Photoelectric Effect
Overview
Source: Yong P. Chen, PhD, Department of Physics && Astronomy, College of Science, Purdue University, West Lafayette, IN
Photoelectric effect refers to the emission of electrons from a metalwhen light is shining on it. In order for the electrons to be liberated from the metal, the frequency of the light needs to be sufficiently high such that the photons in the light have sufficient energy. This energy is proportional to the light frequency.The photoelectric effect provided the experimental evidence for the quantum of light that is known as photon.
This experiment will demonstrate the photoelectric effect using a charged zinc metal subject to either a regular lamp light, or ultraviolet (UV) light with higher frequency and photon energy.The zinc plate will be connected to an electroscope, an instrument that can read the presence and relative amount of charges. The experiment will demonstrate that the UV light, but not the regular lamp, can discharge the negatively charged zinc by ejecting its excess electrons.Neither light source, however, can discharge positively charged zinc, consistent with the fact that electrons that are emitted in photoelectric effect.
Principles
A metal contains many mobile electrons. It is relatively easy to excite these electrons, and if they are excited with enough energy, they can leave the metal. When such an excitation is made with light, the ejected electrons are known as photoelectrons and this effect is known as the photoelectric effect. It has been observed that in order for this to happen, the frequency (f) of the light must exceed some minimal threshold (f0), or equivalently, the light wavelength (λ), which is related to the frequency f by:

(with c ≈ 3x108 m/s being the speed of light) needs to be below some threshold (λ0), that is, f > f0 (λ < λ0). Otherwise, if f < f0 (λ > λ0), no photoelectrons will be emitted even with intense light illumination.
Albert Einstein was able to explain these observations using the concept of photons, the quanta of light. Light consists of many of such particle-like photons, and each photon has energy:

with h ≈ 6.63x10-34 Js, called Planck’s constant, which relates the light frequency to photon energy.
The microscopic process of the photoelectric effect is that an individual photon is absorbed by the metal and its energy is used to excite an electron. The electron will be emitted from the metal if the photon energy,
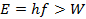
where W is known as the “work function” and represents the minimal energy needed to liberate the electron from the metal. If,

even if the light is intense (meaning it contains a large number of photons) and even if the light is shone for a long time, no photoelectrons will be produced since the individual photons do not have sufficient energy to liberate electrons.
Einstein’s explanation of the photoelectric effect was historically significant as it provided key support for the theory of photons (quanta of light), which shows that light can behave as particles in addition to as electromagnetic waves, and possess the dual particle-wave nature.
For example, zinc (Zn) metal to be used in this experiment has a work function of W ≈ 4.3 eV (with 1 eV ≈ 1.6x10-19 J). This means that the threshold frequency for the photoelectric effect for Zn will be:
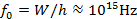
corresponding to a threshold wavelength,

In order to produce photoelectrons out of Zn, light must have a frequency exceeding f0 ≈ 1015 Hz, or a wavelength below λ0 ≈ 300 nm. Such a short wavelength corresponds to UV (since the visible light has a wavelength exceeding ~ 400 nm, which corresponds to violet color).
Since an electron carries a negative charge, the photoelectric effect will remove negative charges from a metal (effectively adding positive charges to it). If the metal is originally negatively charged, this will make it less charged. If the metal is originally positively charged, this will make it more charged. Such effects will be studied in this experiment.
Procedure
1. Obtain the Needed Components for This Experiment
- Obtain an electroscope (Figure 1), which is a device that monitors the charge on the metal plate connected to the electroscope.Due to the coulomb repulsion force between the charges, the needle inside the electroscope will deflect more (or less) if there are more (or less) charges on the plate, and will not move if there are no charges.
- Obtaina zinc metal plate.Use sandpaper to polish its surface (this removes the zinc oxide on the metal surface and makes it easier to lose electrons through the photoelectric effect).
- Place the zinc plate on and in direct contact with the top of the electroscope (Figure 1).
- Obtain a UV light source,which has a wavelength component below 300nm and a regular lamp providing visible light.Obtain a sunglass with UV protection.
- Obtain an acrylic rod and a piece of fur commonly used to produce charges. Rubbing the rod with the fur will add electrons to the rod, making the rod negatively charged.

Figure 1: Diagram showing an uncharged (a) and a charged (b) (indicated by the deflection of the needle) electroscope, with a zinc metal plate placed on and connected to its top plate.(The charged situation for b is drawn for positive charges as an example.A similar observation holds true for negatively charged electroscope.
2. Photoelectric Effects on Negatively Charged Zinc
- Rub the rod with the furfive times.This will make the rod negatively charged.
- Bring the rod close to the zinc plate, without touching it.Use another hand to briefly touch the zinc plate.This will charge the zinc plate to be positively charged through induction (the negatively charged rod attracts some positive charges from the hand onto the zinc metal, and the positive charges remain on the zinc metal after its contact with the hand is removed).The needle of the electroscope should deflect to indicate that the metal plate, along with all the parts in the electroscope connected to it, is charged (Figure 2a).If needed, repeat steps2.1 and 2.2 to add more charges to the plate.
- Turn on the visible lamp and bring it close to the electroscope to shine its light on the zinc plate (Figure 2b).Observe the response of the electroscope.
- Turn off the regular lamp and now put on the UV protecting sunglass. Turn on the UV light and bring it close to the electroscope.Shine the UV light on the zinc metal(Figure 2c).CAUTION: Point the UV light away from the eyes and avoid looking directly into the UV lightto protect the eyes from UV.Observe the response of the electroscope.Then turn off the UV light.

Figure 2: Diagram showing (a) positively charging the zinc metal by the negatively charged rod through induction; and bringing (b) regular lamp light and (c) UV light to observe their effects on the charge state of the zinc, as monitored by the electroscope connected to it.
3. Photoelectric Effects on Positively Charged Zinc
- Rub the rod with the furfive times again.This will make the rod negatively charged.
- Bring the rod in direct contact with the zinc metal plate and rub the rod on the plate five times.This will transfer some negative charges onto the zinc, indicated by the deflection of the needle of the electroscope (Figure 3a).
- Put away the rod and do not use the hand or any other objects to touch the zinc metal.
- Turn on the regular (visible) lamp and bring it close to the electroscope to shine its light on the zinc plate (Figure 3b).Observe the response of the electroscope.
- Turn off the regular lamp and now turn on the UV light and bring it close to the electroscope to shine UV light on the zinc metal (Figure 3c).CAUTION:Point the UV light away from the eyes and avoid looking directly into the UV light to protect the eyes from UV.Observe the response of the electroscope.Then turn off the UV light.
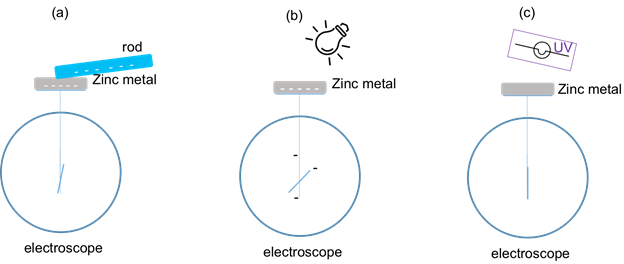
Figure 3: Diagram showing (a) negatively charging the zinc metal by the negatively charged rod through direct contact; and bringing (b) regular lamp light and (c) UV light to observe their effects on the charge state of the zinc, as monitored by the electroscope connected to it.
Results
For steps 2.1-2.4, the electroscope remains charged (needle remain deflected) for both the regular lamp and UV light illumination (Figure 2b and 2c), indicating that the zinc plate remains positively charged.This is because the charged zinc plate (which has already lost some electrons in the first place to become positively charged) further losessome photoelectrons by the UV light to make it further positively charged. In thiscase, itmay be noticeablethat the needle of the electroscope deflects a bit further in Figure 2c.The regular visible light doesnot change the positive charges on the zinc plate and the electroscope remains charged as well (Figure 2b).
For steps 3.1-3.5, when the zinc plate is negatively charged, it can be observed that the regular lamp light again has no effect on the electroscope (Figure 3b), while the UV light causes the needle of the electroscope to collapse and return to the uncharged position with no deflection, Figure 3c. This is because only the UV light photons have enough energy (above the workfunction of zinc) to eject photoelectrons, thus to discharge the zinc that has been previously charged to be negative (with excess electrons).
Application and Summary
In this experiment, we haveused an electroscope to show that UV light can discharge a negatively charged zinc metal through the photoelectric effect.In contrast, a positively charged zinc sample (which has already lost some electrons) will not be discharged, nor will a visible light (which cannot cause the photoelectric effect) discharge either negatively or positively charged zinc.
The photoelectric effect played important roles in the development of quantum physics in the 20th century as it provided experimental evidence that light is made of particles that we call photons andcarry quanta of the light energy proportional to light frequency.
Practically, the photoelectric effect has also been used to make various optoelectronic devices, such as photosensitive electrical switches-where the blocking or unblocking of a light beam shining on a metal turns off or on an electrical current due to the absence or presence of photoelectrons.This is commonly used in many mechanical-position sensors (for example opening or closing of a door that unblocks or blocks a light beam).
Skip to...
Videos from this collection:

Now Playing
Photoelectric Effect
Physics II
32.7K Views

Electric Fields
Physics II
77.5K Views

Electric Potential
Physics II
105.0K Views

Magnetic Fields
Physics II
33.6K Views

Electric Charge in a Magnetic Field
Physics II
33.7K Views

Investigation Ohm's Law for Ohmic and Nonohmic Conductors
Physics II
26.2K Views

Series and Parallel Resistors
Physics II
33.2K Views

Capacitance
Physics II
43.8K Views

Inductance
Physics II
21.6K Views

RC/RL/LC Circuits
Physics II
142.9K Views

Semiconductors
Physics II
29.8K Views

Reflection and Refraction
Physics II
36.2K Views

Interference and Diffraction
Physics II
91.2K Views

Standing Waves
Physics II
49.8K Views

Sound Waves and Doppler Shift
Physics II
23.5K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved