Method Article
Three-Dimensional Printing Assisted Physician-Modified Fenestrated Endografts for Thoracic Endovascular Aortic Aneurysm Repair in Zone 1
* These authors contributed equally
In This Article
Summary
This protocol describes an approach for repairing aortic arch aneurysms in Zone 1 using physician-modified fenestrated endografts with the assistance of three-dimensional printing.
Abstract
Aortic arch aneurysm is a life-threatening cardiovascular disorder that requires timely medical intervention. Aneurysms in Zone 1 typically involve multiple branch arteries, making repair challenging. Open surgical repair often results in significant surgical trauma, massive blood loss, and prolonged operative time. With advancements in endovascular technology, fenestrated/branched thoracic endovascular aortic repair (F/B TEVAR) has been employed for aortic arch repair and branch artery reconstruction. Stent grafts for F/B TEVAR require personalized modification and fabrication based on patient anatomy. Physician-modified fenestrated endografts (PMEGs) offer a feasible approach for personalized aortic arch aneurysm repair in Zone 1. However, fabricating PMEGs demands a thorough understanding of anatomy and extensive experience, making it challenging for most surgeons. To simplify this process, three-dimensional printing is used to assist in precise fenestration. PMEGs guided by three-dimensional printing enhance branch artery patency and reduce post-operative endoleaks following F/B TEVAR. Further follow-up is necessary to assess the long-term benefits and efficacy of this technique.
Introduction
Aortic aneurysms are common life-threatening aortic diseases that require timely evaluation and therapeutic intervention1. Aortic arch aneurysms often involve major arterial branches, including the innominate artery, left common carotid artery, and left subclavian artery1. According to the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines, the aorta is divided into 11 landing zones2. Repairing aortic arch aneurysms in Zone 1 requires reconstruction of the aortic arch branch arteries, posing significant anatomical challenges.
The initial approach for aortic arch repair was open surgical repair. DeBakey et al. first successfully repaired an aortic arch aneurysm in 19573. However, several limitations restrict the application of open surgical repair4, including severe surgical trauma, significant blood loss, high complication rates, and prolonged operative duration5. With advancements in endovascular technology, thoracic endovascular aortic repair (TEVAR) has been proven effective for treating thoracic aortic aneurysms and dissections6,7,8. Building on conventional TEVAR, fenestrated/branched thoracic endovascular aortic repair (F/B TEVAR) was developed to address thoracic aortic aneurysms involving branch arteries9,10. Notably, F/B TEVAR has demonstrated high technical success and acceptable post-operative mortality in patients with post-dissection thoracoabdominal aneurysms11,12.
F/B TEVAR can restore normal physiological blood flow and achieve high patency rates in branch arteries following thoracic aortic aneurysm repair13,14. Precise fenestration on the main body stent graft is essential for reconstructing branch arteries. Aortic arch aneurysms in Zone 1 typically involve multiple branch arteries and require stent grafts with triple fenestrations. However, current commercial stents cannot be customized based on individual patient anatomy. Physician-modified fenestrated endografts (PMEGs) offer a viable alternative for personalized treatment of thoracic aortic aneurysms in Zone 115,16.
Successful fabrication of PMEGs requires extensive practice and experience, which can be challenging for many surgeons. To simplify the preparation process, this article presents a method for fabricating PMEGs for F/B TEVAR. Three-dimensional (3D) printing was used to achieve precise fenestration on the main body stent graft, followed by the attachment of branch stents in the appropriate orientation. This study reports a case series of 21 patients who underwent F/B TEVAR using PMEGs, providing novel insights into the efficacy and applicability of this technique.
Protocol
The surgical protocols described here were approved by the ethics committee of Nanjing Drum Tower Hospital, affiliated with Nanjing University Medical School. Written was obtained from the patients participating in this study. The details of the reagents and the equipment used are listed in the Table of Materials.
1. Pre-operative assessment
- Apply the following inclusion criteria: Patients aged over 18 years; confirmed diagnosis of aortic aneurysms in Zone 1 of the aorta using computed tomography angiography (CTA); no contraindications for F/B TEVAR; informed consent for the surgery.
- Apply the following exclusion criteria: Patients with other aortic diseases (such as aortic ulcer and dissection); patients with severe concurrent diseases (such as kidney or hepatic failure, uncontrolled diabetes, severe active infections); pregnant women; patients who have undergone aortic arch surgery.
- Perform pre-operative CTA and reconstruct the aorta to evaluate the position of the aortic aneurysm (Figure 1A,B).
- Conduct additional examinations, including routine physical examination, blood tests, and urine tests.
2. Preparation of the 3D-printed model
- Import the original CTA data (DICOM format) of the patient into the 3D reconstruction software.
- Click on SEGMENT and perform thresholding by defining the maximum and minimum threshold values.
- Click on Calculate Part to create a 3D preview of the aorta.
- Click on Edit Masks to optimize the 3D model.
- Export the 3D model as an STL format file.
- Import the STL format file into the simulation analysis software.
- Click on Edit-Select Outliers to delete most of the invalid points.
- Next, click on Edit-Discontinued Components. Recognize the remaining invalid points using the Separation and Size options, then delete them.
- Click on Points-Reduce Noise and select Free-form shapes to reduce noisy data.
- Click on Points-Wrap to generate a 3D model with a polygon wrap.
- Click on Polygons-Fill Holes-Fill Single to fill holes in the 3D model.
- Click on Decimate to simplify the model according to curvature.
- Click on Smooth-Relax/Sandpaper to refine the model surface.
- Click on Repair-Defeature to further optimize the model.
- Design fenestrations by clipping branch arteries on the 3D model.
- Export the final 3D model as an STL format file (Figure 1C).
- Import the STL format file into a 3D printer and print the 3D model of the aorta using biocompatible clear MED610 materials.
- Sterilize the model with ethylene oxide to prepare it for modification of the PMEG.
3. Intraoperative fabrication of PMEGs
- Place the delivery sheath with the main body stent graft into the sterile 3D-printed model before the surgical procedure.
- Release the main body stent graft inside the 3D-printed model.
- Mark the fenestrations of branch arteries using a marker pen (Figure 2A).
NOTE: Place markings between metal edges to prevent interference with stent expansion. - Create fenestrations at the marked locations using an electrocautery pen (Figure 2B).
- Suture metal coils as selection markers at the fenestrations.
- Constrain the main body stent graft using a guidewire, restricting the diameter to 50%-70% of the original.
NOTE: Introduce the guidewire through the distal delivery sheath of the stent graft after completing the stent modification. Symmetrically place 4-0 non-absorbable sutures on the posterior aspects of both sides of the fenestration and securely anchor them to the guidewire. - Reinstall the prepared PMEG into the delivery sheath of the main body stent.
- Inflect the PMEG before implantation to facilitate its delivery into the aorta (Figure 2C).
4. Surgical procedure
- Anesthetize the patient using anesthetic induction agents (propofol 20 mg/mL), analgesics (fentanyl 50 µg/mL), and muscle relaxants (vecuronium 10 mg/mL) administered via intravenous injection (following institutionally approved protocols).
- Position the patient in the supine position and locate the arteries serving as surgical access points using percutaneous landmarks.
NOTE: Use femoral arteries as access for the main body stent graft and the left common carotid and brachial arteries as access for the branch stents. - Expose the arteries and create access by inserting delivery sheaths.
NOTE: Use a long delivery sheath (18-20 F) for the main body stent graft and delivery sheaths (4-6 F) for branch stents. - Implant a 150 cm guidewire and a 4 F catheter. Perform digital subtraction angiography (DSA) to evaluate the aortic arch aneurysm.
- Administer systemic heparinization (heparin, 1 mg/kg).
- Replace the guidewire with a supportive guidewire to facilitate device placement and exchange.
- Deliver the PMEG to the aortic arch through the femoral artery approach and position it in the planned location (Figure 3A).
- Slowly release the anterior segment of the main body stent graft while keeping the stent in its constricted state (Figure 3B).
NOTE: The constricted state allows positional adjustments. Withdrawing the guidewire reliably releases the bilateral sutures, enabling the controlled expansion of the stent graft to align fenestrations with branch arteries. - Insert a catheter through each branch artery access. Advance catheters selectively into the respective fenestrations of the innominate artery, left common carotid artery, and left subclavian artery (Figure 3C).
- Pull out the constraining wire and fully release the main body stent graft.
- Implant and release branch stents in the innominate artery, left common carotid artery, and left subclavian artery.
- Dilate expansion balloons at the bridging sites between the main body stent and branch stents.
- Verify the patency of each branch artery and check for endoleaks using DSA (Figure 3D).
- Remove catheters, guidewires, and delivery sheaths. Suture the arteries using non-absorbable sutures (6-0 or 7-0).
5. Post-operative monitoring and care
- Transfer the patient to the post-anesthesia care unit (PACU) or intensive care unit (ICU).
- Monitor vital signs, including blood pressure, heart rhythm, respiratory function, and blood oxygen saturation.
- Assess for complications, such as stroke, spinal cord ischemia, and endoleaks.
- Initiate pain management and rehabilitation care.
Results
Twenty-one patients, aged 35 to 87 years, underwent F/B TEVAR for aortic arch aneurysm repair using PMEGs. Blood flow in all aortic arch branch arteries (innominate artery, left common carotid artery, and left subclavian artery) was restored through triple fenestrations in all cases (Figure 4). The average operative time was 234.3 min ± 70.4 min. Intraoperative blood loss was 150 mL (IQR = 300). The post-operative ICU stay averaged 1.2 ± 2.2 days, while the total hospital stay was 9.0 ± 4.9 days. The peri-operative mortality rate was 4.8%, and none of the patients required reintervention. The average follow-up duration was 17.4 ± 8.6 months (Table 1).
One patient died post-operatively from an unknown cause. Two patients experienced a stroke after surgery, resulting in a stroke incidence rate of 9.5%. Additionally, two patients developed post-operative paraplegia due to spinal cord ischemia. Endoleaks occurred in three patients (two type Ia and one type Ic) during the peri-operative period, with an overall endoleak incidence of 14.3%.

Figure 1: Pre-operative assessment and three-dimensional (3D) model construction. (A,B) Computed tomography angiography (CTA) scans of the aortic arch aneurysm in Zone 1. (C) Designed 3D model of the aortic aneurysm based on CTA. Please click here to view a larger version of this figure.

Figure 2: Preparation of the physician-modified endograft (PMEG). (A) Marking fenestrations on the main body stent graft. (B) Prepared PMEG with fenestrations. (C) Inflecting the PMEG before implantation. Please click here to view a larger version of this figure.
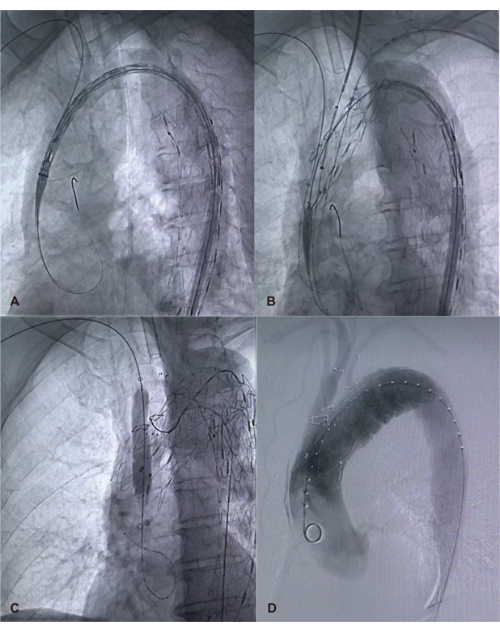
Figure 3: Deployment of the PMEG and repair of the aneurysm. (A) Gradual release of the PMEG. (B) Selective advancement of catheters into fenestrations. (C) Deployment of branch stents. (D) Verification of aortic and branch artery patency using digital subtraction angiography (DSA). Please click here to view a larger version of this figure.
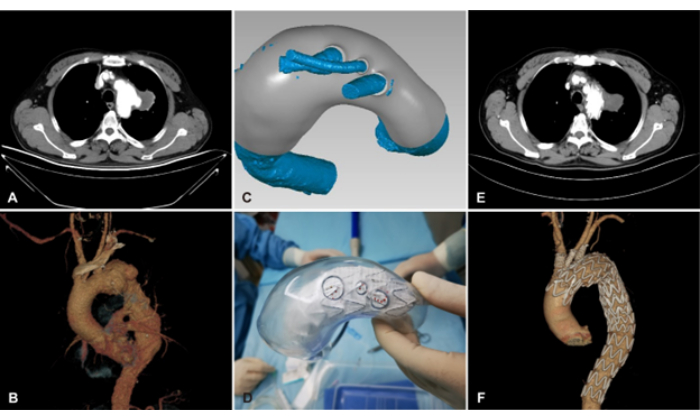
Figure 4: Fenestrated and branched endovascular aortic repair (F/B EVAR) assisted by 3D printing for aortic arch aneurysm repair in Zone 1. (A,B) Pre-operative CTA image and 3D reconstruction of the aortic arch aneurysm. (C) Designed 3D model of the aortic aneurysm based on CTA. (D) Release of the main body stent graft in the 3D-printed model. (E,F) Post-operative CTA image and 3D reconstruction of the aortic arch aneurysm. Please click here to view a larger version of this figure.
| Outcome measures | Patients with aortic arch aneurysms in Zone 1 (N = 21) |
| Average operation duration (min) | 234.3 ± 70.4 |
| Intraoperative blood loss (mL) | 150 (300) |
| Postoperative ICU stay (days) | 1.2 ± 2.2 |
| Postoperative hospital stays (days) | 9.0 ± 4.9 |
| Perioperative mortality rate (%) | 4.8 |
| Incidence of stroke (%) | 9.5 |
| Total incidence of endoleak (%) | 14.3 |
| Average follow-up duration (months) | 17.4 ± 8.6 |
Table 1: Outcome measures of fenestrated and branched endovascular aortic repair (F/B EVAR) using physician-modified endografts (PMEGs). Data are presented as mean ± standard deviation or median (interquartile range) for continuous variables.
Discussion
F/B TEVAR is a suitable approach for repairing aortic arch aneurysms in Zone 1, effectively maintaining branch artery patency. Compared with open surgical repair, F/B TEVAR is associated with lower peri-operative morbidity and mortality15,17. However, endoleaks are likely to occur at fenestration bridging sites post-operatively, potentially requiring reintervention18. Studies have shown that a greater number of fenestrations increases the likelihood of target vessel-related endoleaks19. Therefore, accurate fenestration is essential for triple-fenestration F/B TEVAR to minimize endoleaks.
Personalized fenestrated stent grafts enable precise fenestration for endovascular aortic arch repair. Most commercially available fenestrated stent grafts cannot be tailored to individual patient anatomy or provide precise fenestrations. In contrast, PMEGs have been widely used in F/B TEVAR and have demonstrated favorable outcomes15,20. Moreover, no significant difference in performance has been found between commercialized stents and PMEGs21, supporting the efficacy and safety of PMEGs. While double-fenestrated PMEGs have been used for aortic arch repair in recent studies22,23, reports on triple-fenestrated PMEGs for F/B TEVAR remain limited. Repairing aortic arch aneurysms in Zone 1 requires a triple-fenestrated PMEG when F/B TEVAR is performed with a proximal landing in Zone 0. The presence of additional fenestrations increases technical complexity, making the precise preparation of fenestrated PMEGs a critical concern.
To improve precision and streamline the fenestration process in F/B TEVAR, a 3D-printed model was used to assist in fabricating PMEGs. 3D-printed models have been demonstrated as a feasible approach for modifying stent grafts, particularly in emergency situations where customized stents are unavailable24,25,26. In clinical practice, 3D printing has been shown to shorten PMEG preparation time, which is critical for reducing mortality27. Additionally, it was observed that once one fenestration is selected using a wire guide catheter, the remaining fenestrations tend to align spontaneously during the surgical procedure. Moreover, various commercially available stents can be utilized as the main body stents for PMEG fabrication, highlighting the broad applicability of 3D-assisted F/B TEVAR. Compared with TEVAR using three-vessel inner branch stents28, 3D-assisted F/B TEVAR demonstrated lower mortality (4.8%) and stroke rates (9.5%). The post-operative mortality in patients undergoing F/B TEVAR with PMEGs for aortic arch disease repair was lower than that reported by Zhu et al.29. Additionally, the incidence of endoleak (14.3%) was improved compared to previous studies30. These findings suggest that 3D-assisted PMEGs are a safe and effective approach for repairing aortic arch lesions in Zone 1.
Despite its advantages, 3D-assisted F/B TEVAR has several limitations. First, intra-operative PMEG fabrication extends anesthesia and surgical duration. Second, modification and fenestration of stents require surgical expertise. Third, the production of 3D-printed models necessitates computer technical support. Lastly, the safety and reliability of this approach require further validation.
Disclosures
All of the authors declare no conflict of interest.
Acknowledgements
This work was supported by the Standardization Research and Innovative Application of Regional Vascular Surgery Disease Clinic Database, Jiangsu Provincial Drug Administration Drug Supervision Scientific Research Program Project (No. 202014).
Materials
| Name | Company | Catalog Number | Comments |
| 3D printer | Stratasys | Eden260VS | Used for printing 3D models |
| Ankura TAA Stent Graft System | Lifetech | TAA2622B100 | Used as the main body stent grafts |
| Biocompatible PolyJet material | Stratasys | MED610 | |
| Fluency Plus Endovascular Stent Graft | Bard Peripheral Vascular | FEM10100 | Used as the branch stents |
| Geomagic Wrap software | OQTON | Used for simulation analysis of vascular remodeling after stent implantation | |
| GORE DRYSEAL Flex Introducer Sheath | W.L. Gore & Associates | DSF1065 | Used as the delivery sheaths |
| GORE VIABAHN Endoprosthesis | W.L. Gore & Associates | VBHR051002A | Used as the branch stents |
| Hi-Torque Supra Core peripheral extra supportive guide wires | Abbott | 1002703 | Used as the guidewires |
| INFINITI DIAGNOSTIC CATHETER | Cordis | SRD6642 | Used as the catheters |
| Lunderquist Extra-Stiff Wire Guide | COOK MEDICAL | G49228 | Used as the guidewires |
| Mimics software | Materialise | Used for performing 3D reconstructions of the aorta | |
| Nester Embolization Coil | COOK MEDICAL | G47332 | Used as the coils |
| PROLENE Polypropylene Suture | Johnson&Johnson MedTech | SXPP1B201 | Used as the operative suture |
| RADIFOCUS Angiographic Catheter | Terumo Interventional Systems | RF-DB1500GM | Used as the catheters |
| RADIFOCUS Guide Wire M | Terumo Interventional Systems | RF-GA18153M | Used as the guidewires |
| SurVeil Drug-Coated Balloon | Abbott | SRV03513504010 | Used as the expansion balloons |
| V-18 & V-14 ControlWire Guidewire | Boston Scientific Corporation | 39216-71822, 46-850 | Used as the guidewires |
| Valiant thoracic stent graft with Captivia delivery system | Medtronic | VAMF2626C100TU | Used as the main body stent grafts |
References
- Bossone, E. Eagle, K. A. Epidemiology and management of aortic disease: Aortic aneurysms and acute aortic syndromes. Nat Rev Cardiol. 18 (5), 331-348 (2021).
- Isselbacher, E. M. et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: A report of the American Heart Association/American College of Cardiology joint committee on clinical practice guidelines. Circulation. 146 (24), e334-e482 (2022).
- De Bakey, M. E., Crawford, E. S., Cooley, D. A., Morris, G. C. Successful resection of fusiform aneurysm of aortic arch with replacement by homograft. Surg Gynecol Obstet. 105 (6), 657-664 (1957).
- Kavanagh, E. P. et al. Hybrid repair versus conventional open repair for aortic arch dissection. Cochrane Database Syst Rev. 7 (7), Cd012920 (2021).
- Hsieh, R. W. et al. Comparison of type b dissection by open, endovascular, and medical treatments. J Vasc Surg. 70 (6), 1792-1800.e3 (2019).
- Liu, J. et al. Comparisons of open surgical repair, thoracic endovascular aortic repair, and optimal medical therapy for acute and subacute type b aortic dissection: A systematic review and meta-analysis. BMC Cardiovasc Disord. 25 (1), 86 (2025).
- Seike, Y., Green, S. B., Mori, K., Reid, K., Matsuda, H. Outcomes of thoracic endovascular aortic repair for complicated type b acute aortic dissection from a multicenter Japanese post-market surveillance study. Gen Thorac Cardiovasc Surg. 10.1007/s11748-025-02123-4 (2025).
- Parodi, J. C., Palmaz, J. C., Barone, H. D. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg. 5 (6), 491-499 (1991).
- Liu, D., Luo, H., Lin, S., Zhao, L., Qiao, C. Comparison of the efficacy and safety of thoracic endovascular aortic repair with open surgical repair and optimal medical therapy for acute type b aortic dissection: A systematic review and meta-analysis. Int J Surg. 83, 53-61 (2020).
- Xodo, A. et al. Peri-operative management of patients undergoing fenestrated-branched endovascular repair for juxtarenal, pararenal and thoracoabdominal aortic aneurysms: Preventing, recognizing and treating complications to improve clinical outcomes. J Pers Med. 12 (7), 1018 (2022).
- Gorgatti, F. et al. Post-dissection thoraco-abdominal aortic aneurysm managed by fenestrated or branched endovascular aortic repair. Eur J Vasc Endovasc Surg. 68 (3), 325-334 (2024).
- Gallitto, E. et al. Fenestrated and branched endografts for post-dissection thoraco-abdominal aneurysms: Results of a national multicentre study and literature review. Eur J Vasc Endovasc Surg. 64 (6), 630-638 (2022).
- Motta, F. et al. Outcomes and complications after fenestrated-branched endovascular aortic repair. J Vasc Surg. 70 (1), 15-22 (2019).
- Arnaoutakis, D. J. et al. Comparative outcomes of open, hybrid, and fenestrated branched endovascular repair of extent II and III thoracoabdominal aortic aneurysms. J Vasc Surg. 71 (5), 1503-1514 (2020).
- Scali, S. T. et al. Outcomes of surgeon-modified fenestrated-branched endograft repair for acute aortic pathology. J Vasc Surg. 62 (5), 1148-1159.e2 (2015).
- Yang, G. et al. Endovascular repair of postdissection aortic aneurysms using physician-modified endografts. Ann Thorac Surg. 112 (4), 1201-1208 (2021).
- Tenorio, E. R., Lima, G. B., Marcondes, G. B., Oderich, G. S. Sizing and planning fenestrated and branched stent-grafts in patients with chronic post-dissection thoracoabdominal aortic aneurysms. J Cardiovasc Surg (Torino). 61 (4), 416-426 (2020).
- Dossabhoy, S. S. et al. Reinterventions after fenestrated or branched endovascular aortic aneurysm repair. J Vasc Surg. 68 (3), 669-681 (2018).
- Chen, Z. et al. Risk factors for target vessel endoleaks after physician-modified fenestrated or branched endovascular aortic repair for postdissection thoracoabdominal aortic aneurysms. J Vasc Surg. 77 (3), 685-693.e2 (2022).
- Doumenc, B. et al. Management of type ia endoleak after evar by explantation or custom-made fenestrated endovascular aortic aneurysm repair. Eur J Vasc Endovasc Surg. 61 (4), 571-578 (2021).
- Dossabhoy, S. S. et al. Fenestrated endovascular aortic aneurysm repair using physician-modified endovascular grafts versus company-manufactured devices. J Vasc Surg. 67 (6), 1673-1683 (2018).
- Lounes, Y. et al. Endovascular aortic arch repair with a pre-cannulated double-fenestrated physician-modified stent graft: A benchtop experiment. Interact Cardiovasc Thorac Surg. 32 (6), 942-949 (2021).
- Canaud, L. et al. Double homemade fenestrated stent graft for total endovascular aortic arch repair. J Vasc Surg. 70 (4), 1031-1038 (2019).
- Coles-Black, J., Barber, T., Bolton, D., Chuen, J. A systematic review of three-dimensional printed template-assisted physician-modified stent-grafts for fenestrated endovascular aneurysm repair. J Vasc Surg. 74 (1), 296-306.e1 (2021).
- Tong, Y. H. et al. Use of 3D printing to guide creation of fenestrations in physician-modified stent-grafts for treatment of thoracoabdominal aortic disease. J Endovasc Ther. 27 (3), 385-393 (2020).
- Tong, Y. et al. Three-dimensional printing to guide the application of modified prefenestrated stent grafts to treat aortic arch disease. Ann Vasc Surg. 66, 152-159 (2020).
- Katsargyris, A., Uthayakumar, V., Marques De Marino, P., Botos, B., Verhoeven, E. L. Aneurysm rupture and mortality during the waiting time for a customised fenestrated/branched stent graft in complex endovascular aortic repair. Eur J Vasc Endovasc Surg. 60 (1), 44-48 (2020).
- Tenorio, E. R. et al. Multicenter global early feasibility study to evaluate total endovascular arch repair using three-vessel inner branch stent-grafts for aneurysms and dissections. J Vasc Surg. 74 (4), 1055-1065.e4 (2021).
- Zhu, J. et al. Fenestrated thoracic endovascular aortic repair using physician-modified stent grafts (PMSGS) in zone 0 and zone 1 for aortic arch diseases. Cardiovasc Intervent Radiol. 42 (1), 19-27 (2019).
- Marecki, H. L. et al. Characterization and management of type ii and complex endoleaks after fenestrated/branched endovascular aneurysm repair. J Vasc Surg. 78 (1), 29-37 (2023).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved