Method Article
Construction, Characterization, and Regenerative Application of Self-Assembled Human Mesenchymal Stem Cell Aggregates
In This Article
Summary
This study describes a method to construct aggregates based on the self-assembly of human mesenchymal stem cells and identifies the morphological and histological characteristics for the regenerative treatment of cranial bone defects.
Abstract
Mesenchymal stem cells (MSCs), characterized by their self-renewal ability and multilineage differentiation potential, can be derived from various sources and are emerging as promising candidates for regenerative medicine, especially for regeneration of the tooth, bone, cartilage, and skin. The self-assembled approach of MSC aggregation, which notably constructs cell clusters mimicking the developing mesenchymal condensation, allows high-density stem cell delivery along with preserved cell-cell interactions and extracellular matrix (ECM) as the microenvironment niche. This method has been shown to enable efficient cell engraftment and survival, thus promoting the optimized application of exogenous MSCs in tissue engineering and safeguarding clinical organ regeneration. This paper provides a detailed protocol for the construction and characterization of self-assembled aggregates based on umbilical cord mesenchymal stem cells (UCMSCs), as well as an example of the cranial bone regenerative application. The implementation of this procedure will help guide the establishment of an efficient MSC transplantation strategy for tissue engineering and regenerative medicine.
Introduction
Mesenchymal stem cell (MSC) condensation is an essential stage to ensure the normal growth and development of the body in early organogenesis1,2, especially in the formation of bone, cartilage, teeth, and skin1,3,4. In the last few decades, tissue engineering therapies using cultured postnatal MSCs combined with biodegradable scaffolds have made important advances in osteogenic5 and cartilaginous regeneration6. However, the use of scaffolds may have some disadvantages, such as immune rejection, as well as low cellular affinity and plasticity7. In this regard, we have investigated the feasibility of applying a spheroid cell culture method to provide scaffold-free self-assembled aggregates mimicking the developing condensation phenomenon, which contain only MSCs and the deposited extracellular matrix (ECM)8. The formation of aggregates increases applicative plasticity to match the defect shape and avoids scaffold implantation and digestion by proteolytic enzymes to harvest MSCs for transplantation9.
MSC aggregates have been used widely for regeneration of the bone, dental pulp, periodontium, and skin10, among other tissue and organs. Many different types of MSCs can be selected as candidates for seed cells, including but not limited to bone marrow MSCs (BMMSCs), umbilical cord MSCs (UCMSCs), adipose tissue-derived stromal cells (ADSCs), and dental MSCs (e.g., dental pulp mesenchymal stem cell [DPSCs], mesenchymal stem cells from the deciduous teeth [SHED]11, and periodontal mesenchymal stem cells [PDLSCs])12. Many technologies for three-dimensional cell clusters have been developed in the past decade, including assisted and self-assembled aggregation. However, assisted aggregation approaches are often weak in producing ECMs and forming homogeneous and tight aggregates, and are therefore not suitable for mimicking physiological conditions13,14,15. Moreover, some assisted aggregation methods require cell-material interactions to form stable structures16,17,18,19, whereas this self-assembled aggregation method is generally available for a wide range of MSCs. Notably, in our recent clinical trials, MSC aggregates have been successfully used to regenerate the pulp-dentin complex and the periodontium after implantation into injured human incisor teeth, which have achieved de novo tissue regeneration with physiological structure and function20,21.
This paper provides a thorough procedure for MSC aggregate construction and characterization, as well as in vivo transplantation. This approach will attract the attention of researchers when they aim to repair defects in tissues, such as the teeth, bone, cartilage, and skin, based on stem cell applications. This method is simple, convenient, and completely composed of cells and ECM without additional scaffolds, which can be cultured for a long time to obtain dense and stable aggregates22. Meanwhile, the aggregates cultured in this way are rich in ECM, which mimics the developing niche for these high-density cells and thus promotes tissue regeneration23. The construction process can be divided into two stages: cell preparation and culture, and self-assembled formation and harvest of cell aggregates. The characterization of aggregates includes morphological identification via an inverted optical microscope and a scanning electron microscope (SEM), and histological analysis via hematoxylin and eosin (HE) and Masson's staining. The formed aggregates were demonstrated for regenerative implantation to repair the cranial bone defect. The implementation of this procedure will help guide the establishment of an efficient MSC transplantation strategy for tissue engineering and regenerative medicine.
Protocol
NOTE: All animal procedures were approved by the Animal Care and Use Committee of the Fourth Military Medical University and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Cryopreserved human UCMSCs that were obtained from a commercial source were used for the present study (see Table of Materials). The use of human cells was approved by the Ethics Committee of the Fourth Military Medical University. UCMSCs were taken as an example to describe the procedure. The cranial defect was taken as an example for showing the need of repair to describe the implantation procedure. All the experiments were repeated three times.
1. Construction of UCMSC aggregates
- Preparation and culture of UCMSCs
- Add 10 mL of prewarmed phosphate-buffered saline (PBS) into a new 15 mL centrifugal tube in a sterile biosafety cabinet.
- Remove the freezing tube containing frozen UCMSCs from liquid nitrogen and quickly place it into a 37 °C water bath. Shake the tube gently to facilitate rapid and thorough cell thawing.
CAUTION: The whole procedure must be finished within 1 min, because dimethyl sulfoxide (DMSO) in the freezing solution may cause cytotoxicity. - Slightly suspend the cells in the freezing tube and transfer all the contents into the centrifuge tube containing 10 mL of PBS (step 1.1.1). Centrifuge the sample at 100 × g for 5 min. Discard the supernatant and wash the cells with 10 mL of PBS.
- Centrifuge the sample at 100 × g for 5 min. Discard the supernatant, and gently resuspend the cells with complete alpha-minimum essential medium (α-MEM) containing 10% fetal bovine serum (FBS), 1% penicillin-streptomycin, and 584 mg/L glutamine.
- Seed the resuspended cells in 10 cm culture dishes (often one to two dishes for one tube, depending on the total amount of cells after centrifugation, at a density of 5 × 106 cells per dish). Add complete α-MEM medium in the dishes to a total volume of 10 mL.
- Gently shake the dishes to distribute the cells. Incubate the dishes at 37 °C with 5% CO2 in a humidified chamber. After overnight incubation,discard the supernatant medium and add 5 mL of PBS 1-2x to remove the nonadherent cells. Add 10 mL of fresh complete α-MEM medium. Change the culture media every 2 days.
- For cell passaging, when the cells reach 80%-90% confluence, discard the supernatant and wash the cells with 5 mL of PBS to remove the medium thoroughly. Incubate the cells in 2 mL of 0.25% trypsin-1 mM ethylenediaminetetraacetic acid (EDTA) at 37 °C for 2 min.
- When the cells become round with broken intercellular junctions under a microscope, separate the cells from the dishes without too much disruption of the cell structure. Neutralize the trypsin by adding 4 mL of complete α-MEM medium.
- Collect the cells from the dishes repeatedly and gently by pipetting in a fresh 15 mL centrifuge tube. Centrifuge the sample at 100 × g for 5 min. Discard the supernatant, resuspend the cells with complete α-MEM medium, and seed them in fresh culture dishes or well plates. Change the culture media every 2 days.
NOTE: According to the size of the defect area, a 25 mm3 defect requires the use of a well of 6-well plate cells cultured to form aggregates.
- Formation and harvest of aggregates
- Prepare cell aggregate-inducing media by adding 50 µg/mL vitamin C into α-MEM with 10% FBS, 1% penicillin-streptomycin, and 584 mg/L glutamine.
CAUTION: Direct light exposure should be avoided when using vitamin C, as vitamin C oxidizes when exposed to light and becomes ineffective. - When the cells in step 1.1.12 reach 95% confluence (Supplemental Figure S1), change the media to cell aggregate-inducing media; change fresh cell aggregate-inducing media every 2 days.
- After 7-10 days of inducing (the length of inducing time depends on the stem cell types and is often ~7 days for the edge of UCMSCs), look for shaped aggregates under the microscope, with thick edges separated from the dish bottom.
- Discard the culture media and gently wash the cells 2x with PBS. Gently push the aggregates from the edge to the middle using small sterile forceps, folding the aggregates.
- On some occasion, UCMSCs can spontaneously curl toward the center to form a solid condensation. This structure is equivalent to the aggregate obtained in step 1.2.4.
- Prepare cell aggregate-inducing media by adding 50 µg/mL vitamin C into α-MEM with 10% FBS, 1% penicillin-streptomycin, and 584 mg/L glutamine.
2. Morphological identification of aggregates
- General and microscopic observation
- Observe that the aggregates are dense and integrated and are not easily damaged by mechanical forces, such as pulling during detachment and transplantation.
- Under a microscope, look for a membrane-like structure of a certain thickness with woven pattern.
- Live/dead cell staining
- Seed the cells in 6-well plate at a density of 1 × 106 cells per well.
- Prepare 2 µM calcein AM and 4 µM EthD-1 working solution and using PBS.
- Add 0.3% triton to the cells for 5 min, wash with PBS three times to induce the cells to death, as a control for staining.
- Wash the cells, aggregates, and dead-inducing cells with PBS twice.
- Add 100 µL of PBS and 100 µL of prepared staining solution in step 2.2.2 to each well.
- Incubate the sample for 30 min at RT. Wash the sample with PBS twice.
- Add 200 µL of 10 µg/mL Hoechst to each well and incubate the sample for 5 min at RT. Wah the sample with PBS twice.
- Observe under a fluorescence microscope.
- SEM observation
- Seed and induce cell the aggregates obtained in step 1.2.4 or 1.2.5. Wash the cells with PBS after the aggregates are formed in step 1.2.4. or 1.2.5.
- Fix the cells using glutaraldehyde for at least 4 h.
- Wash the samples with PBS, then dehydrate through a gradient concentration of ethanol from 30%, 50%, 70%, 80%, 90%, to 100%, each for 5 min. Treat 2x with anhydrous ethanol.
- Immerse the aggregates in 3 mL of hexamethyldisilazane for 30 min. Aspirate all fluid and naturally dry the samples thoroughly.
CAUTION: The whole process needs to be carried out in a fume hood because hexamethyldisilazane is volatile and highly toxic. - Stick the dried samples onto the sample table with double-sided carbon tape and coat the surface with metal. Store the sample at room temperature (RT) for several days.
- Observe under an SEM.
- Replace the sample inside the machine. Adjust the machine voltage to 5 k, and the focal length to ~12 mm. Adjust the focus bar until a clear picture appears.
- Capture the images at low magnification. Adjust the magnification and refocus to obtain a higher magnification image.
CAUTION: The surface of the aggregates cannot be touched before observation to avoid destruction of surface morphology.
3. Histological analysis of aggregates
- Fixation and sectioning of samples
- Fix the aggregates obtained in step 1.2.4 or 1.2.5with 4% paraformaldehyde at 4 °C for at least 24 h. After the fixation, wash with PBS and place the sample into embedding cassettes overnight to rinse with running water.
- Dehydrate the samples using automatic dehydration machines through gradient ethanol, xylene, and paraffin, following the manufacturer's instructions. The whole process is as follows: 85% ethanol for 2 h at RT, 95% ethanol for 2 h at RT, 95% ethanol for 2 h at RT, 100% ethanol for 2 h at RT, 100% ethanol for 1.5 h at RT, xylene for 45 min at RT, xylene for 45 min at RT, paraffin for 30 min at RT, paraffin for 1.5 h at RT, and finally paraffin for 2 h at 60 °C.
- Embed the samples with paraffin. Prepare approximately 5 µm thick sections, adhere them to cationic slides, and dry the slides for 2 h at 45 °C.
- Dewax sequentially through xylene for 15 min, twice, and 100%, 90%, 80%, and 70% ethanol (each for 5 min).
- Slowly rinse the slides 3x with running water for 5 min.
- HE staining
- Stain with hematoxylin for 3-5 min, rinse with water, and carefully remove excess fluid around the tissue.
- Fractionate with ethanol containing 1% hydrochloric acid for 3-5 s and rinse with running water, during which the color becomes a little lighter and blue. Remove excess water around the tissue.
- Stain with eosin for 1 min, rinse with running water, and remove excess fluid around the tissue.
- Dehydrate with 70%, 80%, 90%, and 100% ethanol for 10 s each, xylene for 15 min, twice, and dry naturally in the fume hood before sealing the sheet.
- Add neutral resin drops and seal the slides with coverslips. Observe under a light microscope.
- Masson's trichrome staining
- Stain with Weigert's iron hematoxylin for 5 min, rinse with water, and wipe dry.
- Fractionate with ethanol containing 1% hydrochloric acid for 3-5 s and rinse with running water, during which the color becomes a little lighter and blue. Wipe the surface dry.
- Stain with Lixin red acidic magenta solution for 5-10 min and rinse quickly with distilled water.
- Treat with phosphomolybdic acid aqueous solution for 3-5 min.
- Directly re-stain with aniline blue solution for 5 min without washing with water.
- Treat with 1% glacial acetic acid for 1 min.
- Dehydrate with 70%, 80%, 90%, and 100% ethanol for 10 s each, xylene for 15 min, twice, and dry naturally in the fume hood before sealing the sheet.
- Add neutral resin drops and seal the slides with coverslips. Observe under a light microscope.
4. Implantation
- Anesthetize the nude mouse by an intraperitoneal injection of 50 mg/kg pentobarbital sodium.
NOTE: Confirm the mice are properly anesthetized based on the absence of corneal reflex and limb reaction when the footpad is pinched. Apply eye ointment to prevent dryness. - Cut out a transverse incision (~3-4 cm) in the skin behind the ears of the nude mouse with sterile small scissors after disinfection with iodophor.
- Pull the skin forward to expose the cranium. Lightly draw the edges of the planned defect area with an 11# blade and remove the bone fragment. The defect area is a circular area of approximately 4 mm in diameter. Use saline to flush the wound and provide adequate hemostasis.
- Dip the aggregates obtained in step 1.2.5 or 1.2.6 with sterile gauze to absorb surface moisture before implantation. Place the aggregates into the area and slightly pressurize to fill the defect.
- Replace the skin after pulling to make sure the implant is fixed. Close the incision with 1/2, 4 × 10 angled stitches and 4-0 sutures.
- After the surgery, place the animals on the rewarming blanket until they are active and then return them into the cage.
NOTE: The animals must not be left unattended until they regain sufficient consciousness to maintain sternal recumbency. The animals that have undergone surgery are not returned to the company of other animals until fully recovered.
Results
Aggregates can be successfully constructed from UCMSCs according to the experimental workflow (Figure 1). The quality of aggregates must be evaluated prior to use, via morphological observation and histological analysis. The lamellar structure formed should be complete and dense, with the cells interlaced to form a woven pattern by microscopic observation (Figure 2A). Edge curling can be discovered during aggregation; overcurling edges indicate unsuccessful aggregation leaving cellular gaps (Figure 2B). The final aggregates can be viewed by visual inspection and easily detached (Figure 2C). The aggregates can be observed live using live/dead staining agent (Figure 2D). The cytoplasm of living cells can be stained by calcein AM and appear green, while the nuclei of dead cells can be stained by EthD-1 and appear red. All cell nuclei can be stained by Hoechst and appear blue. As analyzed under a SEM, the surface scanning shows that the aggregates have a normal cellular structure and are abundant in ECM (Figure 3A), while the cross-section scan indicates that the typical morphological characteristics of aggregates of a certain thickness containing a few layers of cells (Figure 3B). HE and Masson's staining show that cells form laminated structures within the aggregates, with a certain amount of space, which may facilitate the penetration of nutritional fluids (Figure 4A). Meanwhile, aggregates indeed contain abundant ECM composed of collagen fibers (Figure 4B). The aggregates can be implanted into cranial bone defects for regenerative applications (Figure 5).

Figure 1: Illustration of the protocol to construct MSC aggregates. The upper image illustrates the whole process of aggregate construction, including cell culture, induction, and harvest. The lower image in the dashed rectangle illustrates that the aggregates can be applied to regenerate skin wounds, bone defects, and dental diseases. Abbreviation: MSC = mesenchymal stem cell. Please click here to view a larger version of this figure.

Figure 2: Morphological identification of the MSC aggregates. (A) Cell morphology under a microscope. Left: UCMSCs show a spindle shape 24 h after seeding. Middle: cells reach 80%-90% confluence after culturing for 48-72 h. Right: cells form a compact, interwoven pattern after inducing for 48 h. Scale bars = 500 µm. (B) Edge morphology under a microscope. Normal edge (left), curling edge (middle), overcurling edge with cellular gaps (right) can be observed. Scale bars = 500 µm. Yellow arrow: cell curling edges. Black arrow: intercellular gap without cells. (C) The formed aggregate is integrated and dense, and easily detached from the dishes or well plates. Abbreviation: MSC = mesenchymal stem cell. (D) Live/dead staining. Cells (left), aggregates (middle), dead-inducing cells stained with calcein AM and EthD-1 (right). Green: calcein AM; red: EthD-1; blue: Hoechst. Scale bar = 100 µm. Please click here to view a larger version of this figure.
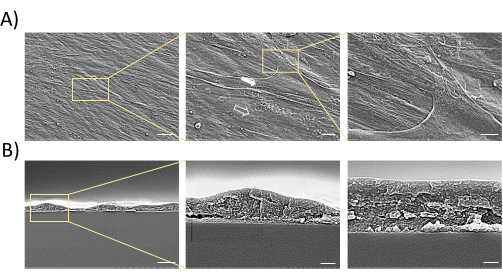
Figure 3: Morphological observation of MSC aggregates by SEM. (A) SEM observation demonstrates aggregates with a certain thickness, multiple layers, and a large amount of ECM. Solid arrow: cells; hollow arrow: ECM. Scale bars = 30 µm (left), 6 µm (middle), and 3 µm (right). (B) Cross-section scan of aggregates. The cross-section scan illustrates aggregates with a certain thickness and multiple layers of cells. Scale bars = 3 µm (left), 0.6 µm (middle), and 0.3 µm (right). Abbreviations: MSC = mesenchymal stem cell; SEM = scanning electron microscopy; ECM = extracellular matrix. Please click here to view a larger version of this figure.
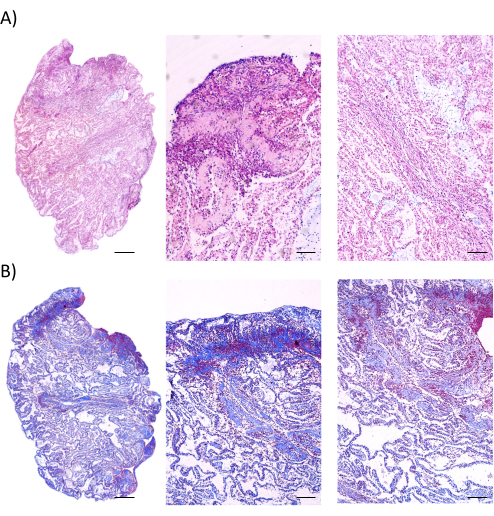
Figure 4: Histological analysis of MSC aggregates. (A) HE staining implies continuous cell layers within the aggregates. Scale bars = 500 µm (left), 100 µm (middle), and 200 µm (right). (B) Masson's staining demonstrates rich and compact ECM containing collagen deposited in the aggregates. Scale bars = 500 µm (left), 100 µm (middle), and 200 µm (right). Abbreviations: MSC = mesenchymal stem cell; ECM = extracellular matrix; HE = hematoxylin and eosin. Please click here to view a larger version of this figure.

Figure 5: An example of a procedure demonstrating surgical implantation of aggregates for the repair of cranial bone defect. (A) Exposing the cranium. (B) Making a defect about 4 mm in diameter. (C) Implanting the MSC aggregates. (D) Representative micro computed tomography (micro-CT) image of the cranial bone defect treated with PBS after 7 days of modeling. (E) Representative micro-CT image of the cranial bone defect treated with MSC aggregates after 7 days of modeling. Abbreviation: MSC = mesenchymal stem cell. Please click here to view a larger version of this figure.
Supplemental Figure S1: Cells reaching 80%-90% confluence. Please click here to download this File.
Discussion
With the advances of tissue engineering biotechnology, strategies to construct an implantable structure with high plasticity and containing long-term-surviving cells that can achieve optimal regeneration have been the focus of many scientists. There are a variety of current implantation methods of MSCs, such as cell-only methods, scaffolds complemented with cytokines6,24, or the combination of stem cells and scaffolds5. This paper presents an efficient method of MSC aggregate construction and implantation, which involves cellular self-assembly to form a condensed cluster induced by specific media, with the advantage of an abundant cell ECM that is convenient to harvest and apply. The deposited ECM acts as a natural scaffold to enhance the exchange of biomolecules between the host and the implanted aggregates, which promote the delivery and the engraftment of MSCs25,26. Obtaining aggregates in this way, the cells can be tightly encapsulated by the naturally deposited ECM, which provides a more intact and physiologically mimetic cell niche for tissue regeneration27,28. In our protocol, we use live/dead cell staining to characterize the viability of the aggregates. Besides this, metabolic activity such as WST-1 can also be applied to verify the viability of the aggregate. The cranial bone regenerative effect of the method can be examined by micro-CT and histological staining. Furthermore, this protocol has been applied clinically, and the implanted aggregates have achieved the regeneration of dental pulp21.
There are some critical points in the procedure. First, cellular senescence as well as poor cellular function can be evidenced by a slow proliferation rate and flattened cell morphology of MSCs instead of a spindle-shaped morphology29. These characteristics will suppress the formation of aggregates. Second, the coherence and denseness of the polymeric matrix is important in the determination of successful construction. During harvest, if the aggregates are found to be less ductile, it implies that the ECM of the aggregates is not dense enough to facilitate regeneration. Third, the aggregates must be moist before implantation, but without excess fluid, so as to avoid the production of cracks inside the ECM or the impairment of cell adhesion30. However, since no scaffold is added to these aggregates, the application is certainly limited in the regeneration of large area defects.
Conventionally, we routinely use vitamin C as an induction reagent, as it can not only promote the proliferation and differentiation of MSCs but also enhance the formation of the ECM31. Vitamin C is an antioxidant that can promote the quality of secreted collagen, as well as induce telomerase activity in MSCs, which may improve the cell regenerative capacity32,33,34. In addition to the application of vitamin C for aggregate induction, other small-molecule drugs can be added in this procedure to achieve optimal effects. For instance, Osthole, a compound extracted from Chinese herbs, was applied and improved cell aggregate formation, enhancing the osteogenesis of PDLSCs even under the circumstances of periodontitis35. In another preclinical study, resveratrol (RSV), a natural phytoalexin, enhanced the regenerative potential of aggregates of both control MSCs and periodontitis-patient-derived MSCs36. In addition, a melatonin-based strategy was able to prevent cellular senescence and preserve the self-renewal of BMMSCs and differentiation properties of aggregates after long-term passaging29. Moreover, licochalcone A (LA) improved the osteogenic differentiation and mineralized the formation potential of BMMSC aggregates in treating osteoporotic bone fractures37. In subsequent experiments, aggregates formed by composite cell sources should be established to achieve more favorable regeneration effects in complicated tissue defects, such as incorporating endothelial cells for improved angiogenesis38,39.
In summary, this protocol provides a basic guide to construct and characterize aggregates based on MSCs, including the analysis of morphological characterization and histological features, as well as the regenerative practice. The protocol will help establish a basis for further regenerative experiments.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81930025, 82100969, and 82071075) and the National Key Research and Development Program of China (2022YFA1104400 and 2021YFA1100600). We are grateful for the assistance of the National Experimental Teaching Demonstration Center for Basic Medicine (AMFU).
Materials
| Name | Company | Catalog Number | Comments |
| 0.25% Trypsin-EDTA (1x) | Sigma | T4049 | Cell passage |
| Automatic Dehydration Machine | LEICA | ASP200s | Dehydrate aggregate |
| Centrifuge | Eppendorf | 5418R | Centrifugation |
| Centrifuge tube | Thermo Nunc | 339650 | Centrifugation |
| Culture dish | Thermo | 150466 | Culture of UCMSCs |
| Ethanol | SCR | 10009218 | Dehydrate aggregate |
| Fatal bovine serum | Sijiqing | 11011-8611 | Culture of UCMSCs |
| Forcep | JZ | JD1080 | Harvest aggregate |
| Glutaraldehyde | Proandy | 10217-1 | Fixation of aggregate |
| Hematoxylin and Eosin Staining Kit | beyotime | C0105S | HE staining |
| Hexamethyldisilazane | SCR | 80068416 | Dry aggregate surface |
| Hoechst33342 | Sigma | 14533 | Cell nuclei stain |
| L-glutamine | Sigma | G5792 | Culture of UCMSCs |
| Live/dead Viability/Cytotoxicity Kit | Invitrogen | L3224 | Live/dead cell stain |
| Masson's Staining Kit | ZHC | CD069 | Masson Staining |
| Minimum Essential Medium Alpha basic (1x) | Gibco | C12571500BT | Culture of UCMSCs |
| Paraffin | Leica | 39601006 | Tissue embedding |
| Paraformaldehyde | Saint-Bio | D16013 | Fixation of aggregate |
| PBS (1x) | Meilunbio | MA0015 | Resuspend and purify UCMSCs |
| Penicillin/Streptomycin | Procell Life Science | PB180120 | Culture of UCMSCs |
| Pentobarbital sodium | Sigma | P3761 | Animal anesthesia |
| Polysporin | Pfizer | Prevent eye dry | |
| Scanning Electron Microscope | Hitachi | s-4800 | SEM observation |
| Scissor | JZ | Y00030 | Animal surgical incision |
| Six-well plate | Thermo | 140675 | Culture of UCMSCs |
| Stitch | Jinhuan | F603 | Close wounds |
| Suture | Xy | 4-0 | Close wounds |
| Thermostatic equipment | Grant | v-0001-0005 | Water bath |
| UCMSCs | Bai'ao | UKK220201 | Commercially UCMSCs |
| Vitamin C | Diyibio | DY40138-25g | Aggregate inducing |
| Xylene | SCR | 10023418 | Dehydrate aggregate |
References
- Hall, B. K., Miyake, T. Divide, accumulate, differentiate: cell condensation in skeletal development revisited. The International Journal of Developmental Biology. 39 (6), 881-893 (1995).
- Hall, B. K., Miyake, T. All for one and one for all: condensations and the initiation of skeletal development. Bioessays. 22 (2), 138-147 (2000).
- Salhotra, A., Shah, H. N., Levi, B., Longaker, M. T. Mechanisms of bone development and repair. Nature Reviews: Molecular Cell Biology. 21 (11), 696-711 (2020).
- Mammoto, T., et al. Mechanochemical control of mesenchymal condensation and embryonic tooth organ formation. Developmental Cell. 21 (4), 758-769 (2011).
- Khanarian, N. T., Jiang, J., Wan, L. Q., Mow, V. C., Lu, H. H. A hydrogel-mineral composite scaffold for osteochondral interface tissue engineering. Tissue Engineering. Part A. 18 (5-6), 533-545 (2012).
- Liu, M., et al. Injectable hydrogels for cartilage and bone tissue engineering. Bone Research. 5, 17014 (2017).
- Ai, C., et al. Osteochondral tissue engineering: Perspectives for clinical application and preclinical development. Journal of Orthopaedic Translatation. 30, 93-102 (2021).
- Huang, X., et al. Microenvironment influences odontogenic mesenchymal stem cells mediated dental pulp regeneration. Frontiers in Physiology. 12, 656588 (2021).
- Li, M., Ma, J., Gao, Y., Yang, L. Cell sheet technology: a promising strategy in regenerative medicine. Cytotherapy. 21 (1), 3-16 (2019).
- An, Y., et al. marrow mesenchymal stem cell aggregate: an optimal cell therapy for full-layer cutaneous wound vascularization and regeneration. Scientific Reports. 5, 17036 (2015).
- Miura, M., et al. SHED: stem cells from human exfoliated deciduous teeth. Proceedings of the National Academy of Sciences. 100 (10), 5807-5812 (2003).
- Gronthos, S., et al. Stem cell properties of human dental pulp stem cells. Journal of Dental Research. 81 (8), 531-535 (2002).
- Chen, H., et al. Regeneration of pulpo-dentinal-like complex by a group of unique multipotent CD24a(+) stem cells. Science Advances. 6 (15), (2020).
- Yang, T., et al. hDPSC-laden GelMA microspheres fabricated using electrostatic microdroplet method for endodontic regeneration. Materials Science & Engineering. C: Materials for Biological Applications. 121, 111850 (2021).
- Foty, R. A simple hanging drop cell culture protocol for generation of 3D spheroids. Journal of Visualized Experiments. (51), 2720 (2011).
- Nakao, K., et al. The development of a bioengineered organ germ method. Nature Methods. 4 (3), 227-230 (2007).
- Hasani-Sadrabadi, M. M., et al. An engineered cell-laden adhesive hydrogel promotes craniofacial bone tissue regeneration in rats. Science Translational Medicine. 12 (534), (2020).
- Fan, L., et al. Gravitational sedimentation-based approach for ultra-simple and flexible cell patterning coculture on microfluidic device. Biofabrication. 12 (3), 035005 (2020).
- Singh, R., et al. Cell specificity of magnetic cell seeding approach to hydrogel colonization. Journal of Biomedical Materials Research: Part A. 105 (11), 2948-2957 (2017).
- Guo, H., et al. Odontogenesis-related developmental microenvironment facilitates deciduous dental pulp stem cell aggregates to revitalize an avulsed tooth. Biomaterials. 279, 121223 (2021).
- Xuan, K., et al. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Science Translational Medicine. 10 (455), (2018).
- Sui, B. D., et al. Stem cell-based bone regeneration in diseased microenvironments: Challenges and solutions. Biomaterials. 196, 18-30 (2019).
- Shang, F., et al. Human umbilical cord MSCs as new cell sources for promoting periodontal regeneration in inflammatory periodontal defect. Theranostics. 7 (18), 4370-4382 (2017).
- Arnold, A. M., Holt, B. D., Daneshmandi, L., Laurencin, C. T., Sydlik, S. A. Phosphate graphene as an intrinsically osteoinductive scaffold for stem cell-driven bone regeneration. Proceedings of the National Academy of Sciences. 116 (11), 4855-4860 (2019).
- Wu, M., et al. SHED aggregate exosomes shuttled miR-26a promote angiogenesis in pulp regeneration via TGF-beta/SMAD2/3 signalling. Cell Proliferation. 54 (7), e13074 (2021).
- Li, Z., et al. Apoptotic vesicles activate autophagy in recipient cells to induce angiogenesis and dental pulp regeneration. Molecular Therapy. 30 (10), 3193-3208 (2022).
- Vogel, V. Unraveling the mechanobiology of extracellular matrix. Annual Review of Physiology. 80, 353-387 (2018).
- Huebsch, N., et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nature Materials. 9 (6), 518-526 (2010).
- Shuai, Y., et al. Melatonin treatment improves mesenchymal stem cells therapy by preserving stemness during long-term in vitro expansion. Theranostics. 6 (11), 1899-1917 (2016).
- Sui, B. D., Zhu, B., Hu, C. H., Zhao, P., Jin, Y. Reconstruction of regenerative stem cell niche by cell aggregate engineering. Methods in Molecular Biology. 2002, 87-99 (2019).
- Wei, F., et al. Vitamin C treatment promotes mesenchymal stem cell sheet formation and tissue regeneration by elevating telomerase activity. Journal of Cellular Physiology. 227 (9), 3216-3224 (2012).
- de Clerck, Y. A., Jones, P. A. The effect of ascorbic acid on the nature and production of collagen and elastin by rat smooth-muscle cells. The Biochemical Journal. 186 (1), 217-225 (1980).
- Fujisawa, K., et al. Evaluation of the effects of ascorbic acid on metabolism of human mesenchymal stem cells. Stem Cell Research & Therapy. 9 (1), 93 (2018).
- Wang, T., et al. The histone demethylases Jhdm1a/1b enhance somatic cell reprogramming in a vitamin-C-dependent manner. Cell Stem Cell. 9 (6), 575-587 (2011).
- Sun, J., et al. Osthole improves function of periodontitis periodontal ligament stem cells via epigenetic modification in cell sheets engineering. Scientific Reports. 7 (1), 5254 (2017).
- Wang, Y. J., et al. Resveratrol enhances the functionality and improves the regeneration of mesenchymal stem cell aggregates. Experimental and Molecular. 50 (6), 1-15 (2018).
- Shang, F., et al. The effect of licochalcone A on cell-aggregates ECM secretion and osteogenic differentiation during bone formation in metaphyseal defects in ovariectomized rats. Biomaterials. 35 (9), 2789-2797 (2014).
- Dissanayaka, W. L., Zhu, L., Hargreaves, K. M., Jin, L., Zhang, C. Scaffold-free prevascularized microtissue spheroids for pulp regeneration. Journal of Dental Research. 93 (12), 1296-1303 (2014).
- Dissanayaka, W. L., Zhu, L., Hargreaves, K. M., Jin, L., Zhang, C. In vitro analysis of scaffold-free prevascularized microtissue spheroids containing human dental pulp cells and endothelial cells. Journal of Endodontics. 41 (5), 663-670 (2015).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved