Method Article
Surgical Transplantation of Tumor Cells into the Spinal Cord of Mice
* These authors contributed equally
In This Article
Summary
The present protocol describes the development of a reproducible murine model of spinal cord glioma by injecting tumor cells into the intervertebral space, offering a more effective and less invasive approach for research and therapeutic development.
Abstract
Spinal cord gliomas are commonly malignant tumors of the spinal cord, leading to a high rate of disability. However, uniform treatment guidelines and comprehensive data on spinal cord gliomas remain limited due to the lack of suitable preclinical animal models. Developing a simple and reproducible animal model has become essential for advancing basic and translational research. A murine model is ideal, as the murine spinal cord shares structural similarities with the human spinal cord. This protocol describes the generation of a reproducible murine model of spinal cord glioma by directly injecting tumor cells into the intervertebral space using the spinous process of the seventh cervical vertebra as a guide. Compared to other methods, this approach is more effective and convenient, involving a smaller incision, reduced invasiveness and blood loss, faster recovery, and more stable tumor formation. This model is expected to advance the understanding of disease mechanisms, optimize surgical strategies, and support the development of therapeutic drugs for spinal cord gliomas.
Introduction
Spinal cord gliomas, including those of the cauda equina, are commonly malignant neoplasms of the spinal cord, with 20%-40% classified as astrocytomas and the remainder as ependymomas1. Based on histological features, spinal cord gliomas are categorized into four grades (I-IV). Grade I and II tumors are considered low-grade gliomas, while grade III and IV tumors are classified as high-grade gliomas. Although spinal cord gliomas can occur at any segment of the spinal cord, they are most frequently found in the cervical region (33% of cases) and are relatively rare in other regions, with 26% of cases in the thoracic region and 24% in the lumbar region2.
Surgery, radiotherapy, and alkylating agents are the primary treatment options for spinal cord gliomas, largely extrapolated from clinical trials on brain gliomas3. However, previous research has demonstrated that, although the histological profiles of spinal cord gliomas resemble those of brain gliomas, the presence of distinct molecular signatures differentiates them from their cerebral counterparts4. In our cohort, spinal cord glioma patients derived no significant benefit from either adjuvant chemotherapy or radiotherapy, underscoring the limited effectiveness of current treatments and the need for new therapeutic strategies5. Therefore, reliable and informative animal models are essential for advancing basic research and preclinical studies.
Currently, several well-established spinal cord glioma models exist, including the method described by Minru et al.6. These models primarily utilize thoracic vertebra removal techniques to expose the spinal cord6,7,8. Although rat models have been employed in the past, they are associated with higher costs, smaller sample sizes, and greater management challenges compared to mouse models. Additionally, more genetically modified experimental mouse models are available than rat models. An immune-competent mouse model is particularly valuable for studying the immune response within the spinal tumor microenvironment and for developing immunotherapeutic strategies for spinal cord gliomas. Furthermore, this method is well-suited for generating patient-derived xenograft models for spinal cord gliomas.
This protocol proposes a safe, technically simple, and rapidly reproducible procedure for creating a spinal cord glioma transplantation model in mice. The model is expected to advance research into the largely unexplored mechanisms underlying glioma progression and facilitate the development of therapeutic drugs for spinal cord gliomas.
Protocol
This protocol was conducted in compliance with the guidelines approved by the Institutional Committee for the Ethics of Animal Care and Treatment in Biomedical Research at Capital Medical University (AEEI-2021-187). Female C57BL/6 mice, aged 8 weeks and weighing 19-21 g, were used in this study. The reagents and equipment utilized are detailed in the Table of Materials.
1. Pre-surgical preparation
- Clean and sterilize all surgical instruments thoroughly.
- Spray the surgical table with alcohol and wipe it clean using sterile paper towels.
2. Preparation of GL261-luc and B16-F10-luc cells for transplant
NOTE: The GL261-luc GBM cell line was obtained commercially, while the B16-F10-luc melanoma cell line was a gift from Professor Wang Xi. Both cell lines were confirmed to be free of mycoplasma infection through pre-experimental testing.
- Prepare complete DMEM (Dulbecco's Modified Eagle Medium) by supplementing it with 10% fetal bovine serum (FBS) and 1% penicillin (100 U/mL)-streptomycin (100 µg/mL).
- Culture GL261-luc or B16-F10-luc cells in the complete DMEM medium and collect cells during the logarithmic growth phase for implantation.
- Wash the cells twice with sterile PBS, then incubate them with 0.05% trypsin-EDTA solution for 3 min.
- Transfer the resulting cell suspension into a tube and centrifuge at 500 × g for 5 min at room temperature.
- After centrifugation, discard the supernatant using a pipette, resuspend the cells in sterile PBS, and centrifuge once more.
- Stain the cells with trypan blue and count viable cells using a cell counter.
- Prepare the cell suspension at a concentration of 5 × 106 cells/mL for GL261-luc cells or 5 × 105 cells/mL for B16-F10-luc cells, making it ready for use.
3. Animal preparation
- Weigh and anesthetize the mice by intraperitoneal injection of a 2.5% tribromoethanol solution (10 µL/g). Confirm anesthesia by checking for the loss of the pedal reflex. The entire procedure, from preparation to suturing, should take approximately 5-10 min.
NOTE: Position the animal on a heating pad to maintain body temperature throughout the procedure. - Expose the skin and prepare a clean surgical window (Figure 1A). Shave the hair from the dorsal neck region and a 2 cm area extending bilaterally from the midline using hair clippers.
- To remove any remaining hair, apply a thin layer of depilatory cream to the shaved areas using a cotton swab and leave it for 1-2 min. Afterward, wipe off the depilatory cream with soap-dampened gauze.
- Disinfect the skin using iodine solution, applied in a circular motion for 30 s, followed by wiping with 75% alcohol for deiodination.
4. Exposure of cervical spine and determination of insertion point
- Position the mice with their dorsal side facing upward and secure their limbs to the surgical table using medical tape. Place a 1-2 cm thick gauze pad under the neck area for support, providing better access to the spinal cord.
- Make a longitudinal incision of approximately 1.5 cm along the neck skin using a surgical scalpel and blade (Figure 1B). Gently separate the neck muscles by blunt dissection, taking care to avoid injuring any blood vessels.
- Carefully dissect the muscles adjacent to the cervical vertebrae to expose the seventh cervical vertebral spinous process, a distinct bony landmark in mice (Figure 1C and Figure 1G-I).
- Clear any blood from the surgical area using sterile cotton swabs before proceeding with the injection.
- Set the puncture point at 0.5-0.9 mm from the midline of the spine, adjusting the injection depth to 0.6-0.9 mm based on the body weight of the mice (16-24 g).
NOTE: The spinal cord injection depth is 0.9 mm for mice weighing 22-24 g.
5. Injection of tumor cells
- Rinse a 10 µL flat-needle syringe thoroughly with sterile PBS solution 2-3 times.
- Draw 2 µL of the cell suspension into the syringe, ensuring no air bubbles are present.
- Stabilize the cervical vertebral spinous process by gently grasping and lifting it with forceps. Use a beveled needle (1.87 mm in length and 0.48 mm in diameter) to puncture the dura mater (Figure 1D). Then, switch to a flat needle syringe (0.48 mm in diameter) to inject the tumor cells (Figure 1E).
NOTE: The puncture site is preserved during the needle switch, with accurate placement confirmed by lower limb twitching from nerve stimulation. - Inject the cell suspension slowly to avoid disruption.
- Keep the syringe in place for 30 s post-injection to ensure successful tumor implantation.
6. Post-surgical care
- Close the skin incision by suturing with a 3-0 nylon suture at the end of the operation (Figure 1F).
- Position the mouse on its side and place it on a heated mat to maintain warmth and ensure stable breathing during recovery from anesthesia in the cage.
- Administer Buprenorphine (0.1 mg/kg) subcutaneously twice daily for 3 days to alleviate pain.
- Monitor the mouse to ensure it regains pre-operative activity without signs of bleeding or wound tearing.
NOTE: Temporary spinal cord dysfunction, including hind limb weakness, is common after surgery and typically resolves within 3 h. Approximately 5% of mice may develop paralysis but usually recover within 3 days. For these mice, provide a nutritionally complete diet and gel water directly on the cage floor to ensure adequate accessibility. A small percentage (about 5%) of mice that experience paraplegia may require euthanasia. - Ensure the mice have continuous access to water and food.
NOTE: If the animals exhibit signs of weight loss or paralysis, they should be housed individually.
7. In vivo bioluminescence imaging
- Administer an intraperitoneal injection of 150 mg/kg D-luciferin dissolved in D-PBS to the mice.
- Place the mice in an anesthesia chamber containing isoflurane for induction.
- Transfer the mice to the integral anesthetic manifold to maintain anesthesia during the procedure.
- Perform in vivo bioluminescent imaging as described in the previous report9.
NOTE: The optimal response time for D-luciferin in live imaging of small animals is 10 min post-injection. Ensure imaging is conducted precisely 10 min after the injection.
Results
To establish a stable and reliable animal model of spinal glioma, the intervertebral space between the sixth and seventh cervical vertebrae in C57BL/6 mice was identified as the ideal site for inoculation based on literature review and experimental findings10. The seventh cervical vertebra provides a distinct bony landmark, the spinous process (Figure 1G-I), which aids in accurately locating the injection site and stabilizing the injection process.
The neck region of mice contains numerous muscles and blood vessels, increasing the complexity of the procedure. To minimize surgical trauma, the cervical vertebrae were exposed as thoroughly as possible using blunt dissection after the skin incision (Figure 1C). The prominent spinous process of the seventh cervical vertebra serves as a clear visual marker within the spinal column (Figure 1G-I).
The injection site was precisely located at the intervertebral space between the sixth and seventh cervical vertebrae. A micro-syringe with a sharp tip was employed to accurately position the needle (Figure 1D), followed by the use of a flat-headed microsyringe for the injection to prevent fluid leakage (Figure 1E).
About 10 days post-surgery, mice inoculated with GL261-luc cells exhibited signs of nerve damage, such as hind limb weakness, followed by significant weight loss (approximately 20%, Figure 2D) and eventual hind limb paralysis. Successful inoculation of tumor cells into the spinal cord was confirmed using bioluminescent imaging (Figure 2A). At the experimental endpoint, defined by hind limb paralysis, the mice were euthanized using CO2, and their spinal cords were harvested. Tumors were clearly visible upon exposing the spinal cord (Figure 2B). Histological examination with H&E staining revealed glioma infiltration within the spinal cord tissue (Figure 2E). A survival analysis (Figure 2C) demonstrated that mice inoculated with GL261-luc cells succumbed to the disease within 18 days, whereas PBS-inoculated mice showed no signs of morbidity and survived the entire observation period.
Melanoma, a disseminated tumor known for potential intrathecal metastasis, was modeled using B16-F10-luc cells to observe tumor progression in the spinal cord more distinctly. Bioluminescence and gross imaging confirmed the establishment of B16-F10-luc melanoma in the spinal cord (Figure 3A,B). Similar to the GL261 model, mice bearing B16-F10 tumors exhibited weight loss and died within 18 days, while PBS-inoculated mice survived and gained weight (Figure 3C,D), aligning with the results of the GL261 model.
Analysis of the immune microenvironment of spinal cord gliomas showed the distribution of tumor-associated macrophages (TAMs), specifically CD163+ and CD86+ populations, within the spinal cord tumors. Immunofluorescence staining (Figure 4) indicated that most TAMs within the tumors expressed CD86, a marker associated with the pro-inflammatory M1 phenotype.
Most mice experienced mild spinal cord injuries, evidenced by transient hind limb weakness lasting 1-3 h, which indicated some degree of spinal cord damage. After 3 h, these mice recovered their pre-surgery activity levels. To assess the repeatability and stability of the procedure, three independent experiments were conducted, each involving 5 mice implanted with tumor cells and 5 mice injected with PBS. These trials confirmed the reproducibility and stability of the procedure in causing spinal injury and generating tumors. In total, 60 mice underwent surgery during the experiments: 30 were implanted with PBS, 15 with GL261-luc tumor cells, and 15 with B16-F10-luc tumor cells. Of the PBS group, one mouse died during surgery, while one mouse each from the B16-F10-luc and GL261-luc groups died within 1-3 days post-surgery due to severe complications. The remaining 57 mice were included in the survival and body weight analyses.
Among these, 54 mice exhibited mild postoperative spinal cord dysfunction that resolved within 3 h, while 3 mice experienced temporary paralysis (moderate injury) that resolved within 3 days (Figure 2F and Figure 3E). Mice with moderate injuries showed more significant body weight loss compared to those with mild injuries (Figure 3F). However, no differences in survival time or tumor progression were observed between the two groups. These findings indicate that the injuries caused by surgery did not influence tumor progression, highlighting the reliability and reproducibility of the model.

Figure 1: Surgical procedure for tumor cell implantation into the spinal cord of mice. (A) Surgery area after shaving and depilating. (B) Skin incision. (C) Exposure of the cervical vertebra. (D) Puncture performed using a microsyringe with a pointed tip. (E) Cell injection using a microsyringe with a flat head. (F) Wound closure with sutures applied to the skin. (G) General view of the spinal column. (H,I) Spinous protrusion of the seventh cervical vertebra highlighted for clarity. Scale bar: 5 mm. Please click here to view a larger version of this figure.

Figure 2: Representative results of the GL261-Luc spinal glioma model. (A) Bioluminescence imaging showing GL261-luc tumors on day 12 post-implantation. Scale bar: 5 mm. (B) Gross image of the mouse spinal cord implanted with GL261-luc on day 14. Scale bar: 5 mm. (C) Survival curve of C57BL/6 mice implanted with PBS or GL261-luc cells (n = 14-15 per group; pooled data from three experiments). (D) Body weight changes in mice over time. Two-way ANOVA was used to calculate P values. Data are presented as mean ± SD (n = 14-15 per group). (E) Representative H&E-stained spinal cord sections from mice on day 14 post-implantation with PBS or GL261-luc. Yellow outlines indicate tumor areas. Scale bar: 500 µm. (F) Observed severity of spinal cord dysfunction in mice post-surgery. Survival data were analyzed using Kaplan-Meier analysis with the log-rank test for group comparison. Please click here to view a larger version of this figure.
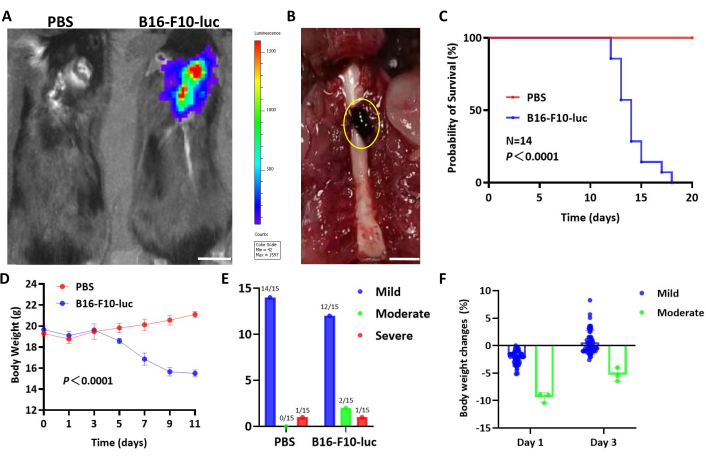
Figure 3: Representative results of the B16-F10-Luc spinal glioma model. (A) Bioluminescence imaging showing B16-F10-luc tumors on day 12 post-implantation. Scale bar: 5 mm. (B) Gross image of mouse spinal cords implanted with B16-F10-luc on day 14. Scale bar: 5 mm. (C) Survival curve of C57BL/6 mice implanted with PBS or B16-F10-luc cells (n = 14 per group; pooled data from three experiments). (D) Body weight changes in mice over time. Two-way ANOVA was used for statistical analysis. Data are presented as mean ± SD (n = 14 per group). (E) Observed severity of spinal cord dysfunction in mice post-surgery. (F) Body weight changes on day 1 and day 3 in mice exhibiting mild or moderate dysfunction. Survival data were analyzed using Kaplan-Meier analysis, with the log-rank test for group comparison. Please click here to view a larger version of this figure.
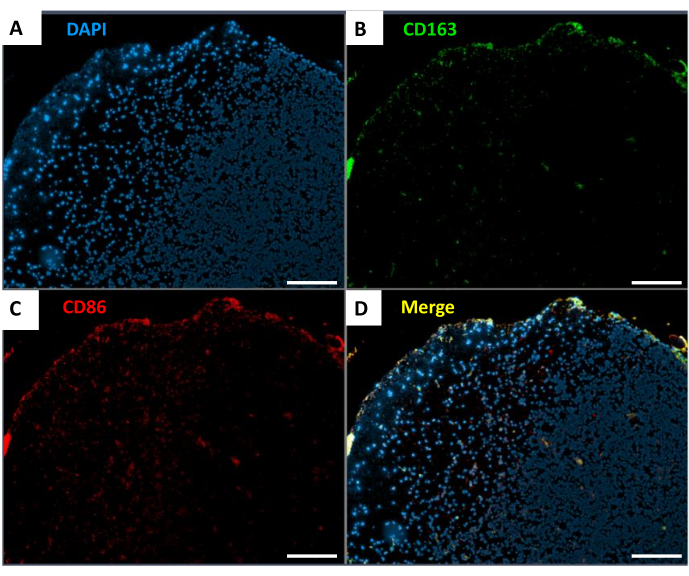
Figure 4: Distribution of TAMs in spinal cord gliomas. (A) DAPI staining (blue). (B) CD163 staining (green). (C) CD86 staining (red). (D) Merged image of the markers. Scale bar: 500 µm. Please click here to view a larger version of this figure.
Discussion
Spinal cord glioma is the most common type of primary malignant tumor in the spinal cord, accounting for over 80% of intramedullary tumors. Pathologically, spinal cord gliomas are primarily classified as ependymomas or astrocytomas, with a particular focus on astrocytomas11. Among astrocytomas, some harbor H3K27M mutations, also known as diffuse midline gliomas (DMGs), which are associated with poor prognoses. A defining feature of spinal cord gliomas is their infiltrative growth pattern, which makes complete surgical resection difficult due to the lack of clear boundaries between the tumor and surrounding healthy tissue. Additionally, the densely packed nerve fibers in the spinal cord can lead to significant sensory and motor dysfunction as the tumor progresses.
Current treatments for spinal cord glioma are similar to those used for brainstem gliomas and typically include surgery and radiotherapy, with chemotherapy regimens such as Temozolomide (TMZ) serving as supplementary therapies12,13. However, the immune microenvironment of spinal cord gliomas differs from that of brain gliomas, and the experience gained from immunotherapy in brain gliomas offers limited insights for spinal cord gliomas14. Despite ongoing advancements in targeted therapies and immunotherapies for central nervous system tumors, treatment options for spinal cord gliomas remain limited15,16,17,18. The primary challenge lies in the absence of a rapid, effective, and reproducible animal model for spinal cord glioma research, which has hindered the development of novel therapeutic strategies.
The injection process for mouse spinal cord experiments is often time-consuming. A percutaneous, direct posterolateral approach is commonly used for tumor cell injection19. However, the success of this model heavily relies on the operator's skills and experience, making it difficult to replicate. In some cases, team collaboration is required during the injection, leading to prolonged procedural times20,21. Additionally, certain researchers remove one or two vertebrae to directly expose the spinal cord for tumor cell injection, which increases the risk of injury and infection in mice. In Feng's experimental model, a surgical approach was implemented involving vertebra removal to enhance the precision of tumor cell implantation into the spinal cord22. While this method improves modeling accuracy, it raises concerns regarding tumor cell leakage during transplantation, spinal instability, and a higher risk of postoperative infection. Furthermore, for small animal models like mice, this approach demands advanced surgical expertise from the operator. Weng et al. introduced the use of a stereotaxic device to facilitate injections by exposing only the mouse spine and delivering tumor cells through the intervertebral space10. This technique enables precise control over the needle's position and depth, reducing procedural variability. However, its applicability may be limited by differences in mouse strains and ages. Additionally, using a stereotaxic device can extend the injection time, thereby increasing the risk of mortality and infection. Studies also recommend maintaining the needle in place for a certain period post-injection to prevent cell leakage, which has been demonstrated to improve transplantation outcomes23. Findings suggest that by using an appropriate injection depth and a small injection volume, the required waiting time can be reduced without observing overflow upon needle removal.
This study introduces a rapid and effective method for creating a spinal cord glioma model in mice, emphasizing precision and reduced procedure time. During the surgical process, gently separating the muscles attached to the spine minimized excessive bleeding, resulting in a clearer and more complete exposure of the spinal cord. The seventh cervical vertebra was identified as an optimal landmark due to its distinct bony protrusion and the relatively wider intervertebral space between the sixth and seventh cervical vertebrae, making it ideal for needle insertion. To replicate the infiltrative growth characteristic of gliomas, accurate implantation of tumor cells into the white matter was essential. Using methylene blue injections, the optimal depth of 0.9 mm was determined for cell inoculation into the spinal cord. To maintain this depth while avoiding irreversible spinal cord damage, a capillary hose was fitted over the microinjection needle, leaving a controlled distance to ensure precise injection depth. This approach achieved results comparable to those obtained using stereotaxic instruments but significantly reduced the injection time per mouse, enhancing overall efficiency.
The choice of injection needle also played a crucial role. Using a single oblique needle led to cell leakage outside the spinal cord and inoculation failure, while a flat needle alone was insufficient to penetrate the dura mater, potentially harming the spinal cord. To overcome these challenges, a combination of oblique and flat needles was utilized. The oblique needle, modified with a capillary hose for depth control, was used to puncture the dura mater, with lower limb twitching in the mice, confirming successful depth penetration. The flat needle was then employed for precise tumor cell injection into the spinal cord, ensuring effective and consistent model establishment.
The study has several limitations that must be acknowledged. Initially, efforts to inject tumor cells into the thoracic and lumbar regions of the mouse spine resulted in a loss of limb mobility, but no discernible tumor lesions were observed in the spinal cord. Considering the relatively dense structure of the cervical spinal cord and the loose structure of the lumbar spinal cord near the conus medullaris and filum terminate, these areas may not be conducive to tumor cell attachment and growth. Consequently, we modified the protocol to inject tumor cells into the cervical spinal cord, where tumors were clearly visible upon exposure. This raises an important question about whether different regions of the spinal cord influence tumor growth. Further investigation is needed to explore this aspect in greater detail. Additionally, the GL261 tumor cell line used in this study does not carry the characteristic H3K27M mutation found in primary spinal cord tumors. As a result, the model may not fully replicate the conditions of diffuse midline glioma (DMG), which is associated with poor prognosis. To address this, future plans involve developing a glioma cell line carrying the H3K27M mutation, allowing for the exploration of how this driver gene affects tumor progression and model characteristics.
In conclusion, the primary objective of this study was to develop a stable, reliable, and efficient mouse model for spinal cord glioma. By analyzing the spinal cord structure, an optimal puncture site was identified, and determining the appropriate puncture depth improved the stability and reproducibility of the model. The simplification of the modeling procedure has significantly expedited the experimental process, making it a more efficient tool for studying spinal cord glioma. This method provides a valuable resource for advancing research on potential therapeutic strategies for this challenging condition.
Disclosures
No conflicts of interest were declared.
Acknowledgements
This work was supported by the National Natural Science Foundation of China General Program (Fund No. 8207317). R&D Program of Beijing Municipal Education Commission (Fund No. KZ202210025040). Chinese Institutes for Medical Research, Beijing (Grant No. CX24PY08).
Materials
| Name | Company | Catalog Number | Comments |
| A nutritionally complete food and water gelled diet (Nutra-Gel) | Bio-Serv | N/A | |
| Adhesion microscope slides | CITOTEST | 188105 | |
| AffiniPure Fab Fragment Goat Anti-Mouse IgG (H+L) | Jacksonimmuno | 115-007-003 | |
| B16-F10-luc | Professor Wang Xi's laboratory | N/A | |
| Buprenorphine Related Compound A | Sigma-Aldrich | 457071-73-7 | |
| CD163 (ABT-CD163) mouse mAb | Immunoway | YM6146 | |
| CD86 rabbit pAb | Immunoway | YT7823 | |
| Cell counter | Bio-rad | 1450102 | |
| Cell Counting Slides | Biorad | 1450011 | |
| DAPI/Sealant Dual Solution (Anti-Quenching) | Immunoway | YS0014 | |
| Dilator | Jinzhong | D22178 | |
| D-Luciferin | PerkinElmer | 122799 | |
| DMEM | Gibco | C11995500BT | |
| D-PBS | Solarbio | D1040 | |
| Fetal Bovine Serum, qualified | Gibco | 10270-106 | |
| GL261-luc | Shanghai Zishi Biotechnology | N/A | |
| Goat anti-Mouse IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 | Invitrogen | A11029 | |
| Goat anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 647 | Life | A21244 | |
| Goat Serum | Beyotime | C0265 | |
| Hamilton microinjector 10 µL fixed 701N | Hamilton | 80383 | |
| In vivo bioluminescent imaging (IVIS Spectrum) | PerkinElmer | N/A | |
| Methanol | Fuyu Chemical | 67-56-1 | |
| Micro Scissors | Jinzhong | WAA320 | |
| Microliter Syringes (10 µL, pointed tip) | Shanghai Gaoge | N/A | |
| Microscope cover glass | CITOTEST | 10212440C | |
| needle holder 12.5 cm | Jinzhong | JCZ200 | |
| Ophthalmic Forceps 10 cm | Jinzhong | JD1060 | |
| Ophthalmic Scissors 10 cm | Jinzhong | Y00030 | |
| PBS, 10× | Solarbio | P1022 | |
| Penicillin-Streptomycin Liquid | Solarbio | P1400 | |
| Scalpel Blades | Jinzhong | J0B050 | |
| super pap pen | ZSGB-Bio | ZLI-9303 | |
| Surgical Knife Handle | Jinzhong | J11010 | |
| Surgical scissors 12.5cm straight tip | Jinzhong | J21010 | |
| Nylon Surgical Sutures with thread, size 3-0 | UNIFY | N/A | |
| Tissue-Tek O.C.T. Compound | SAKURA | 4583 | |
| Tribromoethanol | Sigma-Aldrich | T48402 | |
| Triton X-100 | Servicebio | GC204003 | |
| Trypan Blue Stain Solution, 0.4% | Solarbio | C0040 | |
| Trypsin Digestion solutions, 0.25% (without phenol red) | Solarbio | T1350 | |
| Tween-20 | Solarbio | T8220 |
References
- Ostrom, Q. T., et al. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2015-2019. Neuro Oncol. 24 (Suppl 5), v1-v95 (2022).
- Kane, P. J., el-Mahdy, W., Singh, A., Powell, M. P., Crockard, H. A. Spinal intradural tumours: Part II--Intramedullary. Br J Neurosurg. 13 (6), 558-563 (1999).
- Horbinski, C., et al. NCCN guidelines insights: Central Nervous System Cancers, Version 2.2022. J Natl Compr Canc Netw. 21 (1), 12-20 (2023).
- Chai, R. C., et al. The molecular characteristics of spinal cord gliomas with or without H3 K27M mutation. Acta Neuropathol Commun. 8 (1), 40 (2020).
- Zhang, Y. W., et al. Clinicopathological characteristics and survival of spinal cord astrocytomas. Cancer Med. 9 (19), 6996-7006 (2020).
- Muir, D., et al. Assessment of laminin-mediated glioma invasion in vitro and by glioma tumors engrafted within rat spinal cord. J Neurooncol. 30 (3), 199-211 (1996).
- Ren, T. J., et al. Establishment of intramedullary spinal cord glioma model in rats. Chin Med J (Engl). 123 (18), 2580-2585 (2010).
- Hsu, W., et al. Animal model of intramedullary spinal cord glioma using human glioblastoma multiforme neurospheres. J Neurosurg Spine. 16 (3), 315-319 (2012).
- Lim, E., et al. In vivo bioluminescent imaging of mammary tumors using IVIS spectrum. J Vis Exp. (26), e1210 (2009).
- Weng, Z., et al. A reproduceable in situ xenograft model of spinal glioma. J Neurosci Methods. 346, 108928 (2020).
- Chamberlain, M. C., Tredway, T. L. Adult primary intradural spinal cord tumors: a review. Curr Neurol Neurosci Rep. 11 (3), 320-328 (2011).
- Watanabe, G., et al. Diffuse Midline H3K27-Altered Gliomas in the Spinal Cord: A Systematic Review. J Neurooncol. 166 (3), 379-394 (2024).
- Chalif, E. J., et al. Impact of extent of resection and adjuvant therapy in diffuse gliomas of the spine. Spine J. 23 (7), 1015-1027 (2023).
- Ellis, J. A., et al. Unique microenvironmental responses to PDGF stimulation in brain and spinal cord gliomas determine tumor phenotype. J Neurooncol. 123 (1), 27-33 (2015).
- Zhou, D., et al. Harnessing immunotherapy for brain metastases: insights into tumor-brain microenvironment interactions and emerging treatment modalities. J Hematol Oncol. 16 (1), 121 (2023).
- Sampson, J. H., Gunn, M. D., Fecci, P. E., Ashley, D. M. Brain immunology and immunotherapy in brain tumours. Nat Rev Cancer. 20 (1), 12-25 (2020).
- Jha, P., et al. Analysis of PD-L1 expression and T cell infiltration in different molecular subgroups of diffuse midline gliomas. Neuropathology. 39 (6), 413-424 (2019).
- Majzner, R. G., et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature. 603 (7903), 934-941 (2022).
- Cossigny, D. A. F., Mouhtouris, E., Dushyanthen, S., Gonzalvo, A., Quan, G. M. Y. An in vivo mouse model of intraosseous spinal cancer causing evolving paraplegia. J Neurooncol. 115 (2), 189-196 (2013).
- Carbajal, K. S., Weinger, J. G., Whitman, L. M., Schaumburg, C. S., Lane, T. E. Surgical transplantation of mouse neural stem cells into the spinal cords of mice infected with neurotropic mouse hepatitis virus. J Vis Exp. (53), e2834 (2011).
- Minehan, K. J., Brown, P. D., Scheithauer, B. W., Krauss, W. E., Wright, M. P. Prognosis and treatment of spinal cord astrocytoma. Int J Radiat Oncol Biol Phys. 73 (3), 727-733 (2009).
- Feng, S., et al. Establishing a mouse contusion spinal cord injury model based on a minimally invasive technique. J Vis Exp. (187), e64538 (2022).
- Keirstead, H. S., et al. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 25 (19), 4694-4705 (2005).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved