Method Article
Measuring the Migration and Biofilm Formation of Various Bacteria
In This Article
Summary
Here, we present a practical method for the isolation and identification of microorganisms within the host. In this way, the physicochemical properties of microorganisms and possible ways of living in the host are clearly described.
Abstract
As microbes that thrive in the host body primarily have adaptive abilities that facilitate their survival, methods for classifying and identifying their nature would be beneficial in facilitating their characterization. Currently, most studies focus only on one specific characterization method; however, the isolation and identification of microorganisms from the host is a continuous process and usually requires several combinatorial characterization methods. Herein, we describe methods of identifying the microbial biofilm-forming ability, the microbial respiration state, and their chemotaxis behavior. The methods are used to identify five microbes, three of which were isolated from the bone tissue of Sprague-Dawley (SD) rats (Corynebacterium stationis, Staphylococcus cohnii subsp. urealyticus, and Enterococcus faecalis) and two from the American Type Culture Collection (ATCC)-Staphylococcus aureus ATCC 25923 and Enterococcus faecalis V583. The microbes isolated from the SD rat bone tissue include the gram-positive microbes. These microbes have adapted to thrive under stressful and nutrient-limiting environments within the bone matrix. This article aims to provide the readers with the specific know-how of determining the nature and behavior of the isolated microbes within a laboratory setting.
Introduction
The mammalian host represented by the human body contains a large number of microorganisms. These microorganisms are widely distributed in the mouth, digestive tract, intestine, and blood of the host and have different effects on the host's health. The oral cavity is host to a plethora of microbes that can modulate the host's susceptibility to infections. Microbes such as Streptococci (e.g., S. mitis/oralis, S. pseudopneumoniae, and S. infantis) and Prevotella spp. colonize the oral cavity, forming a multispecies biofilm on the tongue surface causing bad breath and functioning as a microbial reservoir for microbial infection. These pathogens can infect the jawbone by infiltrating the periodontal ligaments that hold the tooth root in the jawbone1. The characterization of these microbes isolated from the host body is often a tedious process, particularly when the microbes exhibit individual traits requiring specific treatment and growth conditions. Microbes, such as the pathogenic Helicobacter pylori, Clostridium difficile, and Fusobacterium nucleatum, thrive in the gut's harsh environment, with specific oxygen, nutrient, and growth requirements, presenting a challenge in the characterization processes, particularly in investigating the pathogenicity of these microbes. Therefore, a standardized method of isolating and investigating these microorganisms is needed for scientists and medical practitioners to develop new medical treatments.
This protocol uses microbes that thrive in the bone matrix of rats. Traditionally, with the exception of the osseous system represented by the jaw, where the presence of teeth makes the bone matrix more susceptible to infection than other bones1, it is generally believed that the host's healthy bone is a sterile environment. However, studies have found that microorganisms enter the systemic circulation through the intestinal wall, ultimately affecting bone mineralization2. As a proof-of-concept, we use the described protocol to characterize the biochemical properties of microbial isolates from the femur and tibia of healthy SD rats (Corynebacterium stationis, Staphylococcus cohnii subsp., and Enterococcus faecalis). These microbial isolates were selected as the bone is a closed and hypoxic environment, and characterizing these microbes from bone can be a challenging task. Various articles have detailed the processes used in studying these microbes; however, there are few that provide a complete protocol to identify host-isolated microorganisms.
In establishing the proper culture conditions, the oxygen requirements of the microbe need to be understood via the use of Fluid Thioglycollate Medium (FTM). Microbes with different oxygen requirements form stratified layers in the clear FTM liquid3. Based on the stratification profile, the oxygen requirement of the microbe is then used to investigate the growth of the cells. Microbes that thrive on the surface of the FTM liquid are obligate aerobes, whereas microbes that grow at the bottom are obligate anaerobes. Microbes that grow as a suspension in the FTM liquid are either facultative or aerotolerant anaerobes. The microbial growth rate is established by observing the exponential growth phase of the microbial cells. The growth profile is then compared to the biofilm formation of the microbe. Biofilms are largely composed of multiple species that directly and indirectly affect each other's health. During this process, beneficial interactions among microbial communities select for attachment, providing a spatial structure that favors the evolution of active reciprocal interactions. For example, the co-culture of Paenibacillus amylolyticus and Xanthomonas retroflexus exhibit facultative symbiotic interactions in a static environment, promoting rapid biofilm growth13. Microbes adapt to the host tissues via biofilm formation for sustained localization, protecting themselves against harsh environments and evading the host's immune system4,5,6,7. Biofilms are usually dense and multilayered structures that help microorganisms resist external subcritical stimuli; for example, E. faecalis in dental pulp enhances its resistance to antibiotics by increasing biofilm formation when faced with subconcentrations of tetracycline and vancomycin8.
Chemotaxis enables microorganisms to move according to chemical gradients, and signaling pathways are widely distributed in a variety of pathogenic bacteria. Some pathogenic microorganisms migrate to specific sites, under the guidance of chemical signals, to cause infectious diseases14. For example, Xanthomonas spp. express 19 chemoreceptor and 11 flagellin proteins in the host, triggering bacterial binding and, ultimately, ulceration15. There are also specific proteins in the bacteria (pectin-binding proteins) that guide the specific migration of bacteria to nutrients, which can lead to better growth16. This is also critical for bacteria that may exist in nutrient-poor environments. Microbial cells often rely on chemotactic motility to draw themselves to a conducive growth environment while evading other predatory cells and toxins that harm cellular viability. Building on previously established soft agar approaches to determine the chemotaxis, we develop a diffusible method to generate a chemoattractant gradient for testing the microbial chemotaxis.
This paper describes the methods for determining the growth conditions, biofilm formation, and chemical tropism of bacteria, using Corynebacterium stationis, Staphylacoccus cohnii, Enterococcus faecalis, Staphylacoccus aureus ATCC 25923, and Enterococcus faecalis V583 as examples (see Figure 1). The optimization of microbial growth conditions uses FTM to determine the oxygen requirements of the microbe, while biofilm formation uses glass surfaces as a solid backing, and the biofilm mass is counterstained with crystal violet. The microbe's chemical tropism relies on its chemotactic behavior, where through 3D printing (Supplemental Figure S1), a standardized method is used to generate a chemical reservoir for the chemoattractant in a soft agar matrix (Supplemental Figure S2).
Protocol
NOTE: See the Table of Materials for details about all the materials and equipment used in this protocol. Use aseptic techniques to avoid contamination.
1. Bacterial recovery to get a single colony
- Prepare solid agar plates.
- Prepare Terrific Broth (TB) medium containing agar, with each liter of liquid containing 11.8 g of tryptone, 23.6 g of yeast extract, 9.4 g of K2HPO4, 2.2 g of KH2PO4, and 15 g of agar, pH = 7.
- Autoclave the solution at 121 °C for 20 min using an air-permeable cap or bottle-sealing film with an air vent.
- Before the liquid cools and solidifies, pour the TB broth containing agar into 10 cm plastic Petri dishes at a volume of 25 mL per plate.
NOTE: The broth should be poured into the plate before it solidifies at temperatures lower than 60 °C. Use aseptic techniques to prevent contamination.
- Bacterial inoculation to the plate
- Take out the target strains stored at −80 °C and thaw them at room temperature in a biological safety cabinet.
- Use a 10 µL pipette to take up 10 µL of bacterial suspension and spread it on the TB plate by streaking.
- Seal the plate to prevent contamination. Place the plate in a biochemical incubator at 37 °C. Check colony growth visually after ~24 h. Pick single colonies and amplify them by PCR to confirm that they contain only one type of bacteria.
2. Bacterial liquid culture to logarithmic growth phase
- Prepare liquid culture medium.
- Refer to the preparation of solid agar plates in Step 1.1.1. to prepare TB liquid medium, 1 L of which contains 11.8 g of tryptone, 23.6 g of yeast extract, 9.4 g of K2HPO4, and 2.2 g of KH2PO4, pH ~7.
NOTE: Do not add agar. Bacteria grow faster in a liquid nutrient environment. - Autoclave the solution at 121 °C for 20 min using an air-permeable cap or bottle-sealing film with an air vent.
- Store the liquid culture medium at 4 °C.
- Refer to the preparation of solid agar plates in Step 1.1.1. to prepare TB liquid medium, 1 L of which contains 11.8 g of tryptone, 23.6 g of yeast extract, 9.4 g of K2HPO4, and 2.2 g of KH2PO4, pH ~7.
- Bacterial inoculation and cultivation
- In the biological safety cabinet, pick a single colony with an inoculating loop or a 10 µL pipette and inoculate the bacteria in the prepared TB liquid medium in an Erlenmeyer flask.
- Seal the Erlenmeyer flask with gauze and place it in a shaker (200 rpm) at 37 °C to cultivate the bacteria.
NOTE: The liquid medium becomes gradually turbid over 5 h; the time taken to become turbid varies with different bacteria.
3. FTM experiment and growth curve determination
- Fluid Thioglycollate Medium (FTM) preparation
- Prepare Thiolglycollate Medium (TM), each liter of which contains 15 g of tryptone, 5 g of yeast extract, 5 g of glucose, 0.5 g of L-cysteine, 0.5 g of sodium thioglycolate, 2.5 g of sodium chloride, 0.001 g of resazurin, and 0.75 g of agar.
NOTE: Commercially available solid medium can easily be reconstituted with water and sterilized (Step 3.1.2.). - Weigh 29.3 g of solid Thioglycollate Medium, add 1 L of distilled water, heat and stir until completely dissolved and until the solution turns pink.
- Add TM (19 mL) to a test tube with a sealing plug. After sealing, autoclave the solution at 121 °C for 20 min using an air-permeable cap or bottle-sealing film with an air vent.
NOTE: After heating, the solution becomes light yellow.
- Prepare Thiolglycollate Medium (TM), each liter of which contains 15 g of tryptone, 5 g of yeast extract, 5 g of glucose, 0.5 g of L-cysteine, 0.5 g of sodium thioglycolate, 2.5 g of sodium chloride, 0.001 g of resazurin, and 0.75 g of agar.
- Inoculation, cultivation, and observation of bacteria
- Prepare the bacterial suspension to grow to OD 0.6-0.8 in liquid TB medium (see Step 2.2.).
- Open the test tube sealing plug under aseptic conditions in the biological safety cabinet, add 1 mL of the bacterial culture to the test tube containing FTM as soon as possible, and immediately seal the test tube again.
- Shake the tube gently to make sure the bacteria are homogeneously distributed in the liquid and place the test tube at 37 °C for static culture.
- After 48 h of incubation, observe the growth of different bacteria in FTM in various test tubes and take photographs.
- Determination of growth curve under aerobic conditions
- Prepare the initial bacterial culture with a suitable OD value (see Step 2.2.), and culture the bacteria until they reach the logarithmic growth phase.
NOTE: Corynebacterium stationis, Staphylacoccus cohnii, Enterococcus faecalis, Staphylacoccus aureus ATCC 25923, and Enterococcus faecalis V583 used in this protocol took ~5 h to reach the log phase. - Add 200 µL of the bacterial culture (from Step 3.3.1) in TB liquid medium to each well of a 96-well plate in triplicate. Use a microplate reader to determine the OD value at 600 nm, and dilute the different bacterial cultures so that the OD difference between the different bacteria-containing wells and TB liquid medium (blank) is between 0.01 and 0.02.
- Take a new 96-well plate, add TB medium, transfer the different diluted bacterial cultures from Step 3.3.2., in triplicate, to the plate, and place it on an ultra-clean workbench.
- Seal the 96-well plate with sealing tape, put it in the microplate reader, and set the program as follows:
- Set the temperature to 37 °C ± 0.5.
- Set the Kinetic Loop to a total of 60 cycles, each for 30 min.
- Set the absorbance to 600 nm.
- Set Shaking to 1,600 s in a cycle.
- Run the program and stop it when the growth curve plateaus.
- Prepare the initial bacterial culture with a suitable OD value (see Step 2.2.), and culture the bacteria until they reach the logarithmic growth phase.
- Determination of growth curve under anaerobic conditions
- Follow Step 3.3. but keep the 96-well plate with the TB medium and bacterial suspensions in an anaerobic incubator before sealing the plate with sealing tape. Pump and fill the anaerobic box with nitrogen to reduce the oxygen concentration to <3%. Seal the 96-well plate with sealing tape and leave it in the anaerobic box.
4. Biofilm-formation ability test
- Culture the bacteria to logarithmic growth phase in liquid medium.
- Refer to Section 2 to inoculate the bacteria in the TB liquid medium.
- Cultivate the bacteria overnight in a shaker at 37 °C, ensuring that the OD600 of the bacterial culture medium is between 0.6 and 0.8.
- Divide the bacterial culture and cultivate under different conditions.
- When the OD600 of the bacterial culture is between 0.6 and 0.8, add 10 mL of this bacterial suspension to a glass test tube.
- Divide each bacterial culture into two groups: aerobic and anaerobic conditions, and perform all operations in triplicate.
- For the aerobic group, wrap the test tube containing the bacterial suspension with tin foil and incubate it in a 37 °C water bath at a constant temperature.
- For the anaerobic group, wrap the tube containing the bacterial suspension with tin foil and transfer it to an anaerobic incubator. Remove the tin foil in the anaerobic incubator and reduce the oxygen concentration in the incubator to <3% by pumping and filling it with nitrogen. Close the test tube with a frosted glass stopper, take it out after sealing, and incubate it in a water bath at 37 °C.
- Every 6 h, take three test tubes from each of the two groups (aerobic/anaerobic) to observe the growth of the biofilm at the bottom, and stain following Step 4.3.
- Discard the bacterial suspension and dry the biofilm.
- Take out the test tubes at different time points and carefully aspirate the upper layer of the bacterial suspension. Observe the white biofilm adhering to the bottom of the test tube.
NOTE: Take care not to disturb the biofilm when aspirating the bacterial suspension. - Carefully add 2 mL of phosphate-buffered saline (PBS) along the wall of the test tube, shake it gently to wash away the planktonic bacteria adsorbed in the biofilm, and carefully aspirate the PBS buffer.
- Put the test tube with the biofilm adsorbed on the bottom into the oven to dry for 30 min.
- Take out the test tubes at different time points and carefully aspirate the upper layer of the bacterial suspension. Observe the white biofilm adhering to the bottom of the test tube.
- Staining
- Take out the test tubes, add 2 mL of 0.1% crystal violet staining solution to each test tube, and stain the biofilms for 30 min at room temperature.
- Use a pipette to carefully aspirate the crystal violet dye solution. Carefully add distilled water along the wall of the test tube to wash away the remaining crystal violet dye solution, repeating 3x-5x until the added distilled water became almost colorless. Observe the purple biofilm at the bottom of the test tube.
- Dry the test tube again.
- Dissolve the biofilm with 95% ethanol and measure the OD600 of the biofilm solution.
- Take out the dried test tube, add 10 mL of 95% ethanol to each test tube, and shake thoroughly to make sure that the crystal violet was fully dissolved in the ethanol.
- With a volume of 200 µL per well, add crystal violet in ethanol to a 96-well plate, and measure its absorbance at a wavelength of 600 nm.
NOTE: According to Beer-Lambert's law, the absorbance is linearly related to the concentration of the solution, so the value of OD600 can reflect the concentration of crystal violet, which reflects the amount of biofilm in the bacterial suspension at different times.
5. Bacterial chemotaxis test
- Prepare differential eutrophic agar plates.
- Refer to Step 1 to prepare a half-dose MH medium containing 0.5% agar, each liter of which contains 11.8 g of tryptone, 23.6 g of yeast extract, 9.4 g of K2HPO4, 2.2 g of KH2PO4, and 5 g of agar, pH ~7.
- Refer to Step 1 to prepare 5x TB medium containing 0.5% agar, each liter of which contains 59 g of tryptone, 118 g of yeast extract, 47 g of K2HPO4, 11 g of KH2PO4, and 5 g of agar, pH ~7.
- Autoclave the solution at 121 °C for 20 min using an air-permeable cap or bottle-sealing film with an air vent.
- Before the liquid is cooled and solidified, pour 25 mL of half-dose MH broth (containing 0.5% agar) per plate, cover the plate with a 3D printed lid, and place it in a biological safety cabinet. After cooling, remove the cover to observe the cylindrical well (1.4 cm diameter) in the semisolid medium.
NOTE: The 3D printed lid with resin material cannot be sterilized using high-temperature steam. To kill the surface bacteria, it can be sprayed with 95% ethanol and then irradiated with a UV lamp for 30 min in a biological safety cabinet. When removing the lid, apply force in the vertical direction and lift it carefully to prevent damage to the semisolid agar. - Before the 5x TB broth (containing 0.5% Agar) solidifies, use a pipette to add the 5x TB broth to the well in the semisolid plate until the broth is flush with the surface of the medium.
- Cool the plate in a biological safety cabinet to obtain a semisolid agar plate containing eutrophic bodies. Take care to avoid plate contamination by environmental microorganisms.
NOTE: The semisolid medium is still somewhat fluid and, hence, must be fully cooled before being stored upside down.
- Bacterial inoculation, cultivation, and photography
- Place the agar plate with the semisolid culture medium in a biological safety cabinet and cool it for ~30 min under UV irradiation to prevent bacterial contamination.
- Use a 10 µL pipette to take up 4 µL of the bacterial suspension cultured to an OD of ~0.6-0.8, insert the pipette tip into the center of the plastic Petri dishes, and pipet out the bacterial suspension.
- Wait for the bacterial suspension to be absorbed into the medium for 1 min. When the bacterial suspension has been fully absorbed, divide the plate into two groups (aerobic/anaerobic) in triplicate.
- Seal the aerobic group with sealing tape and place those plates upside down in a 37 °C biochemical incubator for static culture. Seal the anaerobic group in an anaerobic box as described in Step 4.2, inverting the plates at 37 °C for growth.
- Check the spread and migration of bacteria every 12 h (at 0 h, 12 h, 24 h, 36 h, and 48 h), and use the imaging system to take images of the plate.
- Use ImageJ software to calculate bacterial migration distances.
- Import the image file acquired using the imaging system.
- Set the scale bar and apply it to all the images.
- When taking images, add a ruler, create a line segment, select a straight-line segment (e.g., 1 cm, and then set the length of this line segment to 1 cm), and mark the length of the board.
- Open the Set Scale window; click on Analyze | Set Scale.
- Type the actual length in Known distance and Unit of length.
- Check Global.
- Enter the folder address of the original image and the output file address.
- Use the straight line tool to select the communities one by one and adjust the tolerance until the straight tool line fits the boundary of the bacterial colony extending to both sides.
- Measure the migration distance of the colony from the center to both ends separately and derive the straight-line length by clicking on Analyze | Measure.
- Repeat Steps 5.3.3-5.3.4 until all the communities are measured and save the results to a .csv file for further analysis.
Results
This work describes the approaches taken to characterize the isolated microbes from the host microbiome (Figure 1). As a proof-of-concept, three microbes were isolated from the SD rat host (C. stationis, S. cohnii, and E. faecalis), and two commercially acquired microorganisms (S. aureus ATCC 25923 and E. faecalis V583) were tested using this protocol. To establish the oxygen requirements of individual microbes using FTM, we added two control organisms, an obligate anaerobe (Fusobacterium nucleatum ATCC 25586) and a facultative anaerobe (Pseudomonas aeruginosa PAO1). The results showed that the five tested microbes were facultative anaerobes (Figure 2A), consistent with previously reported profiles of these microbes11. To elucidate their oxygen requirements, the microbes were cultured under both aerobic and anaerobic conditions. All tested microbes exhibited a sigmoidal growth curve in both aerobic and anaerobic conditions; however, the microbial density, as indicated by the OD600nm, showed that all the five microbes have a higher preference for oxygen-rich environments (Figure 2B,C). These observations are consistent with the microbial abilities to infiltrate and infect the rat bones, which are generally considered hypoxic.
The five bacterial isolates were found to naturally form biofilms, where the biofilm mass produced was higher under anaerobic conditions. Through the growth curve comparison, the biofilms of E. faecalis, and S. aureus ATCC 25923 were found to be actively formed during the stationary growth phase in both aerobic and anaerobic environments (Figure 3). This observation suggests that biofilm formation depends on the growth state of the cells, possibly regulated by intracellular signaling molecules that trigger the biofilm formation11. This dependency of biofilm formation on the growth conditions is consistent with the nutrient and growth conditions presented by the host bone matrix environment that lacks oxygen and nutrients.
Studies on the pathogenesis of microbes are often linked to the ability of the microbe to migrate to sites that are considered conducive for growth. There are many studies on microbial chemotaxis, but there is no one standardized approach to study it. In addition, the use of nondiffusible chemicals has been considered a limiting factor in studying this phenomenon. Here, we developed a resin cover produced via 3D printing that standardizes the distance between the chemical reservoir and the inoculation point (Supplemental Figure S1 and Supplemental Figure S2).We studied the migration of the microbes to a nutrient-rich environment, where 5x TB was used as the chemical stimulant. The microbes were monitored for 48 h under aerobic and anaerobic conditions, where all five microbes showed different degrees of tropism migration (Figure 4A).
ImageJ was used to measure the migration distance of bacteria (Figure 4B); migration toward the reservoir appeared to be faster than migration in the opposite direction. The ratio of the migration distance toward the reservoir to that in the opposite direction shows the rapid expansion of the microbial colony due to microbial chemotaxis (Figure 5B). In this study, bacteria such as S. aureus ATCC 25923 migrated faster than other microbes such as C. stationis. Interestingly, the migration profile of chemotactic cultures under aerobic and anaerobic conditions showed that some bacteria, such as E. faecalis, showed variation in the migration patterns under aerobic and anaerobic conditions, wherein a rapid expansion was observed under anaerobic conditions over time. Confoundingly, the laboratory strain E. faecalis V583 favored an aerobic environment for chemotaxis. Thus, this suggests that E. faecalis isolated from different sources have different growth requirements adapted to the growth environment.
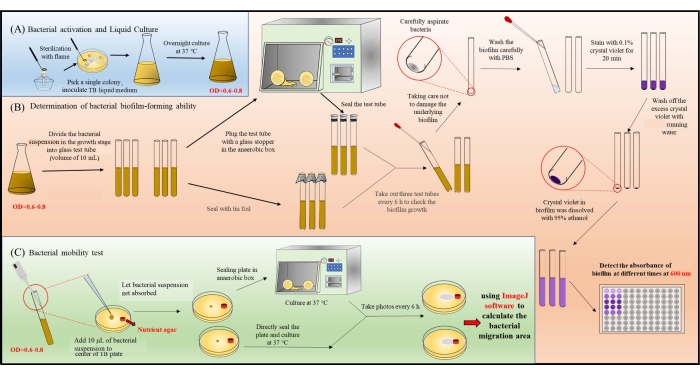
Figure 1: Schematic diagram of the operation process. (A) Bacteria are inoculated on plates and then into liquid medium and grown to the logarithmic growth phase. (B) Determination of the ability of bacteria to form biofilms under different conditions (aerobic/anaerobic). Three samples were selected for each time point (6 h) and repeated in parallel. (C) The protocol for the chemical tropism experiment of bacteria under different conditions (aerobic/anaerobic). Please click here to view a larger version of this figure.

Figure 2: Microbial growth conditions. (A) The growth profile of bacteria cultured in Fluid Thioglycollate Medium (From left to right are I: Fusobacterium nucleatum ATCC 25586, II: Corynebacterium stationis, III: Staphylacoccus cohnii, IV: Enterococcus faecalis, V: Staphylacoccus aureus ATCC 25923, VI: Enterococcus faecalis V583, and VII: Pseudomonas aeruginosa PAO1). (B) Growth curve of microorganisms isolated from the host under aerobic conditions. (C) Growth curve of microorganisms isolated from the host under anaerobic conditions. Please click here to view a larger version of this figure.
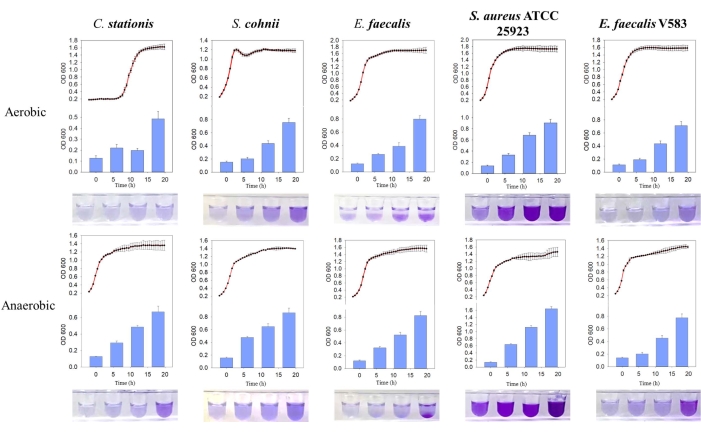
Figure 3: Comparison of growth curves and biofilm formation of different bacteria under different conditions. From left to right are Corynebacterium stationis, Staphylacoccus cohnii, Enterococcus faecalis, Staphylacoccus aureus ATCC 25923, and Enterococcus faecalis V583. The red curve is the growth curve, and the blue histogram is the amount of biofilm produced. Please click here to view a larger version of this figure.
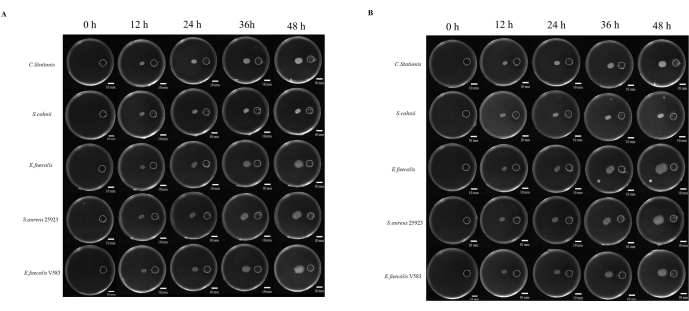
Figure 4: Microbial growth toward eutrophication. (A) Aerobic conditions, (B) anaerobic conditions. Five types of bacteria (from top to bottom are Corynebacterium stationis, Staphylacoccus cohnii, Enterococcus faecalis, Staphylacoccus aureus ATCC 25923, and Enterococcus faecalis V583) tend to grow toward eutrophication on the differential nutrient plate. Scale bars = 10 mm. Please click here to view a larger version of this figure.

Figure 5: Chemotaxis of microbes under different conditions (aerobic/anaerobic). (A) The results of chemical tropism migration of five bacteria on a plate containing nutrient-rich agar; the left group represents aerobic conditions and the right group anaerobic conditions. Each experiment was repeated in triplicate in parallel. (B) Data analysis of chemical tropism. The upper part represents aerobic conditions and the lower part anaerobic conditions. From left to right are Corynebacterium stationis, Staphylacoccus cohnii, Enterococcus faecalis, Staphylacoccus aureus ATCC 25923, and Enterococcus faecalis V583. The red and blue columns are the migration distances from the nutrient body of bacteria migrating toward and away from the nutrient body. The line is the difference between the two values (Red - Blue). Scale bars = 10 mm. Please click here to view a larger version of this figure.
Supplemental Figure S1: Using 3D printed lids to make nutrient difference agar plates. (A) Use software to prepare 3D printing mold renderings. (B) Flat cover mold printed with 8,000 resin. (C) Use the above method to get the nutrient difference agar plates. (D) Production of the nutrient difference agar plates. Please click here to download this File.
Supplemental Figure S2: Construction of nutrient difference agar plates and the measurement of bacterial migration distance using ImageJ. (A) The construction of nutrient difference agar plates uses a 3D printed lid (containing a cylindrical protrusion) to cover the plate. After the agar is cooled, a cylindrical well is formed (the red circle), which is filled with 5x TB agar. This is then cooled to obtain nutrient difference agar plates. (B) Use ImageJ to measure the migration distance of bacteria in different directions. Set scale bar (Analyze | Set Scale) and export migration distance using Straight (Analyze | Measure). Please click here to download this File.
Supplemental Figure S3: Growth patterns of five bacteria on M9 minimal medium-5x TB medium agar. The left group is for aerobic conditions, and the right group is for anaerobic conditions. Please click here to download this File.
Supplemental Figure S4: Control experiments on Fusobacterium nucleatum ATCC 25586 growth curves and chemotaxis of anaerobic bacteria. (A) Determination of growth curves of F. nucleatum ATCC 25586 under aerobic and anaerobic conditions. (B) Chemotaxis experiments of F. nucleatum ATCC 25586 on half-dose MH-5x TB agar plates under different oxygen conditions. Partial enlargement: Growth of F. nucleatum ATCC 25586 on the plate under anaerobic conditions after 48 h (partial magnification). Please click here to download this File.
| Compound | Concentration (g/L) |
| Na2HPO4 | 6.78 |
| KH2PO4 | 3 |
| NaCl | 0.5 |
| NH4Cl | 1 |
| MgSO4.7H2O | 0.493 |
| CaCl2 | 0.011 |
| Glucose | 4 |
Table 1: Components and content of M9 Minimal media.
Discussion
We isolated and identified five species of bacteria by sequential methods. The growth of bacteria has minimal nutrient requirements: the minimal medium-a medium containing only inorganic salts, a carbon source, and water. Although the bacteria in the experimental group were found on MH solid plates, we used half-dose MH medium to verify the chemotaxis of the bacteria and achieved good results. However, we also performed control experiments using minimal medium. M9 basic medium was used in the experiment (see Table 1 for its composition and content), and 5x TB agar was still used for the nutrient agar block. The experimental results are shown in Supplemental Figure S3. Although these bacteria (except E. faecalis) grow slowly and move slowly in M9 medium, there is a generally similar trend (toward growth on nutrient agar), as observed in these experiments. However, E. faecalis V583 did not grow on M9 minimal medium, possibly due to the lack of components required for E. faecalis to grow in M9 medium. Therefore, we recommend using half-dose MH medium as a nutrient-deficient substrate to simulate bacterial growth and migration under nutrient-deficient conditions. This also facilitates the use of ImageJ software to measure the distance over which bacteria tend to migrate.
Biofilms are generally defined as surface-associated microbial communities and are an important microbial survival strategy. Organisms such as Pseudomonas aeruginosa, Haemophilus influenzae, Streptococcus pneumoniae, and Staphylococcus aureus can be found in asymptomatic hosts, and the discovery of adherent microorganisms in vivo is often referred to as colonization, not infection. A very important problem is the lack of an ideal animal colonization model12. Microbes demonstrate initial attachments to charged surfaces before biofilm formation, where materials such as glass and polyvinyl chloride (PVC) are deemed suitable for biofilm-related studies. Studies using PVC 96-well plates have been used to study the microbial biofilms9, wherein single- or multi-species biofilms may result in loosely or densely packed biofilms10. As such, the use of the PVC 96-well plate is considered ill-suited for loosely packed biofilms as the biofilm matrix can easily be washed off during the biofilm staining process. Therefore, we relied on glass test tubes to study microbial biofilm formation (Figure 3).
A key limitation of this method is that it does not work with microorganisms that grow under specific conditions, and not all bacteria can migrate. Moreover, although biofilms are an effective means of helping microorganisms defend against adverse external environments, not all host microorganisms are capable of producing biofilms. For example, F. nucleatum ATCC 25586, mentioned earlier, which is associated with a human oral or gut symbiosis associated with colon cancer development17, only grows specifically in anaerobic environments but does not produce biofilms. We used it in FTM experiments to verify the effectiveness of the FT medium. The FT medium has been shown to be effective in supplying bacteria with gradient concentrations of oxygen.
For comparison, we tested the growth curve and chemical tropism of the anaerobic bacterium F. nucleatum ATCC 25586 (Supplemental Figure S4). In the growth curve experiments (Supplemental Figure S4A), F. nucleatum ATCC 25586 could not grow under aerobic conditions and showed bacterial aggregation at the bottom of the wells under anaerobic conditions. In the chemotaxis experiments (Supplemental Figure S4B) also, F. nucleatum ATCC 25586 did not grow under aerobic conditions, and a low number of bacteria were found after in situ inoculation under anaerobic conditions for 48 h. Similarly, because some bacteria cannot actively seek suitable growth conditions by migrating, they wait for suitable growth conditions by entering a long-term dormant state18. It should be noted that the cultivation of strict anaerobic bacteria requires a special medium, such as ET fermentation medium (semisolid plates must be supplemented with 0.5% agar), which is also different from conventional isolation and identification. This proposed method is a general method applicable to characterize most host microorganisms, with the technique being demonstrated for five microbes, including three microbes isolated from murine bones and two laboratory-grade microbes. This protocol would help the proper identification of microbiota isolates in future studies.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
The development of this technique was supported by the funds from the National Natural Science Foundation of China's Research Fund for International Young Scientists (22050410270), the Shenzhen Special Fund for Innovation and Entrepreneurship of Overseas High-level Talents Peacock Team (KQTD20170810111314625), and the Guangdong Innovative and Entrepreneurial Research Team Program (2019ZT08Y191). We would like to offer our sincere gratitude to Miss Chen Xinyi for her assistance in proofreading the document and laboratory management.
Materials
| Name | Company | Catalog Number | Comments |
| Chemical/Solution | |||
| 1% crystal violet dye solution | Solarbio | G1062 | 100 mL |
| Agar | Sigma-Aldrich | V900500 | Used to obtain semi-solid plates, 20 g |
| Centrifuge tube | Corning | 430790 | 15 mL |
| Fluid thioglycollate medium | Kinghunt | K0001 | 29.3 g |
| Mueller Hinton II Broth medium | Solarbio | NO.11865 | 100 g |
| Petri dishes | Bkman | B-SLPYM90-15 | Plastic Petri dishes, circular, 90 mm x 15 mm |
| Potassium Chloride | VETEC | WXBC4493V | 0.2 g |
| Potassium Dihydrogen Phosphate | aladdin | 04-11-7758 | 0.24 g |
| Sodium chloride | Macklin | S805275 | 8.0 g |
| Sodium phosphate dibasic | aladdin | 7558-79-4 | 1.44 g |
| Terrific Broth medium | Solarbio | LA2520 | 200 g |
| Kits/ Equipment | |||
| Anaerobic incubator | Longyue | ||
| Biochemical incubator | Blue pard | LRH-70 | |
| Microplate reader | Spark | ||
| Tanon 5200multi imaging system | Tanon | 5200CE | |
| Thermostatic water bath | Jinghong | DK-S28 |
References
- Bernardi, S., et al. Combining culture and culture-independent methods reveals new microbial composition of halitosis patients' tongue biofilm. MicrobiologyOpen. 9 (2), 958 (2020).
- Tellez, G., Latorre, J. D., Kuttappan, V. A., et al. Rye affects bacterial translocation, intestinal viscosity, microbiota composition and bone mineralization in Turkey poults. PLOS One. 10, (2015).
- Brewer, J. H. Clear liquid mediums for the "aerobic" cultivation of anaerobes. Journal of the American Medical Association. 115 (8), 598 (1940).
- Azeredo, J., et al. Critical review on biofilm methods. Critical Reviews in Microbiology. 43 (3), 313-351 (2017).
- Liu, C., et al. The regulation of bacterial biofilm formation by cAMP-CRP: a mini-review. Frontiers in Microbiology. 11, 802 (2020).
- Kumar, A., et al. Biofilms: survival and defense strategy for pathogens. International Journal of Medical Microbiology. , (2017).
- Penesyan, A., et al. Secondary effects of antibiotics on microbial biofilms. Frontiers in Microbiology. 11, 2109 (2020).
- Bernardi, S., et al. Subinhibitory antibiotic concentrations enhance biofilm formation of clinical Enterococcus faecalis isolates. Antibiotics. 10 (7), 874 (2021).
- Tsukatani, T., Fumihiko, S., Rieko, K. A rapid and simple measurement method for biofilm formation inhibitory activity using 96-pin microtiter plate lids. World Journal of Microbiology and Biotechnology. 36 (12), 1-9 (2020).
- Hartmann, R., et al. Emergence of three-dimensional order and structure in growing biofilms. Nature Physics. 15, 251-256 (2019).
- Liu, Z., et al. Advances in bacterial biofilm management for maintaining microbiome homeostasis. Biotechnology Journal. 15 (10), 1900320 (2020).
- Hall-Stoodley, L., Stoodley, P. Evolving concepts in biofilm infections. Cellular Microbiology. 11 (7), 1034-1043 (2009).
- Røder, H. L., et al. Enhanced bacterial mutualism through an evolved biofilm phenotype. The ISME Journal. 12 (11), 2608-2618 (2018).
- Matilla, M. A., Tino, K. The effect of bacterial chemotaxis on host infection and pathogenicity. FEMS Microbiology Reviews. 42 (1), 052 (2018).
- Moreira, L. M., et al. Chemotactic signal transduction and phosphate metabolism as adaptive strategies during citrus canker induction by Xanthomonas citri. Functional & Integrative Genomics. 15 (2), 197-210 (2015).
- Konishi, H., et al. Bacterial chemotaxis towards polysaccharide pectin by pectin-binding protein. Scientific Reports. 10 (1), 1-12 (2020).
- Kostic, A. D., et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host & Microbe. 14 (2), 207-215 (2013).
- Kochan, T. J., et al. Updates to Clostridium difficile spore germination. Journal of Bacteriology. 200 (16), 00218 (2018).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved