Using TMS to Measure Motor Excitability During Action Observation
Overview
Source: Laboratories of Jonas T. Kaplan and Sarah I. Gimbel—University of Southern California
Transcranial Magnetic Stimulation (TMS) is a non-invasive brain stimulation technique that involves passing current through an insulated coil placed against the scalp. A brief magnetic field is created by current in the coil, and because of the physical process of induction, this leads to a current in the nearby neural tissue. Depending on the duration, frequency, and magnitude of these magnetic pulses, the underlying neural circuitry can be affected in many different ways. Here, we demonstrate the technique of single-pulse TMS, in which one brief magnetic pulse is used to stimulate the neocortex.
One observable effect of TMS is that it can produce muscle twitches when applied over the motor cortex. Due to the somatotopic organization of the motor cortex, different muscles can be targeted depending on the precise placement of the coil. The electrical signals that cause these muscle twitches, called motor evoked potentials, or MEPs, can be recorded and quantified by electrodes placed on the skin over the targeted muscle. The amplitude of MEPs can be interpreted to reflect the underlying excitability of the motor cortex; for example, when the motor cortex is activated, observed MEPs are larger.
In this experiment, based on a study originally performed by Fadiga and colleagues1 and since replicated by many others,2 we use single-pulse TMS to test the excitability of motor cortex during action observation. It is known that motor cortex can be activated not only when we move, but when we watch others perform movements. A common interpretation of this phenomenon is that it reflects a simulation process that may play a role in the understanding of others' actions. Here we will record MEPs evoked by TMS over the primary motor cortex while subjects observe the movements of others compared with control stimuli.
Procedure
1. Recruit 20 participants.
- Participants should be right-handed and have no history of neurological or psychological disorders.
- Participants should have normal or corrected-to-normal vision to ensure that they will be able to see the visual stimuli properly.
2. Pre-experiment procedures
- Obtain written consent from the participant and explain what is involved in the experiment.
- Explain that the participant will watch a series of short videos while TMS pulses are delivered to their brain. The subject may experience a light tap on the head from the TMS coil, but there should be no great discomfort associated with participation.
3. Prepare the subject for TMS.
- Seat the subject in a comfortable chair in front of a computer screen. Their elbow should be bent at a 90º angle, and the hand should lie comfortably prone.
- Use a chinrest to fix the movement of the head, ensuring that the subject's eyes are about 50 cm from the computer screen.
- Prepare the skin of the hand for EMG electrode placement by cleaning with alcohol.
- Place two EMG electrodes on the first-dorsal interosseous (FDI) muscle of the right hand.
- Locate the peak location of muscular tension by asking the participant to flex their FDI muscle and place the first electrode on that location.
- Place a second reference electrode on the nearby bone of the hand.
- Connect the EMG electrodes to a computer that digitizes, amplifies, filters, and displays the signal.
4. Localize and calibrate TMS.
- Instruct participants to relax their hand, so that there is no muscle tension.
- Use a figure-8 TMS coil to locate the primary motor cortex.
- Place coil against the contralateral (left) surface of the head on the anterior portion of the scalp.
- Deliver a series of individual TMS pulses, systematically moving the location of the coil until twitches are visible in the FDI muscle and the recorded MEPs are stable and reliable. Stimulation intensity may be adjusted as needed during this phase to assist in locating the hotspot.
- Determine the subject's motor threshold.
- Find the minimum stimulator output strength that produces an MEP of greater than 50 µV on 5 out of 10 stimulations. Record this value.
- Measure the resting MEP amplitude.
- Deliver a series of 10 TMS pulses, each separated by 15 s, in the absence of any stimulus to measure the baseline MEP amplitude.
5. Experimental task
- Play a series of 5-s video stimuli, one at a time.
- There are three kinds of videos: hand movement observation, arm movement observation, and control.
- In the control videos, a cup is presented on a table, and a right hand rests nearby. No movement is made.
- In the hand action videos, the right hand reaches and grasps the cup. This action involves contraction of the FDI muscle.
- In the arm action videos, the right arm reaches, is lifted, and moved around the area. There is no grasping action performed, and so the FDI muscle is not involved.
- Play each video 10 times, with 15 s of rest after each one, for a total of 30 videos. Play the videos in random order.
- There are three kinds of videos: hand movement observation, arm movement observation, and control.
- During the video, deliver a TMS pulse at 120% of motor threshold.
- Time the pulse to coincide with the action in the video. To achieve this, the pulse should occur 2 s after the start of the video.
6. Analyze the data.
- For each MEP, calculate the peak-to-peak amplitude.
- Discard MEPs that occur either before TMS stimulation, or more than 100 ms after stimulation to remove spurious spikes.
- For each subject, calculate the average MEP amplitude for the baseline, action observation, and control conditions.
- Perform analysis of variance (ANOVA) on the group data to test the hypothesis that MEP amplitude is affected by action observation.
Results
A comparison of MEP amplitudes reveals a facilitation effect (Figure 1). MEP amplitude recorded from the FDI muscle is significantly greater during the hand action videos compared with control videos. This result suggests that the motor cortex increases in excitability during action observation.
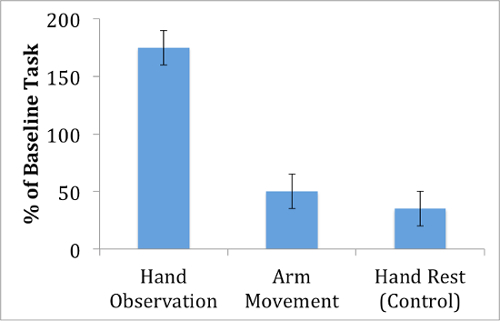
Figure 1: MEP amplitude during action observation. Motor evoked potentials from the first-dorsal interosseous muscle are largest when observing a hand movement, compared with an arm movement or a control video that displays no action.
Notably, the facilitation effect is relatively selective for the videos that involve a grasping action, as MEPs recorded during observation of the arm movement video show smaller MEPs compared with the hand action video. This suggests that the motor facilitation that occurs during action observation does not affect the entire motor cortex, but instead is specific to the muscle movements that are observed. In fact, the motor facilitation effect appears to be specific not only for which muscle is observed, but also to when the muscle is observed. For instance, Gangitano et al. have demonstrated temporal correlation between motor excitability and observed action dynamics.3
Application and Summary
The single-pulse TMS technique lends itself well to the study of the motor cortex, both because of the accessible location of this cortex on the frontal surface of the brain, and also because of the directly observable reaction produced in the muscle in the form of MEPs. The measurement of cortico-spinal motor excitability has provided support further for the phenomenon of motor simulation during action observation in humans. This resonant motor activity may have implications for social behavior, for example in contributing to the process of understanding what others are doing. Furthermore, this same technique has provided evidence for motor activation during the imagination of action,4 a process that may be important for improving performance through mental rehearsal.
The robustness and specificity of the motor facilitation effect may reflect the sophistication of an individual's motor representations. For example, the temporal dynamics of motor facilitation are directly related to motor expertise.5 This effect is also disrupted with disorders of movement, opening up the possibility that measurement of TMS-induced motor potentials can be used as way to assess the health of the motor cortex, as in recovery from stroke or other brain disease.6
Skip to...
Videos from this collection:

Now Playing
Using TMS to Measure Motor Excitability During Action Observation
Neuropsychology
10.3K Views

The Split Brain
Neuropsychology
68.6K Views

Motor Maps
Neuropsychology
27.7K Views

Perspectives on Neuropsychology
Neuropsychology
12.2K Views

Decision-making and the Iowa Gambling Task
Neuropsychology
33.1K Views

Executive Function in Autism Spectrum Disorder
Neuropsychology
18.0K Views

Anterograde Amnesia
Neuropsychology
30.5K Views

Physiological Correlates of Emotion Recognition
Neuropsychology
16.4K Views

Event-related Potentials and the Oddball Task
Neuropsychology
27.7K Views

Language: The N400 in Semantic Incongruity
Neuropsychology
19.7K Views

Learning and Memory: The Remember-Know Task
Neuropsychology
17.3K Views

Measuring Grey Matter Differences with Voxel-based Morphometry: The Musical Brain
Neuropsychology
17.4K Views

Decoding Auditory Imagery with Multivoxel Pattern Analysis
Neuropsychology
6.5K Views

Visual Attention: fMRI Investigation of Object-based Attentional Control
Neuropsychology
42.3K Views

Using Diffusion Tensor Imaging in Traumatic Brain Injury
Neuropsychology
16.9K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved