Introduction to Mass Spectrometry
Overview
Source: Laboratory of Dr. Khuloud Al-Jamal - King's College London
Mass spectrometry is an analytical chemistry technique that enables the identification of unknown compounds within a sample, the quantification of known materials, the determination of the structure, and chemical properties of different molecules.
A mass spectrometer is composed of an ionization source, an analyzer, and a detector. The process involves the ionization of chemical compounds to generate ions. When using inductively coupled plasma (ICP), samples containing elements of interest are introduced into argon plasma as aerosol droplets. The plasma dries the aerosol, dissociates the molecules, and then removes an electron from the components to be detected by the mass spectrometer. Other ionization methods such as electrospray ionization (ESI) and matrix assisted laser desorption ionization (MALDI) are used to analyze biological samples. Following the ionization procedure, ions are separated in the mass spectrometer according to their mass-to-charge ratio (m/z), and the relative abundance of each ion type is measured. Finally, the detector commonly consists in an electron multiplier where the collision of ions with a charged anode leads to a cascade of increasing number of electrons, which can be detected by an electrical circuit connected to a computer.
In this video, the procedure of ICP-MS analysis will be described by the detection of 56Fe as an example.
Principles
ICP-MS combines a high-temperature ICP (inductively coupled plasma) source with a mass spectrometer.
Samples need to be in ionic form prior to entering the mass analyzer in order to be detected. The digestion process of solid samples consists in the incubation of solid samples into strong and oxidizing acid at high temperature and for a prolonged period of time depending on the metal analyte. The sample is introduced as an aerosol into the ICP plasma (temperature of 6,000–10,000 K) to be converted into gaseous atoms, which are ionized.
The most commonly used mass analyzer is the quadrupole mass filter. It works as an electrostatic filter that only allows ions of a single mass-to-charge ratio (m/z) to reach the detector at a given time. It can separate up to 15,000 daltons (Da) per second and therefore is considered to have simultaneous multi-elemental analysis properties. ICP-MS is a very sensitive method that allows the detection of elements with concentrations below particle per billion (ppb), and below particle per trillion (ppt) for certain elements.
Finally, the detector system converts the number of ions striking the detector into an electrical signal. By using calibration standards (samples of known concentration for a certain element), it is possible to assess the concentration of a sample for one or several elements of interest.
Procedure
1. Cleaning of Polycarbonate Tubes
- Use polycarbonate tubes resistant to acidic solutions for sample digestion. In order to remove any contaminating trace of iron, fill all tubes with 5 mL of 0.1 M HCl.
- Place tubes in a water bath for 1 h at 50 °C.
- Wash the tubes with 5 mL of Milli-Q water and dry the tubes in an oven or chemical hood.
2. Sample Preparation and Digestion
- Place 200 µL of sample in 1.8 mL of concentrated nitric acid (65%).
- Place tubes in a water bath overnight at 50 °C. Adjust the protocol by increasing the temperature if a reduction of the overall digestion time is needed.
- Let the tubes cool down at room temperature.
- Dilute the samples by adding 8 mL of Milli-Q water to obtain a final nitric acid concentration bellow 20% (v/v).
- Centrifuge tubes at 3,000 x g for 10 min to pellet any remaining macroscopic residues.
3. Preparation of the Instrument
- Clean the ICP torch using ultrasonication in 5% nitric acid for 15 min. Wipe cones with 5% nitric acid. Change the peristaltic tubing. Check the pump oil level.
- Turn on the argon and chiller, start plasma. Start liquid flow into plasma and wait for instrument to stabilize, about 20 min.
- Optimize lens voltages. Run daily performance check by measuring test solutions containing Mg, In, and Ur to confirm the sensitivity of the ICP-MS instrument. Measure Ce and Ba where oxide form and double charged ions should remain below 3%. Check the mass at 8 and 220 Da to measure the background signal.
- The instrument is now ready for use.
4. Selection of User's Method and Sample List
- Select element and isotopes of interest.
- Select scan mode as peak hopping.
- Choose a dwell time of 100 ms (minimum 50) with 40 sweeps (minimum 15) per reading. Select one reading per replicate and 5 replicates (minimum 3). The total integration time is 4,000 ms. If the amount of sample is limited, reduce dwell time, number of sweeps and replicates keeping the values higher than the minimum values defined above.
- Use a flow rate of ammonia (NH3) at 0.7 mL/min to avoid the interference of 40Ar16O on the determination of 56Fe.
- Prepare calibration curve for the elements of choice.
- Run the samples.
Results
ICP-MS analysis of samples containing iron oxide nanoparticle is shown below. A standard curve was carried out using known concentration of 56Fe (Figure 1). The correlation coefficient being close to 1 (R2 = 0.999989) showed the good linear relationship between the sample concentrations and the intensity measured by the detector. Samples of interests showed values within the calibration range (Figure 2). The concentrations calculated by the software were then adjusted according to the dilution carried out during the protocol. The present protocol described a dilution of 1/50 following the dilution in acid (1/10) and in Mili-Q water (1/5). For example, a concentration of 51.427 µg/L was measured for the sample number 51 (Figure 2). The concentration of the original sample was 50x higher corresponding to 2.57 mg/L.

Figure 1. Calibration curve for 56Fe measurements. Four standard points (0.01, 0.1, 1, and 10 µg/mL) show a correlation coefficient (R2) of 0.999989. This confirms the good linear relationship between the signal intensity detected and the concentrations of reference.

Figure 2. Representative results following ICP-MS measurements on iron oxide nanoparticle samples. The concentration of each diluted sample is automatically calculated according to the defined calibration curve.
Application and Summary
The environmental and geological fields represent the first use for ICP-MS for example to measure contaminants present in water, in the soil, or in the atmosphere. The presence of contaminants at high concentration in tap water such as Fe, Cu, or Al can be monitored using ICP-MS.
The medical and forensic science fields also use ICP-MS detection. In case of suspicion of a metal poisoning such as arsenic, samples such as blood and urine can be analyzed using ICP-MS. This technique can also provide valuable information in case of pathology involving metabolic concerns or hepatological issues resulting in the poor excretion of certain elements.
ICP-MS allows the quantification of metals in any material. In Figure 3, the concentration of Fe was measured in nanoparticles and related to their magnetic resonance imaging (MRI) properties. ICP-MS provides a reliable quantification of Fe of different nanoparticles to discriminate which nanoparticles are the most efficient for imaging application.
Another application is to study the biodistribution of nanoparticles associated with metals. Figure 4 presents the organ biodistribution of nanoparticles containing iron oxide in mice following intravenous injection. At 24 h, each organ was collected and digested in concentrated nitric acid until full organ digestion was achieved. The 56Fe concentration was quantified by ICP-MS. Results show higher concentration of 56Fe in liver and spleen for mice injected with nanoparticles than in organs from naïve animals. Therefore, it was concluded that nanoparticles accumulate mostly into liver and spleen organs.
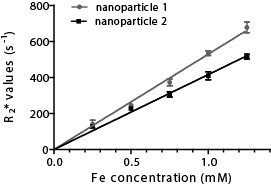
Figure 3. Magnetic resonance imaging (MRI) measurement of nanoparticles function of their Fe concentration. Five concentrations of iron were used (0.25, 0.5, 0.75, 1, and 1.25 mM) that were imaged for their MRI properties (relaxation rate, R2*).

Figure 4. Biodistribution of iron oxide nanoparticles following intravenous injection in mice. Naïve samples show the basal organ level of iron in untreated mice. Following the injection of nanoparticles containing iron oxide, the quantity of iron in certain organ increases which is associated to the accumulation of nanoparticles.
Tags
Skip to...
Videos from this collection:

Now Playing
Introduction to Mass Spectrometry
Analytical Chemistry
112.0K Views

Sample Preparation for Analytical Characterization
Analytical Chemistry
84.3K Views

Internal Standards
Analytical Chemistry
204.4K Views

Method of Standard Addition
Analytical Chemistry
319.5K Views

Calibration Curves
Analytical Chemistry
795.2K Views

Ultraviolet-Visible (UV-Vis) Spectroscopy
Analytical Chemistry
622.2K Views

Raman Spectroscopy for Chemical Analysis
Analytical Chemistry
51.1K Views

X-ray Fluorescence (XRF)
Analytical Chemistry
25.4K Views

Gas Chromatography (GC) with Flame-Ionization Detection
Analytical Chemistry
281.3K Views

High-Performance Liquid Chromatography (HPLC)
Analytical Chemistry
383.3K Views

Ion-Exchange Chromatography
Analytical Chemistry
264.1K Views

Capillary Electrophoresis (CE)
Analytical Chemistry
93.4K Views

Scanning Electron Microscopy (SEM)
Analytical Chemistry
86.9K Views

Electrochemical Measurements of Supported Catalysts Using a Potentiostat/Galvanostat
Analytical Chemistry
51.3K Views

Cyclic Voltammetry (CV)
Analytical Chemistry
124.5K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved