Visualization of Knee Joint Degeneration after Non-invasive ACL Injury in Rats
Overview
Source: Lindsey K. Lepley1,2, Steven M. Davi1, Timothy A. Butterfield3,4 and Sina Shahbazmohamadi5,
1Department of Kinesiology, University of Connecticut, Storrs, CT; 2Department of Orthopaedic Surgery, University of Connecticut Health Center, Farmington, CT; 3Department of Rehabilitation Sciences, University of Kentucky, Lexington, KY; 4Center for Muscle Biology, Department of Physiology, University of Kentucky, Lexington, KY; 5Biomedical Engineering Department, University of Connecticut, Storrs, CT
Anterior cruciate ligament (ACL) injury to the knee dramatically increases the risk of post-traumatic osteoarthritis (PTOA), as approximately one-third of individuals will demonstrate radiographic PTOA within the first decade following ACL injury. Though ACL reconstruction (ACLR) successfully restores knee joint stability, ACLR and current rehabilitation techniques do not prevent the onset of PTOA. Therefore, ACL injury represents the ideal model to study the development of PTOA after traumatic joint injury.
Rat models have been used extensively to study the onset and effect of ACL injury on PTOA. The most widely used model of ACL injury is ACL transection, which is an acute model that surgically destabilizes the joint. Though practical, this model does not faithfully mimic human ACL injury due to the invasive and non-physiological injury procedures that mask the native biological response to injury. To improve the clinical translation of our results, we have recently developed a novel non-invasive model of ACL injury where the ACL is ruptured through a single load of tibial compression. This injury closely replicates injury conditions relevant to humans and is highly reproducible.
Visualization of joint degeneration through micro-computed tomography (µCT) provides several major advancements over traditional OA staining techniques, including rapid, high-resolution, non-destructive 3D imaging of whole joint degeneration. The goal of this demonstration is to introduce the state of the art non-invasive ACL injury in a rodent model and use µCT to quantify knee joint degeneration.
Principles
The ACL is a band-like structure of dense connective tissue that arises from the anterior intercondylar space of the tibia and extends superiorly and laterally to the posterior aspect of the lateral condyle of the femur. Structurally, the ACL serves as both a passive stabilizer of the knee, working in concert with other ligaments as well as thigh musculature to help control the joint during dynamic movement. The ACL is the primary restraint to anterior tibial displacement and plays an essential role in maintaining knee joint stability. Beyond structural support, the ACL also acts as a pathway for neural information between the knee joint and central nervous system. The greatest stress on the ACL occurs when the knee is near extension, and it is during this time that the ACL is at the highest risk of injury.
The ACL is the most commonly injured knee ligament during sport and work-related activities. Non-contact ACL injuries account for nearly 70% of all ACL injuries, and they occur when a person generates sufficient forces and/or moments at the knee that leads to excessive loading of the ACL. Although the mechanism of non-contact ACL injury has been investigated using a variety of research models (prospective, retrospective, observational, in vivo and in vitro), a direct determination of how injury occurs remains elusive. ACL reconstruction is often performed by surgically inserting a portion of the individuals hamstring or patellar tendon into the area of the ACL. The aim of surgical reconstruction is to maximize knee stability and functional capacity that were lost following the injury. Surgical reconstruction facilitates a safe return to sport and promotes long-term knee joint health. However, despite the best efforts of clinicians and researchers, nearly two-thirds of patients with a reconstructed ACLs reconstructed patients do not return back to activity at 12 months post-reconstruction and more than 50% of ACL reconstructed knees have radiographic signs of PTOA 5-14 years after injury.
Animal models provide both a practical and clinically relevant way to study the natural history and response of treatment to joint health. Importantly the knee of a rat has similar anatomy and function to knees in humans, which makes the rat knee a useful model to study PTOA after ACL injury. To improve the clinical translation of our results, we have recently developed a novel non-invasive model of ACL injury, where the ACL is ruptured through a single load of tibial compression. This injury closely replicates injury conditions relevant to humans and is highly reproducible.
The load device consists of two custom-built loading platforms (Figure 1); the top knee stage is rigidly mounted to a linear actuator (DC linear actuator L16-63-12-P, Phidgets, Alberta, CA) that positions the right hindlimb in 30°1-3 of dorsiflexion and 100°1 of knee flexion while providing room for anterior subluxation of the tibia relative to the femur; the bottom stage holds the flexed knee and is mounted directly above a load cell (HDM Inc., PW6D, Southfield, MI). During the injury, rats are anesthetized and then the right hindlimb is subjected to single load of tibial compression at a speed of 8 mm/s.1 ACL injury is noted by a release of compressive force during injury that is monitored via a custom program (LabVIEW, National Instruments, Austin, TX). Post-injury, ACL rupture is clinically confirmed by the Lachman's test, where the femur is secured while an anterior force is applied to the tibia. Excessive anterior tibial translation indicates ACL deficiency. The ACL injured hindlimb can then be extended and secured in a custom 3D printed device to visualize knee joint degeneration. Images are acquired to characterize changes in trabecular structure related to PTOA development.4
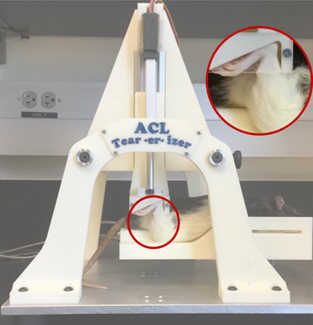
Figure 1: Tibial compressive load causing isolated non-invasive ACL injury.
Procedure
Non-invasive ACL Injury
- Wear proper personal protection equipment. You may use a respiratory mask, but it is not mandatory for this protocol.
- Anesthetize the rats using an induction chamber with 5% isoflurane and 1 L/min oxygen. Maintain the flow of anesthesia using via a nose cone with 1 - 3% isoflurane and 500 mL/min of oxygen. If the apparatus is not set up on a backdraft or downdraft table, ensure that waste gas is scavenged using a tabletop system and charcoal filters.
- Perform a toe-pinch to ensure an adequate depth of anesthetic has been reached. Note that it is not necessary to apply eye lubricant the protocol is performed quickly (< 3 min) and there is minimal risk of cornea dryness.
- Position the right hindlimb at 30° of dorsiflexion and 100° of knee flexion while providing room for anterior subluxation of the tibia relative to the femur.
- Rigidly mount the top knee stage to a linear actuator.
- Position the flexed knee on the bottom stage, which is mounted directly above a load cell.
- Induce ACL injury using a single load of tibial compression at a speed of 8 mm/s.
- ACL injury is noted by a release of compressive force. This is monitored via a custom program.
- Post-injury, while the animal is still under the plane of anesthesia, perform a Lachman's test to clinically confirm an ACL rupture has occurred. A Lachman's test is a clinical test used to evaluate the integrity of the ACL by assessing sagittal plan stability. While stabilizing the femur, pull the tibia forward (in an anterior direction) to assess the amount of motion. An intact ACL will produce a 'firm end-feel' where the researcher will not be able to translate the tibia forward. An injured ACL will produce a 'soft or mushy end-feel,' indicative of a torn ACL.
- Palpate the femur and tibia to detect any gross bone damage. If no contraindications are identified, transfer the animal to its cage and allow it to recover. During this time, monitor the animal to ensure it doesn't display any signs of pain, such as a reluctance to move, vocalization, or abnormal posturing.
µCT imaging of joint degeneration
2-D images are obtained using scanner settings of 70 kV, current 85.5 µA (Figure 2B). Data is collected every 0.6° rotation step at a resolution of 11.5 µm through a complete 180°. Cross-sectional images are reconstructed using a smoothed back-projection algorithm and on the stack of reconstructed images (Figure 2C). Trabecular structure is then analyzed by segmentation in software, whereby a 1.53 mm sphere is centered in the epiphyseal plate of the medial and lateral tibial plateaus and femur to determine trabecular thickness (µm), trabecular separation (µm) and trabecular number (1/mm).5,6
- At 4 weeks post-ACL injury, euthanize the rat with prolonged exposure to CO2 in the induction chamber.
- Extend and secure ACL injured hindlimb in a custom 3D-printed device (Figure 2A).
- Acquire images using µCT.
- Obtain frontal plane radiographs to determine joint space. narrowing (between the femoral condyle and tibial plateau [measured in mm]) compared to the non-injured limb.
- Obtain 2-D images using the following scanner settings: 70 kV and current 85.5 µA.
- Collect the data every 0.6° rotation step at a pixel size of 11.5 µm through a complete 180°.
- Reconstruct cross-sectional images using a smoothed back-projection algorithm on the stack of reconstructed images.
- To ensure a consistent region of interest is measured, place a 1.53 mm sphere in the epiphyseal plate of the medial and lateral tibial plateaus and femur to determine trabecular thickness (µm), trabecular separation (µm), and trabecular number (1/mm).
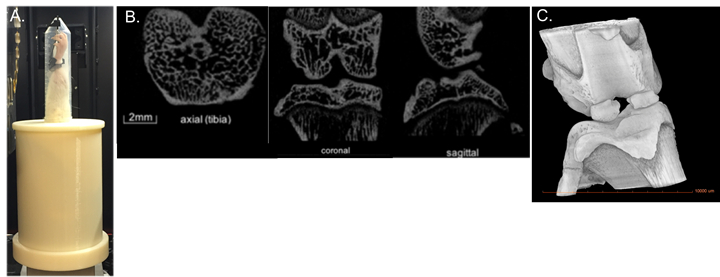
Figure 2: A) Custom printed device to hold hindlimb during μCT, B) 2-D images, and C) 3-D μCT.
Results
Smaller trabecular number, reduced trabecular thickness and greater trabecular spacing, all hallmark characteristics of PTOA onset, were evident 4 weeks after the non-invasive ACL tear (Table 1 and Figure 3). An image of a dissected ACL of healthy limb versus an acute injured limb is shown in Figure 5. The novel non-invasive model of ACL injury, where the ACL is ruptured through a single load of tibial compression, was able to produce an isolated proximal tear of the ACL.

Figure 3: 3-D reconstructed μCT image of an acute ACL-injury (left) and 4 weeks post-ACL injury (right) in a rat.
Table 1: Characteristic measurements of PTOA onset.
| Animal | Tb.N (1/mm) |
Tb.Th (µm) |
TB.Sp (µm) |
| Acute ACL injured | 3.11 | 168.5 | 217 |
| 4 wks post-ACL injury | 2.63 | 166.7 | 213 |
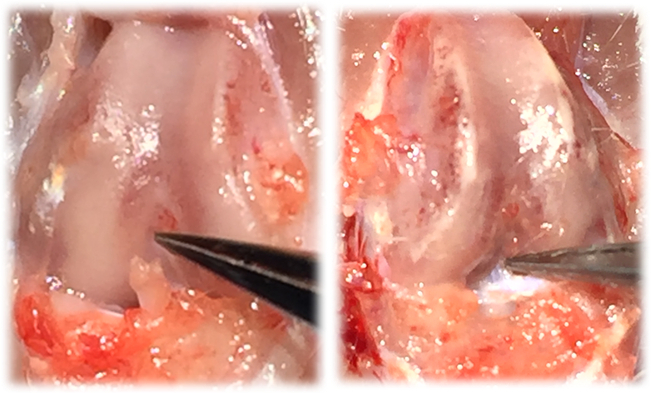
Figure 4: Image of an acute injured ACL-limb (left) and image of intact, healthy ACL (right).
Application and Summary
This video demonstrates how a linear actuator can be used to produce an isolated non-invasive ACL rupture in rats. This injury closely replicates injury conditions relevant to humans and is highly reproducible. To overcome several of the major limitations of traditional OA staining techniques, this method uses µCT to quantify whole joint degeneration and trabecular structure.
Evidence-based interventions to improve musculoskeletal rehabilitative outcomes is a highly significant area that has changed little in the past two decades, even though significant advances in basic biology have suggested that alterations to rehabilitation protocols are long overdue. The issue is that classically rehabilitation specialists have used anecdotal reports to shape clinical practice rather than basic science to provide informed hypotheses that are tested in model organisms before translation to the clinic. The procedures described here provide scientists with a method to closely replicate a traumatic joint injury that is relevant to humans and use µCT to track the progression of joint health.
Materials List:
| Equipment | Company | Catalog Number | Comments |
| Linear actuator | Phidgets | L16-63-12-P | |
| Load Cell | HDM Inc. | PW6D | |
| μCT | Zeiss | XRM Xradia 520 |
References
- Maerz T, Kurdziel MD, Davidson AA, Baker KC, Anderson K, Matthew HW. Biomechanical Characterization of a Model of Noninvasive, Traumatic Anterior Cruciate Ligament Injury in the Rat. Ann Biomed Eng. 2015;43(10):2467-2476.
- Christiansen BA, Anderson MJ, Lee CA, Williams JC, Yik JH, Haudenschild DR. Musculoskeletal changes following non-invasive knee injury using a novel mouse model of post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2012;20(7):773-782.
- Lockwood KA, Chu BT, Anderson MJ, Haudenschild DR, Christiansen BA. Comparison of loading rate-dependent injury modes in a murine model of post-traumatic osteoarthritis. J Orthop Res. 2014;32(1):79-88.
- Blair-Levy JM, Watts CE, Fiorentino NM, Dimitriadis EK, Marini JC, Lipsky PE. A type I collagen defect leads to rapidly progressive osteoarthritis in a mouse model. Arthritis Rheum. 2008;58(4):1096-1106.
- Mohan G, Perilli E, Kuliwaba JS, Humphries JM, Parkinson IH, Fazzalari NL. Application of in vivo micro-computed tomography in the temporal characterisation of subchondral bone architecture in a rat model of low-dose monosodium iodoacetate-induced osteoarthritis. Arthritis Res Ther. 2011;13(6):R210.
- Jones MD, Tran CW, Li G, Maksymowych WP, Zernicke RF, Doschak MR. In vivo microfocal computed tomography and micro-magnetic resonance imaging evaluation of antiresorptive and antiinflammatory drugs as preventive treatments of osteoarthritis in the rat. Arthritis Rheum. 2010;62(9):2726-2735.
Skip to...
Videos from this collection:

Now Playing
Visualization of Knee Joint Degeneration after Non-invasive ACL Injury in Rats
Biomedical Engineering
8.2K Views

Imaging Biological Samples with Optical and Confocal Microscopy
Biomedical Engineering
35.7K Views

SEM Imaging of Biological Samples
Biomedical Engineering
23.5K Views

Biodistribution of Nano-drug Carriers: Applications of SEM
Biomedical Engineering
9.3K Views

High-frequency Ultrasound Imaging of the Abdominal Aorta
Biomedical Engineering
14.4K Views

Quantitative Strain Mapping of an Abdominal Aortic Aneurysm
Biomedical Engineering
4.6K Views

Photoacoustic Tomography to Image Blood and Lipids in the Infrarenal Aorta
Biomedical Engineering
5.7K Views

Cardiac Magnetic Resonance Imaging
Biomedical Engineering
14.7K Views

Computational Fluid Dynamics Simulations of Blood Flow in a Cerebral Aneurysm
Biomedical Engineering
11.7K Views

Near-infrared Fluorescence Imaging of Abdominal Aortic Aneurysms
Biomedical Engineering
8.2K Views

Noninvasive Blood Pressure Measurement Techniques
Biomedical Engineering
11.9K Views

Acquisition and Analysis of an ECG (electrocardiography) Signal
Biomedical Engineering
104.8K Views

Tensile Strength of Resorbable Biomaterials
Biomedical Engineering
7.5K Views

Micro-CT Imaging of a Mouse Spinal Cord
Biomedical Engineering
8.0K Views

Combined SPECT and CT Imaging to Visualize Cardiac Functionality
Biomedical Engineering
11.0K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved