Method Article
Human Subcutaneous Adipose Tissue Sampling Using a Mini-Liposuction Technique
In This Article
Summary
The manuscript and associated video demonstrate a percutaneous biopsy technique to obtain samples of subcutaneous adipose tissue from areas surrounding the umbilicus. This method is a low-risk and efficient way to investigate a range of parameters (e.g., gene or protein expression, enzyme activity, lipid content) within adipose tissue.
Abstract
Studies on adipose tissue are useful in understanding metabolic and other conditions. Human subcutaneous adipose tissue is accessible. With appropriate training and strict adherence to aseptic technique, subcutaneous adipose samples can be safely and efficiently obtained in a non-clinical setting by researchers. Following the administration of local anesthetic lateral to the umbilicus, a 14 G needle attached to a 5 or 10 mL syringe is inserted through the skin into the subcutaneous tissue. Under suction, the syringe is moved in a reciprocating, slicing motion to isolate fragments of adipose tissue. Withdrawing the plunger is enough to ensure that adipose tissue fragments are aspirated through the needle into the syringe. A single biopsy can collect about 200 mg of tissue. This biopsy technique is very safe for both participants and research staff. Following the biopsy, participants can resume most everyday activities, although they should avoid swimming and overly strenuous activities for 48 h to avoid excessive bleeding. Participants can safely undergo 2 biopsies within a single day, meaning that the technique can be applied in before-after acute intervention studies.
Introduction
Adipose tissue can provide useful information on the metabolic function of humans. Human subcutaneous adipose tissue is readily accessible. A technique for subcutaneous adipose tissue extraction was first described in the mid-80s1; since then, the initial protocol has been improved to increase the yield and improve study participant tolerability. Subcutaneous adipose tissue can be obtained from numerous sites, most commonly from the glutei1 and abdominal area2. Samples from the latter may be more desirable as they provide more valuable information in metabolic disease-related contexts3.
Subcutaneous adipose tissue biopsy using the mini-liposuction method can be safely and efficiently performed in a non-clinical setting. Following appropriate training by a board-certified physician and using strict aseptic technique, researchers can routinely perform these biopsies with minimal risk to both participant and investigators. The biopsy team must consist of at least 2 individuals: the person who will perform the biopsy and an assistant.
The person responsible for the biopsy is tasked with confirming the participant's identity, checking the participant can safely undergo the procedure (see protocol steps 2.1-2.4 below), ensuring the participant is comfortable throughout the procedure, ensuring sterile technique is maintained throughout the procedure, carrying out the procedure, and providing the participant with verbal and written after-care procedures. The assistant's role is to handle and rapidly process the adipose tissue obtained for later analysis and/or storage. The assistant also helps by being the "non-sterile hands" and ensuring the participant is at ease throughout the procedure. The purpose of this video and paper is to describe the step-by-step biopsy procedure to safely obtain subcutaneous adipose tissue from the abdominal area.
Protocol
NOTE: The University of Stirling NHS, Invasive, or Clinical Research Committee approved the biopsy procedure described below. All research studies using this procedure must be approved by the appropriate independent ethics committee. The biopsy taker must have completed formal training in the described technique in accordance with their institution's requirements. Typically, this involves observing a demonstration of the described adipose tissue biopsy technique by a board-certified physician, followed by supervised practice. Once the trainee has performed 10 practice adipose tissue biopsies on volunteer subjects under supervision, they will be examined by a board-certified physician to ensure good knowledge and practice of the procedure. The board-certified physician then provides the individual with a signed examination form.
1. Laboratory room preparation
- Ensure that the laboratory has an appropriately private room with clean, wipeable non-porous surfaces and a clean, comfortable (preferably non-porous) bed on which the participant may lie supine. Clean all required surfaces for the biopsy procedure using 70% ethanol spray and clean paper towels. Provide clean pillows or cushions to support the participant if required.
- Keep appropriate sharps disposal bins and biohazard waste bags within easy reach of the area where the biopsy is being performed and within easy reach of the person taking the biopsy.
- Prepare the equipment required for the procedure and set up on a freshly cleaned general medical trolley prior to the participant arriving to the laboratory (Figure 1). For a complete list of consumables required, see the Table of Materials.
2. Participant preparation
- Ensure that all participants provide written informed consent prior to undergoing the procedure in accordance with protocols required by the institution's independent ethics committee. Additionally, ask the participants to complete a written questionnaire to ensure they are not allergic to any materials used in the procedure (namely, nickel, chromium, local anesthetic, iodine, shellfish, and plasters).
- Confirm the identity of the participant. Ensure the participant understands the procedure to be carried out and potential secondary effects, including bruising, pain, and infection (Table 1). Gather verbal consent in addition to previously obtained written informed consent.
- Describe to the participant how the procedure will be carried out, with emphasis on how the administration of the anesthetic and biopsy itself will feel. Ensure that the participant is comfortable with proceeding.
NOTE: Local subcutaneous anesthetic will produce a stinging sensation, similar to a bee sting of short duration. Many participants report the anesthetic administration as the most uncomfortable part of the technique. Once the anesthetic has taken effect, the participant should feel no more than a slight tugging sensation during the biopsy. - Ensure that the participant has no allergies to the local anesthetic (specifically from the amino-amide type, if using lidocaine or similar), certain metals (nickel and chromium), and shellfish (if using iodine-based solutions). Additionally, ensure that the participants are not taking any form of anticoagulant medication.
- Provide the participant with an opportunity to go and empty their bladder if required, to ensure they do not have to interrupt the procedure or experience undue discomfort in step 4.1.
3. Biopsy procedure - instructions for the biopsy taker
- Once the participant is lying in a supine position, identify the biopsy site approximately 5-10 cm lateral to the umbilicus.
NOTE: If the participant is to undergo multiple biopsies on the same day, identify biopsy sites on opposing sides of the umbilicus for each biopsy. This will ensure maximal distance between each biopsy site. - Wash hands with soap and warm water according to standard medical guidelines4.
- Place the sterile sheet on the cleaned trolley or work area, taking care to only touch the outer edges of the sheet.
- Put on sterile surgical gloves using proper aseptic technique. Have the assistant open the rest of the equipment in such a way that it drops onto the prepared sterile sheet without touching/contaminating the equipment. Ensure that the assistant takes care not to touch items when removing tools from their sterile wrappings.
- Instruct the assistant to dispense a small amount of iodine-based solution on some sterile gauze (without oversaturating the gauze) on the work surface.
- Sterilize approximately 5-10 cm2 around the chosen biopsy site using the sterile gauze and iodine-based solution. Ensure the skin is cleaned in a spiraling motion moving outward from the proposed biopsy site. Repeat the skin cleaning procedure twice. Remove excess liquid (e.g., running off sterile area) by wiping with fresh sterile gauze.
- Along with the assistant, verbally confirm the content of the local anesthetic vial (2% lidocaine in this protocol) and that this is within its expiry date. Instruct the assistant to hold the opened vial upside down and draw 5 mL of local anesthetic into a syringe, using a 21 G needle. Dispose of the needle into the sharps bin, and ensure the syringe is free of air bubbles.
- Apply a 26 G needle to the syringe and expel any air bubbles. Gently pinch the abdominal skin and adipose tissue, moving it away from the abdominal wall. Then, insert the needle horizontally into the subcutaneous tissue at an angle no greater than 10° relative to the surface of the skin.
- Withdraw the syringe's plunger an additional 0.5 mL (to ensure the needle is not in a blood vessel). If blood appears in the syringe, withdraw and reinsert the needle at a different angle.
- Raise a bleb of 2-4 mm diameter to anesthetize the insertion area.
- Advance the needle into the subcutaneous tissue and administer ~1 mL of lidocaine in a fan-shaped pattern (Figure 2), taking care to withdraw the plunger each time before injecting the anesthetic.
- Remove and dispose of the 26 G needle, apply a 21 G needle to the syringe, expel any air bubbles, and administer the remaining ~4 mL of lidocaine in a fan-shaped pattern (Figure 2), taking care to withdraw the plunger each time before injecting the anesthetic.
- Wait approximately 5 min for the local anesthetic to take effect. Use a sterile scalpel to gently prod the biopsy area to i) ensure the local anesthetic has taken effect and ii) identify the boundaries of the anesthetized area. If necessary, wait an additional minute or two and reassess.
- Once satisfied that the local anesthetic is working, gently pinch the skin and adipose tissue (as in step 3.8) and use a sterile scalpel to make a small 1-2 mm puncture in the skin.
NOTE: This only needs to be large enough to ease the entry of the 14 G needle and must be small enough that no suture is required to close it. It is common for some bleeding to occur from this point onwards, which can be controlled with a piece of sterile gauze. - First, apply a 14 G needle to a 5 or 10 mL syringe. Then, while gently pinching the skin and adipose tissue, gradually insert the needle through the puncture into the adipose tissue approximately centrally in the anesthetized area and at an angle no greater than 10° relative to the surface of the skin.
NOTE: For all cases of needle advancement in step 3.11, a syringe angle of no greater than 10° must be maintained.- Apply suction by withdrawing the plunger to approximately the 2.5 mL mark. Take the biopsy by moving the needle in a quick backwards and forwards motion to slice fragments of adipose tissue. After approximately 30 s, twist the needle and syringe through 90° and repeat this procedure to break up the fragments of adipose tissue, which are then aspirated into the syringe by the suction.
NOTE: Other syringe sizes can be used. It is essential that the researcher selects a syringe size that permits both a good grip on the syringe and to comfortably maintain plunger retraction for maintenance of the vacuum. Locking syringes are available that maintain the vacuum, which can improve needle control and reduce perceived difficulty for the biopsy taker 5. - After approximately 45-60 s of step 3.11.1, remove the needle and empty the syringe content onto a layer of gauze covering a weighing boat. Ensure that the lumen of the needle is facing down to avoid potential blood spatter.
- Repeat steps 3.11.1 and 3.11.2 for a maximum of 3 times. Check that the participant is content to proceed before each repeat of the above procedure.
- Whilst performing steps 3.11.1 and 3.11.2, instruct the assistant to process and prepare the samples for analysis/storage (see section 5).
- Apply suction by withdrawing the plunger to approximately the 2.5 mL mark. Take the biopsy by moving the needle in a quick backwards and forwards motion to slice fragments of adipose tissue. After approximately 30 s, twist the needle and syringe through 90° and repeat this procedure to break up the fragments of adipose tissue, which are then aspirated into the syringe by the suction.
4. Post-biopsy procedure
- Once a satisfactory sample (i.e., ~200 mg) of adipose tissue has been obtained, place 1-2 layers of sterile gauze over the puncture wound, then place an ice pack over these, and apply firm pressure for approximately 10 min to induce hemostasis.
- When hemostasis has occurred, wipe away any iodine-based solution/dried blood with sterile gauze, and apply an adhesive wound dressing with absorbent pad to the site. Check that the participant feels well and provide verbal and written instructions on biopsy site aftercare.
- Emphasize that the participants will likely exhibit some bruising for the next few days. Inform them that this may be substantial, although it is minimized by the ice pack in step 4.1 and will resolve without lasting effects.
- Recommend that should the participants feel any discomfort/pain once the anesthetic has worn off, they should take analgesics such as paracetamol following the instructions on the packet but refrain from taking analgesics that have anticoagulant activities (e.g., ibuprofen or aspirin).
- Explain that swelling, redness, or discharge from the biopsy site are indications of infection. In the unlikely event that these signs or symptoms occur, instruct the participant to urgently seek medical advice from a doctor or local Accident & Emergency unit. Inform the participant that if they seek medical advice, they must also notify the research team.
NOTE: As research staff, neither the biopsy taker nor the assistant can provide medical advice or treatment; however, it is important that the research team are aware of and record all instances of complications resulting from the biopsy procedure. - Recommend that participants should avoid swimming or overly strenuous activity for 48 h until the site of incision has closed.
- Clear away any used sharps and contaminated materials into designated sharps and/or clinical waste containers.
- Clean all surfaces used in the biopsy procedure using 70% ethanol spray and clean paper towels. Place disposable and non-disposable items of bedding in appropriate clinical bags for disposal or cleaning, respectively.
5. Sample processing - instructions for the assistant
- Use sterile tweezers and 0.9% saline to rinse the adipose tissue sample to remove visible contaminants (i.e., blood, vasculature). Then, weigh the adipose tissue samples using digital scales. Split the tissue into appropriately sized pieces for downstream analysis and place them into appropriate storing tubes using sterile tweezers. Immerse the tubes containing the adipose tissue biopsies in liquid nitrogen at -190 °C to flash-freeze until the samples are stored at -80 °C.
NOTE: The assistant must complete sample processing as quickly as possible, typically within 3 min of sample aspiration, to minimize potential sample degradation.
Results
The described adipose tissue biopsy procedure is an efficient and low-risk technique for researchers to obtain subcutaneous adipose tissue samples from human volunteers. We performed 39 subcutaneous adipose tissue biopsies using the described procedure in 11 healthy, normal weight females (age, 27.4 ± 3.3 years; body mass index (BMI), 22.6 ± 1.5 kg.m2). All participants attended the laboratory between 07:00 and 10:00 following an 8-12 h fasting period. Sample yield using this adipose tissue biopsy procedure was 192.0 ± 97.1 mg (range = 32.8-393.6 mg) (Figure 4). We observed no relationship between the biopsy yield and participant BMI (p= 0.643), although the participants' BMI were all within the healthy weight range (range= 21.1-25.4 kg/m2). Adequate sample weight was typically obtained following 2-3 bouts of tissue collection (i.e., number of repetitions of steps 3.11.1 and 3.11.2). Following adipose tissue biopsies, all participants experienced a bruise, but none experienced excessive pain that was not alleviated by painkillers. Nor were there any other adverse reactions (Table 1). This is consistent with previously reported complication rates for adipose tissue biopsies1,5.
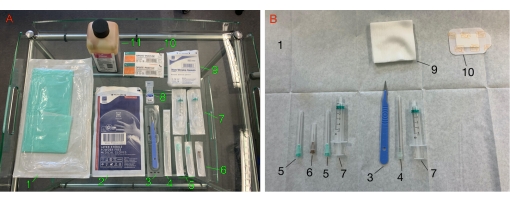
Figure 1: Materials required for the procedure. (A) The trolley laid out with the materials required for the procedure. (B) Materials arranged on the sterile field. 1: sterile field; 2: sterile gloves; 3: scalpel; 4: 14 G needle; 5: 21 G needle; 6: 26 G needle; 7: 5mL syringe; 8: lidocaine 2%; 9: sterile gauze; 10: adhesive wound dressing; 11: iodine-based solution. Please click here to view a larger version of this figure.
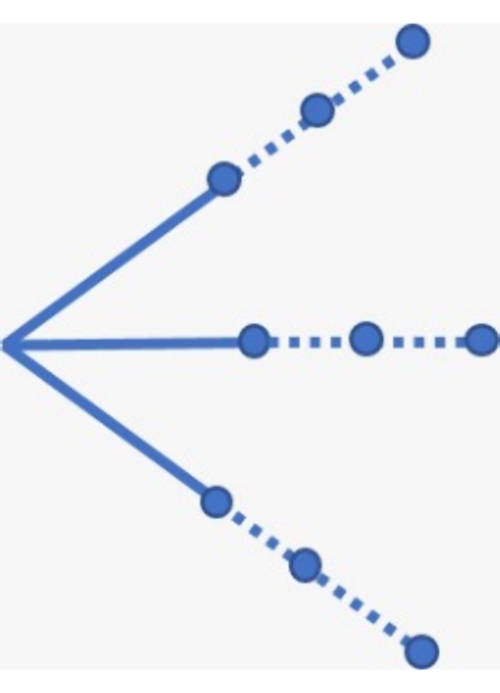
Figure 2: Schematic of the fan-shaped injection sites for administering the local anesthetic. The solid and dotted lines represent where the anesthetic should be administered using the 26 G and 21 G needle, respectively. Please click here to view a larger version of this figure.
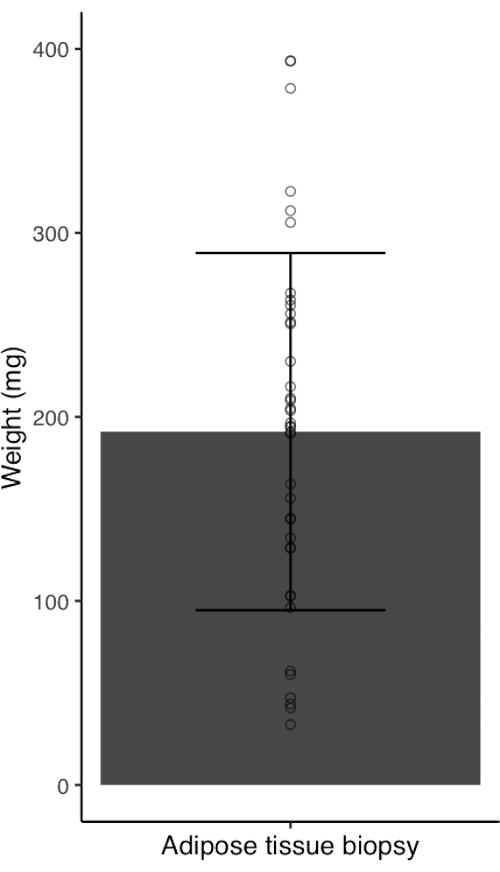
Figure 3: Adipose tissue sample yield from healthy, normal weight women (n= 39). Bar chart with error bars represent mean ± standard deviation. Circles represent individual data points. Please click here to view a larger version of this figure.

Figure 4: An example of a bruise resulting from an early training attempt. Please click here to view a larger version of this figure.
| Complication | Response | |
| Pain | Participant may take analgesics if necessary, following the instructions on the packet (e.g., paracetamol). Participants must refrain from taking analgesics that have anticoagulant activities. | |
| Bleeding | Participant is to be advised that some bleeding is to be expected. | |
| Bruising | Participant is to be advised that bruising is to be expected. | |
| Scar tissue | Participant is to be advised that the development of some scar tissue at the biopsy site is to be expected. | |
| Infection | Participant must be informed of all symptoms of an infection at the biopsy site prior to the biopsy. Participants must be instructed to seek medical advice from a doctor or local Accident and Emergency unit should these symptoms occur and notify the research team retrospectively. | |
Table 1: List of complications that may be experienced by participants.
Discussion
The described protocol and associated video provide a step-by-step overview of a mini-liposuction technique to obtain subcutaneous adipose tissue samples from the abdominal area. This research group has performed a total of 124 biopsies over the course of 19 months with no adverse effects in participants. The procedure is safe and associated with minimum risk to participants or the biopsy team, provided that the described safety measures are followed. Aseptic technique (including opening and dispensing of sterile equipment without contaminating them, appropriately donning/removing sterile gloves, general hand hygiene) must be maintained at all times by the researchers performing the procedure (to minimize the risk of infection to the participant)6. Additionally, disposal of used sharps in an appropriate manner ensures the safety of the researcher and others who handle this waste by reducing the risk of needle-stick injuries7.
Although the procedure can be classed as "low-risk", there are several critical steps in addition to aseptic technique and appropriate waste disposal that need to be followed to minimize adverse effects. Primarily, participants should confirm that they have no allergies to local anesthetics in the amino amide family (e.g., lidocaine) or the drug family of the local anesthetic used, certain metals that may be contained in needles (chromium, nickel, and cobalt), and shellfish/iodine if using an iodine-based skin disinfectant solution (step 2.4). As participants may not be familiar with the name of the anesthetic, and as lidocaine is commonly used in dental procedures, it might be helpful to ask whether they have had a reaction to anesthetic administration in that context. Similarly, participants can be asked whether they had allergic reactions to any jewelry/piercings rather than specifically chromium and nickel. Individuals currently on anticoagulants should not undergo the procedure as they are at increased risk of excessive bleeding. Participants routinely taking low-dose aspirin would not preclude participation in the biopsy protocol; however, participants must inform the biopsy taker as this may affect rate of hemostasis8. While omega-3 fatty acids supplementation would not preclude the biopsy from being performed, participants should confirm whether such supplements (or fatty-rich fish) are part of their routine diet as this may affect blood viscosity9. Prior to commencing the procedure, participants should also be asked whether they have any conditions that might otherwise affect the biopsy. For example, cosmetic surgery (i.e., liposuction) would affect the quantity/quality of tissue sample, and previous scars/tattoo sites should be avoided. Lastly, the biopsy team may want to consider shaving participants with substantial amounts of body hair to make the biopsy area more visible.
When selecting the biopsy area (step 3.1), the researcher should make sure that the site is sufficiently far from the navel (approximately 5-10 cm) as the proximal area is very vascular. Choosing a biopsy site too close to the umbilicus can lead to unnecessarily extensive bruising (e.g., Figure 4). While excessive bruising can be limited by an appropriate choice of biopsy area and the application of an ice pack following the procedure, participants should be informed that some degree of bruising is likely to occur. Within this research group, we anecdotally observed that such contusions dissipate within 3-5 days. In addition, some participants may develop some scar tissue at the biopsy site, presenting as a lump of tissue hard to the touch. Anyone undergoing the biopsy procedure should be made aware that the scar tissue is transient and will resolve itself within 2-3 weeks. To maximize patient tolerability, the researcher should identify the area affected by the local anesthetic (step 3.9): by using a scalpel and gently prodding the biopsy area, the researcher can verbally confirm with the participant that the area has been successfully anesthetized. The limits of the anesthetized area should be confirmed by going beyond the area. Inform the participant that this will be done and that they may feel some very slight discomfort. This is a particularly important step, as placing the biopsy needle in non-anesthetized areas will cause participant discomfort.
The mini-liposuction biopsy technique described here is a low-cost alternative to surgical procedures and does not require specialist tools. Owing to their straightforwardness, these biopsies can be performed routinely with little-to-no problems. The most common issue encountered when performing the adipose tissue sampling is that the shaft of the 14 G needle can become obstructed, preventing adipose tissue aspiration into the syringe. An experienced individual trained in the described biopsy technique will notice the obstruction through changes in the responsiveness of the syringe's plunger (i.e., it "sticks" in place). Should a needle obstruction occur, the researcher is advised in primis to attempt removing the obstruction by forcefully depressing the plunger while the needle bevel is over the weighing boat. If the obstruction is firmly lodged, the second option is to replace the needle and syringe. After the procedure, tissue lodged in a needle can be retrieved by pushing sterile saline through the needle. To prevent sample degradation, the obtained tissue should be cleaned, processed, and stored as soon as possible following the procedure10. To minimize RNA degradation, a stabilization solution can be utilized at the sample processing step17 (please refer to the Table of Materials).
The main limitation of this technique is that while it is relatively fast (~15 min for a trained and experienced individual) and cost-effective, it results in only a moderately sized sample (~200 mg). Whilst this sample size is typically adequate for various metabolic assays, it is recommended that the researcher ensures the expected sample yield is sufficient for the intended sample analysis. The sample yield obtained using the described technique is typically lower than that of surgical techniques11; however, larger incision sites used in surgical biopsies cause more discomfort to participants and may prevent them from engaging in certain day-to-day activities until fully healed11. These techniques are also more likely to discourage participants from enrolling in research studies and require a trained clinician. A key advantage of the mini-liposuction biopsy described in this video is that it can be quickly performed in a non-clinical setting by non-medical researchers. Furthermore, being able to complete multiple biopsies on one participant within the same day enables researchers to perform acute before-after nutritional/exercise intervention studies. It should be noted that in the UK, lidocaine administration requires a prescription; a member of our team is qualified in non-medical prescribing. Local regulations should be checked before the administration of local anesthetic.
Many research groups have applied the mini-liposuction technique for a variety of research questions. These include, but are not limited to, providing adipose tissue hormone profiles in participants with diabetes2, quantifying the variation of adipose tissue miRNA expression in patients with metabolic dysfunction12, and assessing nutritional and exercise interventions in overweight populations13,14. Additionally, the immediate processing of adipose tissue samples permits; isolation of pre-adipocytes for cell culture15; and analysis of ex vivo metabolic parameters, such as lipolytic rate16, hormone secretion13, and mitochondrial respiration14. It must be noted that adipose tissue samples obtained via the described technique have high levels of fragmentation when compared to samples obtained via surgical techniques using a cutting needle or scalpel5. This precludes the successful usage of analytical techniques for the assessment of architectural and morphological parameters5. Should researchers intend to obtain adipose tissue samples for analysis of architectural and morphological parameters, alternative methods are associated with reduced tissue fragmentation18. Nonetheless, obtaining adipose tissue samples via the described technique permits the investigation of a broad range of key physiological processes.
In summary, the present video and paper describe a non-clinical mini-liposuction biopsy technique to obtain subcutaneous abdominal adipose tissue. With appropriate controls in place, the method is relatively pain-free, safe, and time-/cost-effective. This biopsy method is particularly well suited for studies that implement a before-after study design and do not require large amounts of tissue sample.
Disclosures
The authors have no conflicts of interest to declare.
Acknowledgements
The authors have no funding to declare.
Materials
| Name | Company | Catalog Number | Comments |
| Item: 14 G needle 14 G x 3 1/8" 210 mm x 80 mm | B Braun | 4665473 | Per biopsy: 1 |
| Item: 21 G needle 21 G x 1 1/2" 0.8 mm x 38 mm | Terumo | AN*2138R1 | Per biopsy: 2 |
| Item: 26 G needle 26 G x 1/2" microlance needle 0.45 mm x 13 mm | BD | 303800 | Per biopsy: 2 |
| Item: 5 mL syringe 5 mL luer | DB plastipak | 302187 | Per biopsy: 2 |
| Item: Adhesive wound dressing Opsite Post-Op Dressing 9.5 x 8.5 | Smith & Nephew | 6600709 | Per biopsy: 1 |
| Item: Disposable sterile scalpel Disposable Scalpel Sterile Blade no. 10 | Swann Morton | /0501 | Per biopsy: 1 |
| Item: Icepack BlueDot Reusable Hot/Cold Pack 26.5 cm x 13.0 cm | NuCare | F711 | Per biopsy: 1 |
| Item: Iodine based antiseptic Videne antiseptic solution | Ecolab Videne | 3030440 | Per biopsy: q.s |
| Item: Lidocaine 2% w/o epinephrine Lidocaine 2% injection 5 mL | B Braun | 3558553 | Per biopsy: 5 mL |
| Item: Non-sterile gloves Starguard sensitive powder free nitrile gloves | Starguard | SG-N-S | Per biopsy: pair |
| Item: Sodium chloride 0.9% Sodium chlride 0.9% w/v intravenous infusion BP | BBraun | S8004-5384 | Per biopsy: q.s. |
| Item: Stabilization solution* RNAlater Stabilization Solution | ThermoFisher Scientific | AM7020 | Per biopsy: q.s |
| Item: Sterile forceps Sterile forceps | Rocialle | RML109-006 | Per biopsy: 1 |
| Item: Sterile gauze swabs Non woven swabs sterile 7.5 x 7.5 cm | Prestige | 1860 | Per biopsy: 5 |
| Item: Sterile gloves Prestige soft vinyl sterile powder free medical gloves | Prestige | S: P4301 M:P3302 L:P3301 | Per biopsy: pair |
| Item: Sterile Microcentrifuge tubes 1.5 mL Sterile Microcentrifuge Tubes | StarLab | I1415-5510 | Per biopsy: q.s |
| Item: Sterile sheet Paper plain white 90 x 90 cm | Rocialle | RML 126-216 | Per biopsy: 1 |
| Item: Weighing boat Diamond shape weigh boats | Heathrow Scientific | HS1427C | Per biopsy: 1 |
| * denotes optional materials |
References
- Beynen, A. C., Katan, M. B. Rapid sampling and long-term storage of subcutaneous adipose-tissue biopsies for determination of fatty acid composition. American Journal of Clinical Nutrition. 42 (2), 317-322 (1985).
- Moran, C. N., et al. Effects of diabetes family history and exercise training on the expression of adiponectin and leptin and their receptors. Metabolism. 60 (2), 206-214 (2011).
- Jialal, I., Devaraj, S. Subcutaneous adipose tissue biology in metabolic syndrome. Hormone Molecular Biology and Clinical Investigation. 33 (1), (2018).
- WHO Guidelines on hand hygiene in health care: a summary. World Health Organization Available from: https://www.who.int/gpsc/5may/tools/who_guidelines-handhygiene_summary.pdf (2009)
- Kettwich, L. G., et al. New device technologies for subcutaneous fat biopsy. Amyloid. 19 (2), 66-73 (2012).
- Preston, R. M. Aseptic technique: evidence-based approach for patient safety. British Journal of Nursing. 14 (10), 540-546 (2005).
- Handiyani, H., Meily Kurniawidjaja, L., Irawaty, D., Damayanti, R. The effective needle stick injury prevention strategies for nursing students in the clinical settings: a literature review. Enfermeria Clinica. 28, 167-171 (2018).
- Raggio, B. S., Barton, B. M., Kandil, E., Friedlander, P. L. Association of continued preoperative aspirin use and bleeding complications in patients undergoing thyroid surgery. JAMA Otolaryngology-Head & Neck Surgery. 144, 335 (2018).
- Cartwright, I. J., Pockley, A. G., Galloway, J. H., Greaves, M., Preston, F. E. The effects of dietary omega-3 polyunsaturated fatty acids on erythrocyte membrane phospholipids, erythrocyte deformability and blood viscosity in healthy volunteers. Atherosclerosis. 55 (3), 267-281 (1985).
- Hemmrich, K., Denecke, B., Paul, N. E., Hoffmeister, D., Pallua, N. RNA isolation from adipose tissue: an optimized procedure for high RNA yield and integrity. Laboratory Medicine. 41 (2), 104-106 (2010).
- Chachopoulos, V., et al. A technique for subcutaneous abdominal adipose tissue biopsy via a non-diathermy method. Journal of Visual Experiments: JoVE. (127), e55593 (2017).
- Civelek, M., et al. Genetic regulation of human adipose microRNA expression and its consequences for metabolic traits. Human Molecular Genetics. 22 (15), 3023-3037 (2013).
- Chen, Y. C., et al. Feeding influences adipose tissue responses to exercise in overweight men. American Journal of Physiology Endocrinology and Metabolism. 313 (1), 84-93 (2017).
- Mendham, A. E., et al. Exercise training results in depot-specific adaptations to adipose tissue mitochondrial function. Scientific Reports. 10 (1), 3785 (2020).
- Carswell, K. A., Lee, M. J., Fried, S. K. Culture of isolated human adipocytes and isolated adipose tissue. Methods in Molecular Biology. 806, 203-214 (2012).
- Arner, P., Andersson, D. P., Backdahl, J., Dahlman, I., Ryden, M. Weight gain and impaired glucose metabolism in women are predicted by inefficient subcutaneous fat cell lipolysis. Cell Metabolism. 28 (1), 45-54 (2018).
- Mutter, G., et al. Comparison of frozen and RNALater solid tissue storage methods for use in RNA expression microarrays. BMC genomics. 5, 88 (2004).
- Coleman, S. Structural fat grafting: more than a permanent filler. Plastic and Reconstructive Surgery. 118, 108-120 (2018).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved