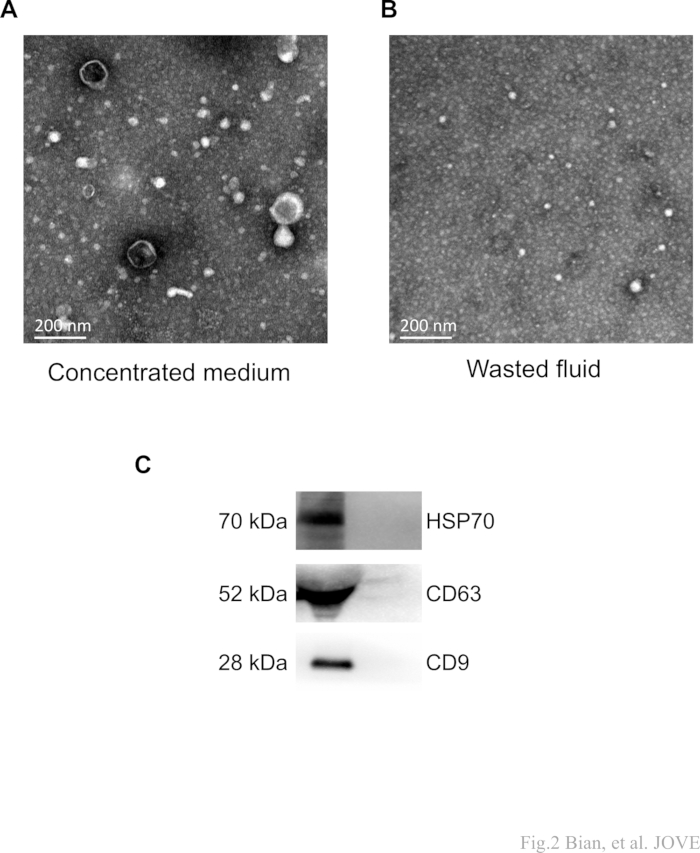
Figure 2: Representative TEM and western blot images of sEVs. (A,B) The protocol aims to obtain a concentrated medium (sample).After concentration and subsequent ultracentrifugation, the wasted fluid denotes the collected waste. The "cup-shaped" MSC-sEVs were detected in the target samples (concentrated medium) and were not observed in the fluid waste. Scale bars: 200 nm. (C) Clear protein bands of CD63, CD9, and HSP70 were observed in the concentrated medium, while no corresponding bands were detected in wasted fluid samples. Please click here to view a larger version of this figure.
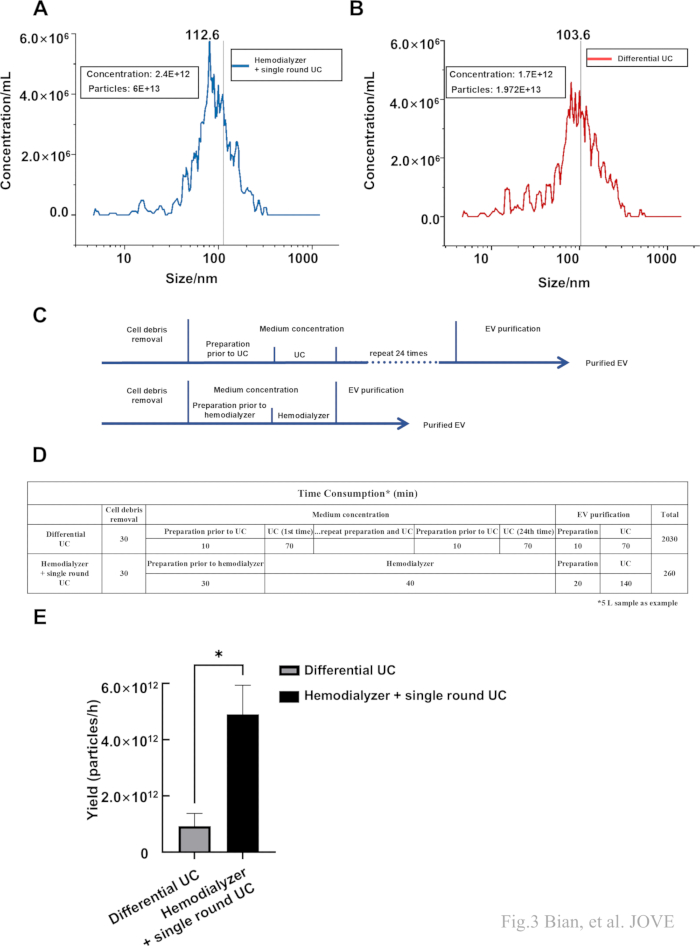
Figure 3: Nanoparticle size and particle number detected by NTA. (A,B) The size and concentration of sEVs isolated by traditional UC and this protocol. (C) The flow chart of the traditional UC (top) and Hemodialyzer + single round UC (bottom). (D) The time consumption of the traditional UC and Hemodialyzer + single round UC. (E) The yield (obtained particles per hour) of two different protocols. Data were represented as mean ± standard deviation (*p < 0.05). Please click here to view a larger version of this figure.
| Test | Result | Unit | Reference | |
| Bacteria | Negative | No bacterial growth observed after 5 days of normal culture | ||
| Fungi | Negative | No fungal growth observed after 5 days of normal culture | ||
| Endotoxin | <0.0050 | EU/mL | ||
| Virus | Hepatitis B virus (HBV) | Below the lowest limit | IU/mL | HBV-DNA quantification,Minimum detection limit 20 |
| Hepatitis C virus (HCV) | Negative | IU/mL | HCV-RNA quantification | |
| Epstein-Barr virus (EBV) | Negative | copies/mL | PCR Fluorescence detection | |
| Cytomegalovirus (CMV) | Negative | copies/mL | PCR Fluorescence detection |
Table 1: The quality control sterility tests.
Discussion
Traditional methods for the isolation of sEVs include differential ultracentrifugation, size exclusion chromatography, and PEG precipitation, each with its own merits and demerits. While amalgamation of these disparate techniques may enhance the yield or purity of sEVs, additional steps often introduce more opportunities for sample contamination. There are integrated systems claiming to extract sEVs in bulk and adhere to GMP standards have emerged on the market21. However, their widespread adoption poses a significant economic challenge, particularly for the growing number of EV research teams. Furthermore, the absence of standardized protocols and recognized procedures for these new devices may introduce a certain level of risk. GMP requires more attempts on both methodology and equipment to ensure simultaneous compliance with yield and purity.
According to a recent review of the clinical applications of sEVs23, 35% percent of the studies exclusively employed this method; an additional 12.5% of the studies utilized ultracentrifugation in combination with other techniques. Its widespread application also implies that ultracentrifuges have become equipment accessible to a considerable portion of researchers24.
In practical terms, the employment of ultracentrifugation helps our method to accurately isolate precise cellular derivatives from other elements such as larger cells, cell fragments, and dead cells25. Furthermore, it should be highlighted that although sEVs have been reported to be effective, the realization of satisfactory therapeutic efficacy typically requires the use of high concentrations of sEVs, particularly in animal experiments, preclinical large animal studies, and clinical trials. Ultracentrifugation is currently the most crucial method for obtaining high-concentration sEVs, which enhances the protocol's relevance to the aforementioned application scenarios. And as a mature method, ultracentrifugation possesses the advantages of being applicable to broad-ranging samples and being cost-effective.
The hemodialyzer has been innovatively utilized for the processing of large volumes of cell culture supernatant samples, enabling the subsequent isolation of sEVs through single-round ultracentrifugation. As described in step 2.5.2, the mode used in ultrafiltration is ISO-UF. The hemodialyzer in this mode is the same as the TFF. Since no exogenous dialysate is used, only small molecules pass out of the filter membrane, such as H2O. TFF is already a widely proven and recommended sEVs enrichment protocol. However, GMP-compliant TFF equipment for sEV isolation is expensive and often lacks operational standards. This kind of equipment is less verified, so the quality of the product is hard to guarantee. In contrast, hemodialyzer is complemented by established consumables, standardized operating protocols, and trained personnel. This method offers a viable solution for concentrating samples, especially for hospital-based research teams engaged in clinical trials. Moreover, as an extension, due to their close physical properties, we believe that this protocol is theoretically suitable for the isolation of sEVs from all MSCs of various lineages, but for certain, further experimental verification is needed.
This protocol provides a more easily met requirement for sterility, which is a crucial requirement in GMP environments. We have conducted comprehensive testing when exploring the protocol, including bacteria, fungi, viruses, and endotoxin in the samples, and the results are all negative. (Table 1) The test here is not the key to the protocol operation we reported, so we choose not to include the results. Sterility is a requirement throughout a protocol, including the sample, operation, equipment, environment, etc. The testing for sterility is essential, especially for products intended for therapeutic use. Besides, other quality control (QC) for GMP compliance should be considered from aspects such as identity and potency. Here, we identified the physical characteristics and protein markers of sEV. The potency of isolated sEVs should also be verified if researchers intend to use the product for further application.
It should also be noted that, first, this protocol incorporates a dual filtration step with 0.45 µm and 0.22 µm filters to remove cellular debris and vesicles larger than 220 nm26. Although alternative methods, such as lower-speed centrifugation, could theoretically precede ultracentrifugation27, this could result in diminished efficiency during large-scale operations. If the cell supernatant samples contain an excessive amount of particulate matter due to operations prior to filtration, the filter membrane used here has a greater probability of reducing the filtration speed due to blockage in the process. Thus, it is also recommended always to have spare sterile filter membranes prepared for replacement in the event of clogging. Moreover, the operation of hemodialysis is not considered complex, especially for trained professionals. However, it is important to ensure that the cell culture medium does not contain phenol red. This precaution is taken not only because evidence suggests that the indicator may potentially affect the biological functions of sEV but also due to its ability to trigger the blood leak alarm of the hemodialysis.
This protocol is unsuitable for tasks with a small volume of samples to be processed. When the isolation can be accomplished by 2 rounds of ultracentrifugation, this protocol does not offer a time advantage. However, as the volume increases, this protocol can become an efficient isolation method. The concentration step of this protocol utilizes solely ultrafiltration without the use of dialysis buffer. It is conceivable that by employing different dialysis buffers, further decontamination of co-isolated substances from sEVs might be achieved. It is not excluded that dialysis buffer may affect the stability of the sEV's outer membrane; thus, more research is needed to verify this.
In summary, we have presented a method for the large-scale isolation of MSC-sEVs that meets GMP standards. It primarily relies on hemodialyzers and ultracentrifuges, which are more accessible to many research teams than some of the newer specialized equipment available on the market. We have characterized the obtained sEVs and, in addition, conducted an examination of the filtration waste to identify critical processes that may lead to the loss of sEVs. The results indicate no significant loss, suggesting the efficiency and stability of the protocol. By fully utilizing existing laboratory equipment to address new demands, this approach also provides a novel research perspective.
Disclosures
The authors declare no competing financial interests.
Acknowledgements
This work was supported by funding from the National Science Foundation of China (822101167, to BB) and the Natural Science Foundation of Chongqing (CSTB2022NSCQ-MSX0020 to BB), Chongqing PhD "Through Train" Scientific Research Project of China (CSTB2022BSXM-JCX0031 to BB) and National Science Foundation of China (82271132 to YL). We are grateful for the assistance of the Department of Nephrology, the First affiliated hospital, Third Military Medical University (Army Medical University), and Institute of Pathology and Southwest Cancer Center, Southwest Hospital, Third Military Medical University (Army Medical University) for the equipment and technical support.
Materials
| Name | Company | Catalog Number | Comments |
| Anti-CD63 | SBI System Biosciences | EXOAB-CD63A-1 | 1:1000 dilution |
| Anti-CD9 | SBI System Biosciences | EXOAB-CD9A-1 | 1:1000 dilution |
| Anti-HSP70 | SBI System Biosciences | EXOAB-Hsp70A-1 | 1:1000 dilution |
| Bicinchoninic Acid Protein Assay Kit | Beyotime | P0012 | |
| Bloodlines | Fresenius Medical Care | AP16641 | |
| Bovine serum albumin 5% | Solarbio | 9048-46-8 | |
| Cell culture supplement | Helios | HPCPLCGL05 | 5% (v/v) in cell culture media |
| Copper grid | Precise | RGRS GP-SMPG-1 | |
| Dialyzer | Helixone | FX8 | 100 kDa MWCO |
| Drainage bag | CZRUIDE | YLD-01 | |
| Goat Anti-Rabbit HRP | SBI System Biosciences | EXOAB-CD63A-1 | 1:10000 dilution |
| Goat Anti-Rabbit HRP | SBI System Biosciences | EXOAB-CD9A-1 | 1:10000 dilution |
| Goat Anti-Rabbit HRP | SBI System Biosciences | EXOAB-Hsp70A-1 | 1:10000 dilution |
| Mesenchymal Stem Cell Basal Medium (MSCBM) | Dakewe | DKW34-BM20500 | |
| Microfiltration membrane | shanghaixingya | WKLM-50-10 | 0.45 μm and 0.22 μm |
| Parafilm | Fisher Scientific | 1337416 | |
| Peristaltic pump | LongerPump | YZ1515x | |
| Phosphate buffer saline | Solarbio | P1022-500ml | |
| Immun-Blot PVDF Membrane | BIO-RAD | 1620177 | |
| SDS-PAGE Gel Quick Preparation Kit | Beyotime | P0012AC | |
| SDS-PAGE Sample Loading Buffer | Beyotime | P0015A | |
| Super ECL Plus Western Blotting Substrate | BIOGROUND | BG0001 | |
| TBST buffer | Solarbio | T1081 | |
| Ultracentrifuge tubes 38.5 mL | Beckman | 344058 | |
| Bio-Rad ChemiDoc MP Imaging System | BIO-RAD | ||
| Hemodialyzer | NIKKISO | DBB-27 | |
| Nanoparticle Tracking Analysis | ZetaView | PMX120 | To measure particle size distribution and particle concentration |
| Transmission Electron Microscopy | JEOL | JEM-1400PLUS | Recommended settings?Exposure: 1.0 s, HT Voltafe 100.00 kV, Beam Curr: 50 μA, Spot Size: 1, Mode: TEM. |
| Ultracentrifuge | BECKMAN COULTER | OPTIMA XPN-100 | SW 28Ti SwingingBucket Rotor |
References
- Tan, F., et al. Clinical applications of stem cell-derived exosomes. Signal Transduct Target Ther. 9 (1), 17 (2024).
- Xia, Y., Zhang, J., Liu, G., Wolfram, J. Immunogenicity of extracellular vesicles. Adv Mater. 36 (33), e2403199 (2024).
- Andaloussi, S. E. L., Mäger, I., Breakefield, X. O., Wood, M. J. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 12 (5), 347-357 (2013).
- Harrell, C. R., Jovicic, N., Djonov, V., Arsenijevic, N., Volarevic, V. Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells. 8 (12), 1605 (2019).
- Lin, Z., et al. Mesenchymal stem cell-derived exosomes in cancer therapy resistance: Recent advances and therapeutic potential. Mol Cancer. 21 (1), 179 (2022).
- Matsuzaka, Y., Yashiro, R. Therapeutic strategy of mesenchymal-stem-cell-derived extracellular vesicles as regenerative medicine. Int J Mol Sci. 23 (12), 6480 (2022).
- Syromiatnikova, V., Prokopeva, A., Gomzikova, M. Methods of the large-scale production of extracellular vesicles. Int J Mol Sci. 23 (18), 10522 (2022).
- Chen, J., et al. Review on strategies and technologies for exosome isolation and purification. Front Bioeng Biotechnol. 9, 811971 (2021).
- Stam, J., Bartel, S., Bischoff, R., Wolters, J. C. Isolation of extracellular vesicles with combined enrichment methods. J Chromatogr B Analyt Technol Biomed Life Sci. 1169, 122604 (2021).
- Bajo-Santos, C., et al. Extracellular vesicles isolation from large volume samples using a polydimethylsiloxane-free microfluidic device. Int J Mol Sci. 24 (9), 7871 (2023).
- Shin, S., et al. Separation of extracellular nanovesicles and apoptotic bodies from cancer cell culture broth using tunable microfluidic systems. Sci Rep. 7 (1), 9907 (2017).
- Zeng, L., et al. Extraction of small extracellular vesicles by label-free and biocompatible on-chip magnetic separation. Lab Chip. 22 (13), 2476-2488 (2022).
- Dong, J., et al. Coupling nanostructured microchips with covalent chemistry enables purification of sarcoma-derived extracellular vesicles for downstream functional studies. Adv Funct Mater. 30 (49), 2003237 (2020).
- Meng, Y., et al. Direct isolation of small extracellular vesicles from human blood using viscoelastic microfluidics. Sci Adv. 9 (40), eadi5296 (2023).
- Martins, T. S., Vaz, M., Henriques, A. G. A review on comparative studies addressing exosome isolation methods from body fluids. Anal Bioanal Chem. 415 (7), 1239-1263 (2023).
- Börger, V., Staubach, S., Dittrich, R., Stambouli, O., Giebel, B. Scaled isolation of mesenchymal stem/stromal cell-derived extracellular vesicles. Curr Protoc Stem Cell Biol. 55 (1), e128 (2020).
- Mohajerani, F., Clark, W. R., Ronco, C., Narsimhan, V. Mass transport in high-flux hemodialysis: Application of engineering principles to clinical prescription. Clin J Am Soc Nephrol. 17 (5), 749-756 (2022).
- Zawada, A. M., et al. Impact of hydrophilic modification of synthetic dialysis membranes on hemocompatibility and performance. Membranes (Basel). 12 (10), 932 (2022).
- Ghannoum, M., Roberts, D. M. Management of poisonings and intoxications. Clin J Am Soc Nephrol. 18 (9), 1210-1221 (2023).
- Khan, M. S., et al. Managing heart failure in patients on dialysis: State-of-the-art review. J Card Fail. 29 (1), 87-107 (2023).
- Chen, Y., et al. Exosome detection via the ultrafast-isolation system: Exodus. Nat Methods. 18 (2), 212-218 (2021).
- Welsh, J. A., et al. Minimal information for studies of extracellular vesicles (misev2023): From basic to advanced approaches. J Extracell Vesicles. 13 (2), e12404 (2024).
- Koch, L. F., et al. Novel insights into the isolation of extracellular vesicles by anion exchange chromatography. Front Bioeng Biotechnol. 11, 1298892 (2023).
- Akbar, A., Malekian, F., Baghban, N., Kodam, S. P., Ullah, M. Methodologies to isolate and purify clinical grade extracellular vesicles for medical applications. Cells. 11 (2), 186 (2022).
- Li, P., Kaslan, M., Lee, S. H., Yao, J., Gao, Z. Progress in exosome isolation techniques. Theranostics. 7 (3), 789-804 (2017).
- Théry, C., Amigorena, S., Raposo, G., Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. Chapter 3 (Unit 3.22), (2006).
- Colombo, M., Raposo, G., Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 30, 255-289 (2014).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved