Method Article
Observation of the Systolic Function of Isolated Right Atria from Guinea Pigs
* These authors contributed equally
In This Article
Summary
The present protocol describes an efficient method for screening drugs that enhance myocardial contractility using isolated right atria from guinea pigs.
Abstract
Common chronic heart failure (CHF) is characterized by impaired ventricular filling and/or ejection function, which leads to insatiable cardiac output and increased incidence. The decline in cardiac systolic function is a key factor in the pathogenesis of CHF. Systolic function is simply the filling of oxygenated blood in the left ventricle, followed by the blood being pumped throughout the body during a heartbeat. A weak heart and the inability of the left ventricle to contract appropriately as the heart beats indicate poor systolic function. Many traditional herbs have been suggested to strengthen the systolic function of the heart in patients. However, stable and efficient experimental methods for screening compounds that enhance myocardial contractility are still lacking in the process of ethnic medicine research. Here, taking digoxin as an example, a systematic and standardized protocol is provided for screening compounds that enhance myocardial contractility by using isolated right atria from guinea pigs. The results showed that digoxin could markedly enhance the contractility of the right atrium. This systematic and standardized protocol is intended to serve as a methodological reference for screening the active ingredients of ethnic medicines in the treatment of CHF.
Introduction
Heart failure is caused by myocardial infarction, myocardiopathy, hemodynamic overload, inflammation, and other causes of myocardial injuries, which modify the myocardial anatomy and activity and, ultimately, lead to failure in the ventricular pumping or filling. Palpitations, tiredness, and fluid retention are the main primary clinical symptoms1. CHF is a chronic heart failure condition that can be maintained, deteriorate, or show decompensation over time, and its incidence and prevalence increase with age2. The decline in cardiac systolic function is a key factor in the pathogenesis of CHF3. The current medical treatment for the disease mainly involves the use of antihypertensive drugs such as angiotensin-converting enzyme inhibitors, β-adrenoceptors (which inhibit the excessive activation of the neurohormonal system, namely the sympathetic system and the renin-angiotensin-aldosterone system), or diuretics (which reduce congestion)4. However, the clinical signs of heart failure caused by reduced cardiac output and reserve are not often addressed in studies examining the impact of these medical treatments5.
Positive inotropic drugs are designed to increase myocardial contractility. Cardiac glycosides, phosphodiesterase inhibitors, and β-adrenergic receptor agonists are used as positive inotropic drugs for treating heart failure. Cardiac glycosides are primarily Digitalis derivatives; an example is, digoxin, which is the most prevalently used Digitalis derivative and is derived from Digitalis lanata (white foxglove)6. They selectively bind to Na+/K+-ATPase on the cell membrane to increase the intracellular calcium concentration and, thus, enhance the cardiac contractility and stroke volume without elevating the oxygen intake, thereby improving cardiac efficiency7. Aside from cardiac glycosides, most positive inotropic drugs, such as phosphodiesterase inhibitors and β-adrenergic receptor agonists, increase the heart rate and myocardial oxygen consumption while increasing the calcium load in myocardial cells to enhance the myocardial contractility, which can result in clinically severe arrhythmias and hypotension and, thus, increased mortality8. Therefore, the clinical application of these inotropic drugs is limited. In order to avoid complications caused by elevated intracellular calcium levels, it is necessary to develop safer and highly effective inotropic modulators for the treatment of CHF (Figure 1).
In recent decades, many studies have been conducted to generate and analyze compounds that can support the positive inotropic properties of cardiac hemodynamics. Many traditional Chinese medicines (TCM), such as Euodia rutaecarpa (Juss.) Benth., Apocynum venetum L., and Sophora alopecuroides L., among others, can enhance myocardial contractility9,10,11. Studies have proven that TCM and its active monomers can exert positive inotropic effects through different mechanisms compared to inotropic drugs. For example, liguzinediol, a form of ligustrazine methylated at C2 and C5 (one active ingredient of Szechwan Lovage Rhizome), which significantly enhances the contractility of isolated rat hearts by enhancing sarcoplasmic reticulum calcium transients without increasing the heart rate, may have fewer side effects and be a better treatment for CHF12. Additionally, matrine is an alkaloid extracted from the TCM plant Sophora flavescens Ait. Matrine can inhibit the upregulation of β3-AR protein expression and diminish eNOS expression in heart failure model rats, thereby enhancing their myocardial contractility13. However, in ethnic medicine research, there is a lack of stable and efficient experimental methods for screening compounds that can enhance myocardial contractility.
It is commonly known that, compared to other rodents, guinea pigs have electrophysiology and calcium handling characteristics that are more similar to those of humans14. On the one hand, the electrocardiogram of guinea pigs is sufficiently similar to that of humans, and their beat-to-beat Ca2+ handling is more similar to human physiology than that of rats or mice15,16. On the other hand, computational models of guinea pig cardiomyocytes have undergone extensive research and include crucial cellular subsystems, including energetics and reactive oxygen species metabolism17. Therefore, isolated right atria from guinea pigs are widely used to screen compounds that enhance myocardial contractility. Here, we take digoxin as an example to provide a systematic and standardized protocol for screening compounds that enhance myocardial contractility by using isolated right atria from guinea pigs. Therefore, this work provides a methodological reference for screening the active ingredients of ethnic medicines in the treatment of CHF.
Protocol
The experimental protocol was conducted in accordance with the requirements of the Use of Laboratory Animals and Institutional Animal Care and Use Committee at Ningxia Medical University. Male Dunkin-Hartley guinea pigs weighing 300-450 g were used for the present study. The effect of digoxin on contractility was observed in isolated right atria from the guinea pigs (Figure 2).
1. Oxygenation preparation for the isolated right atria of guinea pigs
- Prepare the experimental instruments, including a biological signal acquisition and processing system, a JH-2 muscle force transducer, a Magnus bath, an L-shaped ventilation hook, thick scissors, a Petri dish, paraffin, etc. (see Table of Materials).
- Prepare 1,000 mL of Krebs-Henseleit solution (K-H solution) by adding 7.02 g of NaCl (120.0 mM), 2.10 g of NaHCO3 (25.0 mM), 0.30 g of KCl (4.0 mM), 0.07 g of MgSO4 (0.6 mM), 0.07 g of NaH2PO4 (0.6 mM), 0.28 g of CaCl2 (2.5 mM), and 1.98 g of glucose (11.0 mM) into 1,000 mL of double-distilled water, and rinse the Magnus bath two to three times (see Table 1 and Table of Materials).
NOTE: Keep the temperature of the K-H solution at 37 °C ± 1 °C. - Prepare 100 mL of low-calcium K-H solution by adding 0.70 g of NaCl (120.0 mM), 0.21 g of NaHCO3 (25.0 mM), 0.03 g of KCl (4.0 mM), 0.01 g of MgSO4 (0.6 mM), 0.01 g of NaH2PO4 (0.6 mM), 0.01 g of CaCl2 (0.8 mM), and 0.20 g of glucose (11.0 mM) into 100 mL of double-distilled water (see Table 1 and Table of Materials).
- Place approximately 20 mL of the 37 °C K-H solution in the operating basin (see Table of Materials).
- Spread 5 mm of thick paraffin over the bottom of the Petri dish, and then fill the Petri dish with the 37 °C K-H solution (see Table of Materials).
- Install the L-shaped ventilation hook on the latex tube end of the bladder, put it in the Petri dish, and adjust to 1-2 bubbles/s (see Table of Materials).
NOTE: Slowly adjust the bubbles; if the action is too quick, the oxygen may soon run out.
2. Preparation of isolated right atria from guinea pigs
- Weigh guinea pigs on a scale (see Table of Materials).
- Induce anesthesia using an induction box with 5% isoflurane in 100% oxygen, and then switch to a nose cone with 1.5%-3% isoflurane for maintenance (see Table of Materials).
- Cut the carotid artery with rough scissors, and induce exsanguination before placing it on a plate. Then, using scissors, open the thorax (starting with the xiphoid process and completely separating the sides to expose the heart), and peel off the pericardium.
- Hold the heart up with the left hand, use the right hand to cut the heart from the root of the aorta, and quickly place it in the operating basin with the K-H solution. Finally, gently press the ventricle with the hand two to three times, squeeze out the ventricular blood, and place the heart in the Petri dish.
NOTE: The action should be brisk and completed in 2-5 min, and the temperature must be controlled at 35 °C. The temperature of the K-H solution must be controlled at 37 °C.
- Hold the heart up with the left hand, use the right hand to cut the heart from the root of the aorta, and quickly place it in the operating basin with the K-H solution. Finally, gently press the ventricle with the hand two to three times, squeeze out the ventricular blood, and place the heart in the Petri dish.
- Fix the tip of the heart to the paraffin-coated Petri dish with a needle while providing oxygen (60 bubbles/min).
- Identify the right atrium.
NOTE: In guinea pigs, the atria are separated on their ventral surface by the pulmonary artery and dorsally by the aorta. Both the left and right atria are attached to the ventricles like an "inverted triangle", and the right atrium is slightly smaller than the left atrium and has uneven edges. The myocardium of the right ventricle is thin, and the upper end of the collapse is the right atrium; the left ventricle is more puffed, and the distribution of coronary vessels is rich18 (Figure 3). - Gently lift the edge of the right atrium with ophthalmic forceps, and cut along the atrioventricular junction (see Table of Materials).
NOTE: Avoid damaging the sinoatrial node, and try to cut more close to the ventricle while cutting along the atrioventricular junction. Automatic rhythmic contraction of the right atrium can be observed in this step. - Use 4-0 surgical sutures (see Table of Materials) to ligate the top and bottom of the right atrium, respectively (both ends of the "diagonal line"), with one end looped and the other end left with a long thread end that is also looped.
NOTE: Try to ligate as few tissues as possible when ligating both ends of the "diagonal line" of the atria.
3. Measurement and recording of the systolic function of isolated right atria from guinea pigs
- Turn on the computer, and enter the biological signal acquisition and processing system (see Table of Materials). Adjust the gain (50 mV), time constant (DC), filter (20 Hz), and scanning speed (1.00 s/div) after determining the connection channel (first channel, tension).
- Hang one end of the specimen on the L-shaped ventilation hook with the Petri dish and oxygen beside the Magnus bath. Hang the other end of the specimen on the JH-2 muscle force transducer (see Table of Materials).
- Observe the atrial systolic curve, adjust the preload to 0.5-1.0 g, and wait for it to stabilize (about 30 min).
NOTE: Change the K-H solution every 20 min. When normal, take the observed curve as the standard, and mark "normal". If the screen is scanned a little fast, this must be slowed down. - Administer 0.2 mL of low-calcium K-H solution, and observe for 5 min until the curve no longer declines.
- Administer 0.2 mL of 5% digoxin (see Table of Materials), observe for 5 min, wash three times, and then return to normal.
NOTE: Mark the administration. When the effect is obvious, scan the screen faster. When the curve no longer rises, that is, when the contraction amplitude no longer increases, wash three times quickly; otherwise, arrhythmia will occur, which will affect the results of the subsequent drug experiments. - Collect the data, and save it to a floppy disk.
Results
A decrease in myocardial contractility causes insufficient cardiac output, which leads to CHF (Figure 1). This protocol allowed the recording of the effects of different drugs on the systolic function of isolated right atria from guinea pigs and then the rapid screening of compounds from ethnic drugs that enhance myocardial contractility. After connecting the right atrium, the JH-2 muscle force transducer, and the biological signal acquisition and processing system in steps, the parameters were appropriately set, and the systolic curve was observed (Figure 2). The amplitude of the curve represents the intensity of right atrial contraction. The experiments were performed with four animals, and the data were analyzed with descriptive statistics and frequency distributions. As shown in Figure 4, a distinct curve appeared under normal conditions, and after the administration of 0.2 mL of low-calcium K-H solution, the curve gradually decreased until it was no longer changing, indicating that the contractility of the right atrium was weakened. However, after 0.2 mL of 5% digoxin was given, the curve began to rise gradually, suggesting that the contractility of the right atrium was enhanced.

Figure 1: Pathogenesis of CHF and the mechanism of action of digoxin. Myocardial lesions or increased cardiac preload and afterload decrease myocardial contractility and diastolic capacity, leading to insufficient cardiac output and, thus, resulting in CHF. This protocol aims to quickly screen compounds in ethnic drugs that can enhance myocardial contractility. In addition, when the sodium pump is inhibited by digoxin, the intracellular sodium level increases, and the calcium level in the myocardial cells rises, thus increasing the contractile force of the heart. Please click here to view a larger version of this figure.
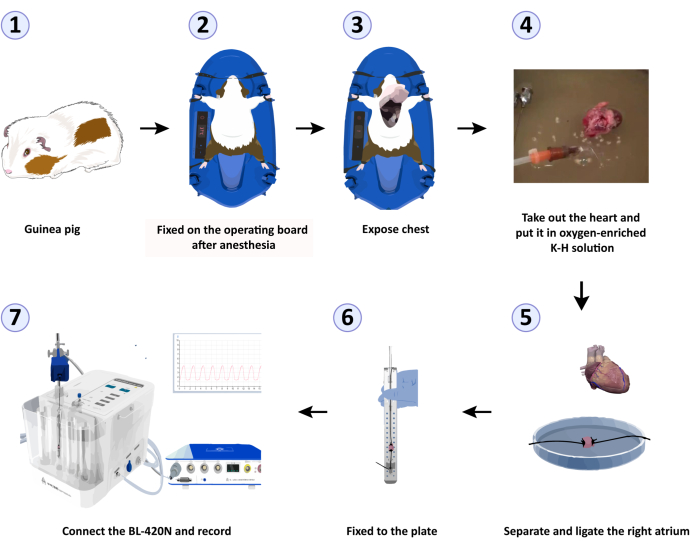
Figure 2: Experimental workflow for recording the systolic function of isolated right atria from guinea pigs. (1) A male Dunkin-Hartley guinea pig was taken. (2) The guinea pig was fixed on the operating board after anesthesia. (3) The guinea pig's chest was exposed. (4) The animal's heart and taken out and put in an oxygen-enriched K-H solution. (5) The right atrium was carefully separated, and its diagonal ends were ligated separately. (6) One end of the right atrium was hung on the L-shaped ventilation hook, and the other end was placed on the JH-2 muscle force transducer to secure it to the plate. (7) The JH-2 muscle force transducer was connected to the biological signal acquisition and processing system, the parameters were adjusted, the relevant solutions were administered, and the data were recorded. Please click here to view a larger version of this figure.

Figure 3: Representation of a guinea pig heart (dorsal aspect). Abbreviations: LA = left atrium; LV = left ventricle; RA = right atrium; RV = right ventricle. Please click here to view a larger version of this figure.
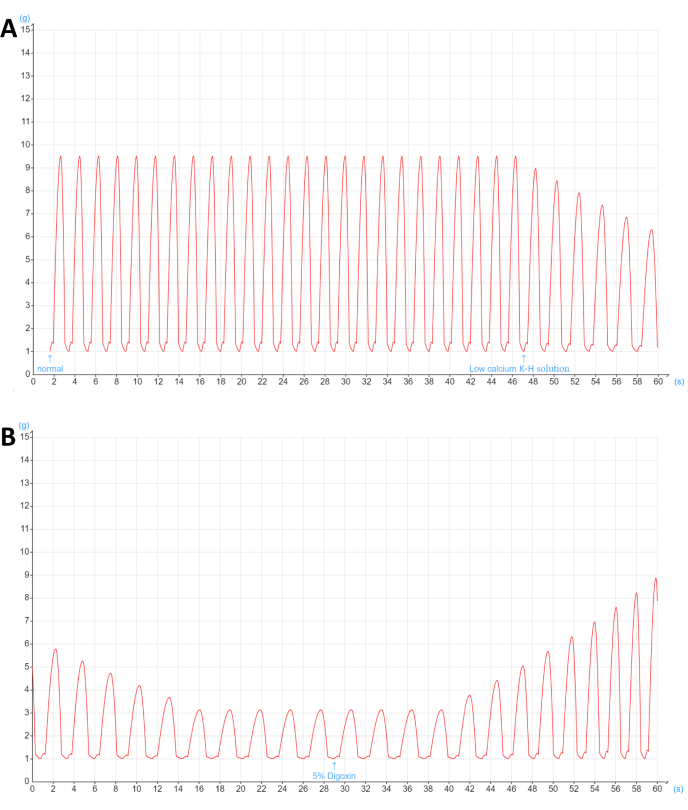
Figure 4: Effects of low-calcium K-H solution and 5% digoxin on the contractility of an isolated right atrium from a guinea pig. The contraction curve of the right atrium is shown with time (s) on the x-axis and tension (g) on the y-axis. (A) A distinct curve appeared under normal conditions, and after the administration of 0.2 mL of low-calcium K-H solution, the curve gradually decreased until it was no longer changing. (B) After 0.2 mL of 5% digoxin was given, the curve gradually rose. Please click here to view a larger version of this figure.
| K-H solution | ||
| Chemicals | g/1000 mL | Composition (mM) |
| NaCl | 7.02 | 120.0 |
| NaHCO3 | 2.10 | 25.0 |
| KCl | 0.30 | 4.0 |
| MgSO4 | 0.07 | 0.6 |
| NaH2PO4 | 0.07 | 0.6 |
| CaCl2 | 0.28 | 2.5 |
| Glucose | 1.98 | 11.0 |
| Low-calcium K-H solution | ||
| Chemicals | g/100mL | Composition (mM) |
| NaCl | 0.70 | 120.0 |
| NaHCO3 | 0.21 | 25.0 |
| KCl | 0.03 | 4.0 |
| MgSO4 | 0.01 | 0.6 |
| NaH2PO4 | 0.01 | 0.6 |
| CaCl2 | 0.01 | 0.8 |
| Glucose | 0.20 | 11.0 |
Table 1: Preparation of the K-H solution and the low-calcium K-H solution.
Discussion
The normal rhythmic activity of the heart requires a suitable physical and chemical environment, as does the activity of isolated right atria. Isolated right atria are isolated from the innervation of the body and the direct influence of systemic humoral factors, meaning changes in the activity of the right atria when changing the drugs they are exposed to can be observed. The fundamental causes of bioelectrical activity in excitable cells are changes in the ion permeability of the cell membrane and the subsequent diffusion of ions along the membrane19. Therefore, a change in ion concentration outside the membranes of cardiomyocytes significantly affects the bioelectrical activity and physiological characteristics of the cardiomyocytes. Among the ions, K+, Na+, and Ca2+ are the most important. When the blood K+ content is excessively high (more than 7.9 mmol/L), the myocardium's excitability, automaticity, conductivity, and contractility decline, which is reflected in impaired contractility, bradycardia, and conduction block. In severe cases, the heart may arrest in diastole20. When the blood's Ca2+ concentration is raised, the myocardium's ability to contract is improved. However, if the concentration of Ca2+ is too high, the ventricle can be arrested in systole. In this case, the blood's Ca2+ level may drop, and the myocardium's contractility may deteriorate21. Moreover, a small change in the Na+ level in the blood has no discernible impact on the myocardium; conversely, a big alteration is required for there to be an impact on the myocardium's physiological characteristics22.
Digoxin is a moderately potent cardiac glycoside that is commonly used to treat a variety of cardiac issues, such as congestive heart failure, atrial fibrillation or flutter, and certain arrhythmias, and it is one of the oldest drugs in cardiology23. It causes the Na+/K+-ATPase enzyme to be temporarily inhibited, which has a number of benefits. The Na+/K+-ATPase enzyme controls the entry and departure of sodium, potassium, and calcium to maintain the intracellular environment (indirectly). The sodium pump is another name for Na+/K+-ATPase. The sodium pump blockage caused by digoxin leads to an increase in the intracellular sodium and calcium levels in the myocardial cells, which enhances the heart's contractile force24. This increases the left ventricular ejection fraction, which is a crucial indicator of cardiac function25. However, myocardial contractility and heart depolarization are affected in low-calcium environments (Figure 4).
The guinea pig is a valuable biological model for evaluating drugs with expected cardiovascular effects26. The ion channels and currents in their cardiomyocytes are very similar to those expressed by and measured in human cardiomyocytes27. This protocol, which uses the isolated right atria of guinea pigs to screen drugs that can enhance myocardial contractility, is simple and easy to operate. After each administration, the systolic curves are observed for 5 min, and then the right atria are washed three times until normal. In particular, it is important to note that strophanthin-K should be given slowly and washed immediately after a slight effect to avoid toxic reactions in the right atria. A constant temperature bath was used throughout the experiment to maintain a 37 °C experimental environment and, thus, ensure good physiological activity in the right atria.
There are various benefits to the model presented here. The isolated guinea pig right atrium model represents a higher level of functional and structural integration than conventional cardiac muscle cells or whole heart tissue but also avoids the confounding complexities associated with in vivo models. Another great advantage observed in this model is the ability to administer multiple drugs at different times, which enables one to monitor the effect of various drugs on the myocardial contractility. Notably, the myocardial contraction values recorded in this model are derived from the longitudinal contraction of the cardiomyocytes. The longitudinal myocardial extension and contraction of the right atrial wall produce a longitudinal pulling motion on the atrioventricular ring28. Additionally, the model offers the benefits of ease of use (i.e., simple operation and a low cost), as stated earlier.
The limitations of this method include the inability to accurately record the effect of drugs on the heart rate because this is an in vitro experiment. Another limitation is the potential side effects of anesthesia if not properly performed. In this experiment, isoflurane inhalation anesthesia was adopted, despite its dose-dependent cardioprotective function. If used at a greater dosage, isoflurane appears to be potentially hazardous. As a result, its dosage must be taken into consideration, and adequate management of the isoflurane dose is needed when conducting experiments. Similarly, it is essential to remember that some steps are technically critical for obtaining reproducible and stable results. This includes quickly preparing the right atrium specimens, preferably within 3 min, to maintain normal systolic and diastolic activity. In order to protect the heart from injury, all preparations should use K-H solution at 37 °C and provide sufficient oxygen. Moreover, successful experiments critically depend on good manual handling during the preparation, isolation, and ligation of the right atrium, which requires practice.
In summary, a systematic and standardized protocol was developed for screening compounds that enhance myocardial contractility using isolated guinea pig right atria. This protocol is reliable and efficient and can serve as a methodological reference for screening the effective components of ethnic medicines in the treatment of CHF. For further studies investigating other potential molecular targets, computer simulations and surface plasmon resonance are both powerful tools for elucidating the mechanisms of drug-induced cardiac contraction29,30.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Ningxia Natural Science Foundation (Grant no. 2023AAC03620), the Scientific Research Project of the Higher Education Department of Ningxia (NYG2022030), and the National Natural Science Foundations of China (Grant no.82160816 and 82260797).
Materials
| Name | Company | Catalog Number | Comments |
| 4-0 surgical suture | Yangzhou Fuda Medical Devices Co., Ltd | ||
| 5% Digoxin (soluble in dimethyl sulfoxide) | TCI Shanghai | D1828 | CAS: 20830-75-5; Purity: >96.0% |
| BL-420N biological signal acquisition and processing system | Chengdu Tai Meng Software Co., Ltd | 1700142S | |
| CaCl2 | Shanghai yuanye Bio-Technology Co., Ltd | S24110 | CAS: 10043-52-4; Purity: 96% |
| Glucose | Shanghai yuanye Bio-Technology Co., Ltd | S11022 | CAS: 50-99-7; Purity: 99% |
| Isoflurane | RWD Life Science Co., Ltd | R510-22-16 | |
| JH-2 muscle force transducer | Institute of Aerospace Medical Engineering, Beijing, China | ||
| KCl | Shanghai yuanye Bio-Technology Co., Ltd | S24120 | CAS: 7447-40-7; Purity: 99.5% |
| Magnus bath | Shanghai Future Experimental Equipment Co., Ltd | L046525 | |
| MgSO4 | Shanghai yuanye Bio-Technology Co., Ltd | S24253 | CAS: 7487-88-9; Purity: 98% |
| NaCl | Shanghai yuanye Bio-Technology Co., Ltd | S24119 | CAS: 7647-14-5; Purity: 99.5% |
| NaH2PO4 | Shanghai yuanye Bio-Technology Co., Ltd | S24161 | CAS: 7558-80-7; Purity: 99% |
| NaHCO3 | Shanghai yuanye Bio-Technology Co., Ltd | S24153 | CAS: 144-55-8; Purity: 99.8% |
| Operating basin | Guangzhou Telekuan Medical Instrument Co., Ltd | 305 mm x 230 mm | |
| Ophthalmic forcep | Suzhou Shuanglu Medical Instrument Co., Ltd | ||
| Ophthalmic operating scissor | Suzhou Shuanglu Medical Instrument Co., Ltd | ||
| Paraffin | Leica Biosystems | 39601095 | |
| Petri dish | Corning | 430167 | 100 mm x 20 mm |
| Rodent anesthesia machine | Shanghai Yuyan Instruments Co., Ltd | ABS type (single channel) | |
| Scale | Shanghai Yueping Scientific Instrument Co., Ltd | YP1002 | |
| Surgical plate | Zhengzhou Ketai Experiment Equipment Co., Ltd | 21 cm x 31 cm | |
| Tissue scissor | Suzhou Shuanglu Medical Instrument Co., Ltd | SL0023 |
References
- Ziaeian, B., Fonarow, G. C. Epidemiology and aetiology of heart failure. Nature Reviews Cardiology. 13 (6), 368-378 (2016).
- Lu, Y. Z., Xia, N., Cheng, X. Regulatory T cells in chronic heart failure. Frontiers in Immunology. 12, 732794 (2021).
- Teerlink, J. R., et al. Omecamtiv mecarbil in chronic heart failure with reduced ejection fraction: Rationale and design of GALACTIC-HF. JACC Heart Failure. 8 (4), 329-340 (2020).
- Edelmann, F., et al. Chronic Heart Failure. Deutsches Arzteblatt international. 115 (8), 124-130 (2018).
- Ahmad, T., et al. Why has positive inotropy failed in chronic heart failure? Lessons from prior inotrope trials. European Journal of Heart Failure. 21 (9), 1064-1078 (2019).
- Hollman, A. Drugs for atrial fibrillation. Digoxin comes from Digitalis lanata. British Medical Journal. 312 (7035), 912 (1996).
- Whayne, T. F. Clinical use of Digitalis: A state of the art review. American Journal of Cardiovascular Drugs. 18 (6), 427-440 (2018).
- Malik, F. I., et al. Cardiac myosin activation: A potential therapeutic approach for systolic heart failure. Science. 331 (6023), 1439-1443 (2011).
- Kobayashi, Y., Hoshikuma, K., Nakano, Y., Yokoo, Y., Kamiya, T. The positive inotropic and chronotropic effects of evodiamine and rutaecarpine, indoloquinazoline alkaloids isolated from the fruits of Evodia rutaecarpa, on the guinea-pig isolated right atria: Possible involvement of vanilloid receptors. Planta Medica. 67 (3), 244-248 (2001).
- Irie, K., et al. Cardiotonic effect of Apocynum venetum L. extracts on isolated guinea pig atria. Journal of Natural Medicines. 63 (2), 111-116 (2009).
- Dai, G., et al. Effects of oxymatrine and matrine on left ventricular contractility using pressure-volume relationship analysis in anesthetized rats. European Journal of Pharmacology. 925, 175014 (2022).
- Chen, L., et al. The novel compound liguzinediol exerts positive inotropic effects in isolated rat heart via sarcoplasmic reticulum Ca2+ ATPase-dependent mechanism. Life Sciences. 91 (11-12), 402-408 (2012).
- Yu, J. B., Yang, S. S., Wang, X., Gan, R. T. Matrine improved the function of heart failure in rats via inhibiting apoptosis and blocking β3adrenoreceptor/endothelial nitric oxide synthase pathway. Molecular Medicine Reports. 10 (6), 3199-3204 (2014).
- Hasenfuss, G. Animal models of human cardiovascular disease, heart failure and hypertrophy. Cardiovascular Research. 39 (1), 60-76 (1998).
- Joukar, S. A comparative review on heart ion channels, action potentials and electrocardiogram in rodents and human: Extrapolation of experimental insights to clinic. Laboratory Animal Research. 37 (1), 25 (2021).
- Bers, D. . Excitation−Contraction Coupling and Cardiac Contractile Force. , (2001).
- Kembro, J. M., Aon, M. A., Winslow, R. L., O'Rourke, B., Cortassa, S. Integrating mitochondrial energetics, redox and ROS metabolic networks: a two-compartment model. Biophysical Journal. 104 (2), 332-343 (2013).
- James, E. B., Esther, M. B., Wagner, J. E., Manning, P. K. Chapter 6-Anatomy. The Biology of the Guinea Pig. , 53-62 (1976).
- García-Navarrete, M., Avdovic, M., Pérez-Garcia, S., Ruiz Sanchis, D., Wabnik, K. Macroscopic control of cell electrophysiology through ion channel expression. ELife. 11, e78075 (2022).
- Fauchier, J. P., Cosnay, P., Latour, F. Coeur et hyperkaliémie [The heart and hyperkalemia]. Archives des Maladies du Coeur et des Vaisseaux. 77, 23-33 (1984).
- Ke, H. Y., et al. Changes in cellular Ca2+ and Na+ regulation during the progression towards heart failure in the guinea pig. The Journal of Physiology. 598 (7), 1339-1359 (2020).
- Firth, J. M., Yang, H. Y., Francis, A. J., Islam, N., MacLeod, K. T. The effect of estrogen on intracellular Ca2+ and Na+ regulation in heart failure. JACC. Basic to Translational Science. 5 (9), 901-912 (2020).
- Patocka, J., Nepovimova, E., Wu, W., Kuca, K. Digoxin: Pharmacology and toxicology-A review. Environmental Toxicology and Pharmacology. 79, 103400 (2020).
- Mangoni, M. E., Nargeot, J. Genesis and regulation of the heart automaticity. Physiological Reviews. 88 (3), 919-982 (2008).
- Ziff, O. J., Kotecha, D. Digoxin: The good and the bad. Trends in Cardiovascular Medicine. 26 (7), 585-595 (2016).
- Bartakova, A., Novakova, M., Stracina, T. Anesthetized guinea pig as a model for drug testing. Physiological Research. 71, S211-S218 (2022).
- Varró, A., Lathrop, D. A., Hester, S. B., Nánási, P. P., Papp, J. G. Ionic currents and action potentials in rabbit, rat, and guinea pig ventricular myocytes. Basic Research in Cardiology. 88 (2), 93-102 (1993).
- Wang, K., Ho, S. Y., Gibson, D. G., Anderson, R. H. Architecture of atrial musculature in humans. British Heart Journal. 73 (6), 559-565 (1995).
- Wang, X. B., et al. Salidroside, a phenyl ethanol glycoside from Rhodiola crenulata, orchestrates hypoxic mitochondrial dynamics homeostasis by stimulating Sirt1/p53/Drp1 signaling. Journal of Ethnopharmacology. 293, 115278 (2022).
- Hou, Y., et al. Salidroside intensifies mitochondrial function of CoCl2-damaged HT22 cells by stimulating PI3K-AKT-MAPK signaling pathway. Phytomedicine. 109, 154568 (2023).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved