Water Quality Analysis via Indicator Organisms
Overview
Source: Laboratories of Dr. Ian Pepper and Dr. Charles Gerba -The University of Arizona
Demonstrating Author: Luisa Ikner
Water quality analysis monitors anthropogenic influences such as pollutants, nutrients, pathogens, and any other constituent that can impact the water’s integrity as a resource. Fecal contamination contributes microbial pathogens that threaten plant, animal, and human health with disease or illness. Increasing water demands and strict quality standards require that water being supplied for human or environmental resources be monitored for low pathogen levels. However, monitoring each pathogen associated with fecal pollution is not feasible, as laboratory techniques involve extensive labor, time, and costs. Therefore, detection for indicator organisms provides a simple, rapid, and cost effective technique to monitor pathogens associated with unsanitary conditions.
Principles
Indicators are easily detectable organisms whose presence correlates directly to one or more pathogens contaminating an environment. In order to be considered an appropriate indicator, an organism must meet the five following criterion:
- The indicator organism must be present when the pathogen is present, and the indicator organism must be absent when the pathogen is absent.
- The indicator organism’s concentration must correlate with the pathogen’s concentration. However, the indicator organism should always be found at higher numbers.
- The indicator organism should be able to survive easier and longer in the environment than the pathogen.
- Detection for the indicator organism should be easy, safe, and inexpensive.
- The indicator organism should be effective for all water types.
Most indicators are enteric organisms or viruses, which are commonly found in warm blooded mammalian and avian gastrointestinal systems, giving a direct connection to fecal contamination. However, many indicators can lack effectiveness due to a poor correlation with certain pathogens. Two of the most widely accepted bacterial indicator organisms are Escherichia coli and coliforms due to their fecal linkages, and ease in laboratory analysis.
Colilert is a defined substrate technology (DST) approach for simultaneous detection, specific identification, and confirmation for E. coli and total coliforms in water samples. This laboratory technique utilizes substrate nutrients specific to each indicator organisms’ metabolic pathway, enumerating only desired microorganisms, which release a signal when the bacteria alter the compound. In the presence of a coliform, the ortho-nitrophenyl-β-D-galactopyranoside (ONPG) nutrient is hydrolyzed by the coliform’s β-galactosidase enzyme. The product compound, ortho-nitrophenyl, is a chromogen that releases a color signal, turning the water yellow (Figure 1).
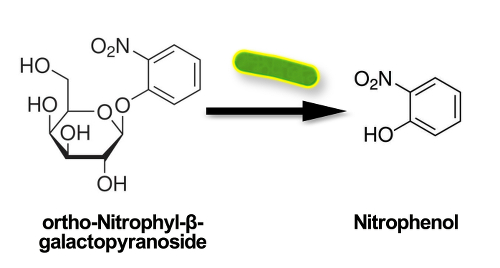
Figure 1. Schematic showing ortho-nitrophenyl releasing a color signal, turning the water yellow.
In the presence of E. coli, the methylumbelliferyl-β-D-glucuronide (MUG) nutrient is cleaved by the bacteria’s glucuronidase enzyme, producing a methylumbelliferone product that fluoresces blue-green under ultraviolet light (Figure 2).
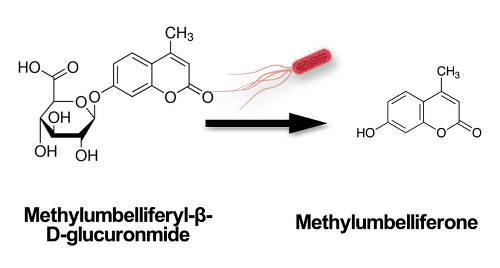
Figure 2. Schematic showing the methylumbelliferyl-β-D-glucuronide (MUG) nutrient cleaved by the bacteria’s glucuronidase enzyme, producing a methylumberlliferone product that fluoresces blue-green under ultraviolet light.
Colilert can be performed as a presence-absence (P-A) test to indicate whether or not the organisms exist in the sample. This test is completed by dissolving the substrate into 100 mL water samples, incubating at 35 ± 0.5 °C for 24 h, and observing the color signals. The indicators’ presence can also be quantified by utilizing a system which determines the most probable number (MPN) for each organism. This procedure involves dissolving the substrate into 100 mL water samples that are sealed into a tray containing 49 large wells and 48 small wells. The tray is incubated at 35 ± 0.5 °C for 24 h, and then the wells containing positive color changes are counted. The ratio of large to small wells containing positive signals is aligned to the MPN chart that provides the quantification for the presence of each indicator organism present. Regulations for drinking water in the United States require that zero coliforms be present in 100 mL of drinking water.
Procedure
1. Colilert Presence – Absence (P – A) Test
- Open the 100 mL plastic Colilert bottle. The bottle includes a small amount of powdered reagent that is necessary for the proper reactions, do not discard this powder.
- Add 100 mL water sample into Colilert bottle.
- Open the pillow tube containing the nutrient substrate and pour the contents into the water sample inside the Colilert bottle.
- Cap and seal the Colilert bottle. Shake the bottle vigorously, repeatedly inverting the bottle until the substrate is completely dissolved.
- Incubate the reagent/water sample mixture inside the bottle at 35 ± 0.5 °C for 24 h.
- Observe the yellow color change in the reagent/water sample mixture. The yellow color indicates coliform is present. Clear water or no change in color indicates that coliforms are absent.
- Expose the reagent/water sample to ultraviolet light and observe the blue fluorescence. Blue fluorescence indicates that E. coli is present. No fluorescence indicates that E. coli is absent (Figure 3).

Figure 3. P-A test negative (left), coliform positive (middle), and E. coli positive (right).
2. Colilert MPN: Quanti-tray 2000
- Open the Colilert bottle, and 100 mL water sample into Colilert bottle.
- Open the pillow tube containing nutrient substrate and pour contents into the water sample inside the Colilert bottle.
- Cap and seal the Colilert bottle. Shake the bottle vigorously, repeatedly inverting the bottle until the substrate is completely dissolved.
- Carefully open Quanti-tray 2000 by squeezing the edges at the top of the tray and pulling back the paper tab. Keep squeezing so that the tray is open.
- Pour the reagent/water sample mixture into the tray, and then incubate the sample inside the tray at 35 ± 0.5 °C for 24 h.
- Observe the yellow color change in the reagent/water sample mixture. Count the number of large and small wells that signal positive presence for coliforms. The yellow color indicates coliform is present. Clear water or no change in color indicates that coliforms are absent.
- Expose reagent/water sample to ultraviolet light and observe blue fluorescence. Count the number of large and small wells that signal positive presence for E. coli. The blue fluorescence indicates that E. coli is present. No fluorescence indicates that E. coli is absent.
- Use the Quanti-tray 2000 MPN sheet (Figure 4) to quantify the concentration for each indicator organism present in 100 mL of water. Use the spreadsheet to compare large: small positive well ratio to enumerate presence for both indicator organisms.

Figure 4. Quanti-tray negative (left), coliform positive (middle), and E. coli positive (right).
Application and Summary
Indicator organisms are employed to rapidly and inexpensively determine environmental contamination. Colilert assays are utilized to analyze water quality for drinking, recreational, and wastewater sources. Water quality must meet legal standards set by the Environmental Protection Agency (EPA) and state regulatory departments in order to be accepted as a resource for human and/or environmental consumption.
Colilert assays are also strategically used as mass balance markers within environmental research, and this data can be analyzed along with other environmental assays to measure the correlation between results. Performing a simple P-A Colilert test gives indication whether a sample is contaminated, which can be analyzed alongside research results. If the P-A sample shows that there is contamination in the water, then the water samples being utilized in research may also have contamination that leads to misinterpreted results, while the MPN Quanti-tray provides a baseline quantification for contamination present. For example, the indicator organisms can be used to correlate indicator quantifications with the number of pathogens found in a water sample. If the quanti-tray enumerates low indicator numbers, this suggests that the water sample should also experience similar trends with low pathogen levels.
Tags
Skip to...
Videos from this collection:

Now Playing
Water Quality Analysis via Indicator Organisms
Environmental Microbiology
29.5K Views

Determination of Moisture Content in Soil
Environmental Microbiology
359.5K Views

Aseptic Technique in Environmental Science
Environmental Microbiology
126.4K Views

Gram Staining of Bacteria from Environmental Sources
Environmental Microbiology
100.3K Views

Visualizing Soil Microorganisms via the Contact Slide Assay and Microscopy
Environmental Microbiology
42.3K Views

Filamentous Fungi
Environmental Microbiology
57.3K Views

Community DNA Extraction from Bacterial Colonies
Environmental Microbiology
28.8K Views

Detecting Environmental Microorganisms with the Polymerase Chain Reaction and Gel Electrophoresis
Environmental Microbiology
44.6K Views

RNA Analysis of Environmental Samples Using RT-PCR
Environmental Microbiology
40.4K Views

Quantifying Environmental Microorganisms and Viruses Using qPCR
Environmental Microbiology
47.8K Views

Isolation of Fecal Bacteria from Water Samples by Filtration
Environmental Microbiology
39.3K Views

Detection of Bacteriophages in Environmental Samples
Environmental Microbiology
40.7K Views

Culturing and Enumerating Bacteria from Soil Samples
Environmental Microbiology
184.5K Views

Bacterial Growth Curve Analysis and its Environmental Applications
Environmental Microbiology
296.0K Views

Algae Enumeration via Culturable Methodology
Environmental Microbiology
13.8K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved