Method Article
Bronchoalveolar Lavage and Oleic Acid-Injection in Pigs as a Double-Hit Model for Acute Respiratory Distress Syndrome (ARDS)
In This Article
Summary
Different, complex animal models exist to study the pathophysiology of acute respiratory distress syndrome (ARDS). Bronchoalveolar lavage and oleic acid injection induced lung injury is suitable as a new double-hit animal model for studying the acute respiratory distress syndrome.
Abstract
The treatment of ARDS continues to pose major challenges for intensive care physicians in the 21st century with mortality rates still reaching up to 50% in severe cases. Further research efforts are needed to better understand the complex pathophysiology of this disease. There are different well-established animal models to induce acute lung injury but none has been able to adequately mimic the complex pathomechanisms of ARDS. The most crucial factor for the development of this condition is the damage to the alveolar capillary unit. The combination of two well-established lung injury models allow us to mimic in more detail the underlying pathomechanism. Bronchoalveolar lavage (BAL) leads to surfactant depletion as well as alveolar collapse. The repeated instillation of fluid volumes causes subsequent hypoxemia. Surfactant depletion is a key factor of ARDS in humans. BAL is often combined with other lung injury approaches, but not with a second hit followed by oleic acid injection (OAI) yet. Oleic acid injection leads to severely impaired gas exchange, a deterioration of lung mechanics and disruption of the alveolo-capillary barrier. The OAI mimics most of the expected effects of ARDS consisting of extended inflammation of lung tissue with an increase of alveolar leakage and gas exchange impairment. A disadvantage of the combination of different models is the difficulty to determine the influence to the lung injury caused by BAL alone, OAI alone or both together. The model presented in this report represents the combination of BAL and OAI as a new double-hit lung injury model. This new model is easy to implement and an alternative to study different therapeutic approaches in ARDS in the future.
Introduction
Acute respiratory distress syndrome (ARDS) is a disease consisting of impaired gas exchange and lung infiltration, which often needs intensive care therapy. The mortality of severe ARDS remains high (up to 50%) worldwide despite almost 50 years of extensive research1. The ARDS is defined by the Berlin Definition, including diagnostic criteria as timing, chest imaging, origin of edema and hypoxemia2. To better categorize patients with different levels of ARDS severity, three different degrees of hypoxemia are defined: mild (200 mmHg < PaO2/FIO2 ≤ 300 mmHg), moderate (100 mmHg < PaO2/FIO2 ≤ 200 mmHg), and severe (PaO2/FIO2 ≤ 100 mmHg)2. Different animal models with a focus on lung injury are widely used and accepted to examine the pathophysiological changes and different therapeutic approaches in ARDS3.
Animal models using endotoxins (e.g., intravenous infusion of bacteria, cecal ligation and puncture to mimic a sepsis-induced lung injury), ischemia/reperfusion models, smoke/burn ARDS models, infusion of oleic acid and bronchoalveolar lavage models are known3. Each model represents only a few pathophysiological changes with advantages and disadvantages to the study results3. This does not reflect the complexity of the ARDS disease. The combination of two proven models allows better conclusions about the pathophysiology of ARDS. In the presented model, we combined bronchoalveolar lavage and oleic acid infusion to mimic the complexity of the human ARDS. Oleic acid is an unsaturated fatty acid and acts directly on the alveolo-capillary unit of the lungs by triggering activation of innate immune receptors subsequently causing neutrophil accumulation, proinflammatory cytokine production and cell death4,5. Oleic acid infusion induces severe hypoxemia, increases in pulmonary arterial pressure and accumulation of extravascular lung water. Hypotension and myocardial depression due to right ventricular failure often occur. The induction of lung injury by repeated bronchoalveolar lavage (BAL) with balanced electrolyte solution reduces the alveolar surfactant lipid concentration3. Surfactants decrease alveolar surface tension and prevent alveolar collapse. BAL causes immediate hypoxemia and an increase of the alveolar-arterial oxygen difference3. Human ARDS is also associated with depletion of surfactant3. The disadvantages of this combined model are the necessity for central venous access, intubation and general anesthesia. Furthermore, the questionable mechanistic relevance (e.g., the oleic acid infusion) for translational aspects remains unclear. At least, it is difficult to determine which part of the lung injury (BAL vs. OAI, or both together) contributes to lung damage. The advantages of this model are its usability in large animals with familiar monitoring and instrumentation similar to human patients (no special equipment required), the good reproduction of the main aspects of ARDS and the possibility to study isolated ARDS without systemic inflammation (e.g., endotoxin models). In the following article, we give a detailed description of the double-hit (BAL and OAI) lung injury in pigs and provide representative data to characterize the stability of the compromises in lung function.
Protocol
All animal experiments described here have been approved by the institutional and state animal care committee (Landesuntersuchungsamt Rheinland-Pfalz, Koblenz, Germany; approval number G18-1-044) and were conducted in accordance with the guidelines of the European and German Society of Laboratory Animal Sciences.
1. Anesthesia, intubation and mechanical ventilation
- Withhold food for 6 hours before anesthesia to reduce the risk of aspiration, but allow free access to water to reduce stress.
- Inject a combination of ketamine (4 mg∙kg-1) and azaperone (8 mg∙kg-1) intramuscularly for sedation while the animal is in the animal box.
- Establish a venous line with a common peripheral venous catheter (20 G) in an ear vein after local disinfection with alcohol.
- Start monitoring the peripheral oxygen saturation (SpO2) by clipping the sensor onto one of the ears or the tail of the animal.
- Inject fentanyl (4 µg∙kg-1), propofol (3 mg∙kg-1) and atracurium (0.5 mg∙kg-1) for induction of anesthesia.
- Place the pig in a supine position on the stretcher.
- Ventilate the pig with a mask suitable for animals with a peak inspiratory pressure below 20 cmH2O, a PEEP of 5 cmH2O, a frequency of 14-16 /min and a FiO2 of 1.0.
- Start a continuous infusion with balanced electrolyte solution (5 mL∙kg-1∙h-1), propofol (8-12 mg∙kg-1∙h-1) and fentanyl (0.1-0.2 mg∙kg-1∙h-1) to maintain anesthesia.
- For the intubation, use a common endotracheal tube suitable for the animal (e.g., weight of 25-30 kg, endotracheal tube ID 6-7) armed with endotracheal tube introducer, and a common laryngoscope with a Macintosh Blade 4. Two people are necessary.
- Person 1: Pull out the tongue with one hand and press the snout down with the other.
- Person 2: Insert the laryngoscope and advance it as usual until the epiglottis can be visualized.
- Pull the laryngoscope upwards to visualize the vocal cords. Sometimes the epiglottis “sticks” to the soft palatine. If so, mobilize it with the tip of the tube.
- Insert the tube through the vocal cords and pull out the introducer.
- Block the balloon of the tube.
- Connect the tube to the ventilator and check correct positioning with capnography and auscultation.
- Start mechanical ventilation (tidal volume 6-8 mL/kg, PEEP 5 cmH2O, FiO2 0.4, frequency to keep etCO2 between 35 – 45 mmHg).
2. Instrumentation
- Retract the hind legs with bandages for catheterizing necessary vessels. An arterial line, an arterial introducer sheath, a central venous line and a venous introducer sheath for pulmonary artery catheter placement are necessary.
- Generously disinfect the femoral area with alcoholic disinfection. Depending on the planned examinations, a more or less aseptic approach is used.
- Prepare catheters by flushing them with saline.
- Place the ultrasound probe on the inguinal ligament and scan for femoral vessels.
- Turn the probe 90° to fully visualize the femoral arteria in the long axis. If necessary, it is also possible in different circumstances to use the short axis view to fully visualize the femoral arteria.
- Cannulate the femoral arteria under in-line ultrasound visualization with the needle of the introducer set in Seldinger’s technique. When pulsating bright blood flows out, introduce the guidewire and retract the needle.
- Visualize the femoral vein and cannulate the vein under in-line ultrasound visualization and continuous aspiration with the needle of the introducer set. When venous blood is aspirable, disconnect the syringe and insert the guidewire. Retract the needle.
- Check position of the wires with ultrasound.
- Insert the arterial and the venous line over the placed guidewires.
- Repeat arterial and venous punctuation on other side and insert the introducer sheaths per Seldinger´s technique as described above.
- Connect the arterial line and the venous line each to a transducer.
- Position both transducers at heart level and switch the three-way stopcocks of both transducers open to the atmosphere to calibrate the system to zero.
NOTE: It is necessary to avoid any air bubbles and bloodstains in the system to generate plausible values. - Switch infusion of propofol and fentanyl to one of the ports of the central venous line.
- Calibrate the probe for ultrafast pO2-measurements and insert it through the arterial introducer sheath.
NOTE: The measurement of pO2 with the probe for ultrafast pO2-measurement is not obligatory but helps visualizing the real-time changes in pO2.
3. Inserting pulmonary artery catheter
- Check the balloon of the pulmonary artery catheter (PAC) for damage.
- Connect to the transducer and calibrate it.
- Insert the PAC through the introducer sheath (balloon deflated).
- When the PAC has passed through the introducer sheath (15-20 cm), inflate the balloon.
- Advance the PAC and monitor the typical waveforms (venous vessels, right atrium, right ventricle, pulmonary arteria, pulmonary capillary wedge pressure). Deflate the balloon and check if blood can be aspirated through all ports of the PAC.
4. Induction of lung injury: first hit by bronchoalveolar lavage
- Prepare sterile balanced electrolyte solution (e.g., Sterofundin) warmed to 40 °C.
NOTE: Sterile balanced electrolyte solution is used to avoid pulmonary pollution. - Change the FiO2 from 0.4 to 1.0 over 10 min before performing the bronchoalveolar lavage.
- Start the ultrafast pO2-measurement.
- Prepare norepinephrine for continuous infusion and for bolus injection (if mean arterial pressure < 60 mmHg). Connect the norepinephrine syringe pump to one of the ports of the central venous catheter without starting it.
- Fill 30 mL∙kg-1 from the warmed sterile balanced electrolyte solution into a funnel. Check that the funnel can be connected to the endotracheal tube.
- Disconnect the tube without PEEP loss in inspiration from the ventilator.
- Connect the funnel to the endotracheal tube.
- Raise the funnel 1 m above the animal manually.
- Open the cap and instill the entire amount of the warmed balanced electrolyte solution from the funnel into the endotracheal tube over 30 s by using the hydrostatic pressure.
- After 30 s, remove the infused solution by lowering the funnel 1 meter below the animal and drain the lavage fluid passively. Reconnect the animal to the ventilator for oxygenation.
- Collect the removed lavage fluid and note the amount. It is needed to calculate the alveolar fluid clearance.
NOTE: Do not reuse the balanced electrolyte solution after a lavage to maximize surfactant wash out. - Aspirate the remainders of the solution in the tube by using suction catheters.
- Closely monitor hemodynamics after the bronchoalveolar lavage and keep norepinephrine at hand. If necessary, give norepinephrine as bolus or continuous infusion to stabilize blood pressure (compare to step 4.4).
- Repeat infusion of 30 mL∙kg-1 balanced electrolyte solution as described in the steps 4.5-4.13 until PaO2/FiO2-ratio is below 250 mmHg. Four to five repetitions of the bronchoalveolar lavage may be necessary.
- If PaO2/FiO2-ratio is lower than 250 mmHg, start with the induction of lung injury by oleic acid injection. Do not change the ventilator settings during this procedure.
5. Induction of lung injury: second hit by oleic acid injection
- Prepare oleic-acid solution: 0.1 mL∙kg-1 of oleic acid in a 20 mL syringe and connect it to a 3-way-stopcock. Take 2 mL of blood in another 20 mL syringe. Add saline to a total volume of 20 mL in both syringes and connect the second syringe also to the 3-way-stopcock.
CAUTION: Use gloves and eye protection when working with oleic acid. - Prepare norepinephrine for continuous infusion and for bolus injection (if mean arterial pressure < 60 mmHg). Connect the norepinephrine syringe pump to one of the ports of the central venous catheter without starting it.
- Continue to monitor the ultrafast pO2-measurement that still measuring. FiO2 is still 1.0.
- Connect the 3-way-stopcock to the proximal port of the PAC.
- Mix the oleic acid and the blood/saline mixture thoroughly by repeated shifting of the solution from one syringe to the other syringe and vice versa via the three-way-stopcock and keep mixing all the time. When it is a homogenous emulsion, inject 2 mL of the emulsion and continue mixing.
NOTE: If mixing stops, the emulsion may separate into a lipophilic and a hydrophilic part. - Closely monitor hemodynamics after injection of oleic acid and keep norepinephrine at hand. If necessary, give norepinephrine as bolus or continuous infusion to stabilize blood pressure (compare to step 5.2).
NOTE: Be vigilant; the animals can die during this procedure. - Repeat injection of 2 mL of the solution every 3 min until PaO2/FiO2-ratio is below 150 mmHg.
- If the syringe is empty before PaO2/FiO2-ratio is between 100 and 200 mmHg, prepare 2 more syringes as described in step 5.1. Repeat the steps 5.5-5.8 until the PaO2/FiO2-ratio falls between 100 and 200 mmHg.
NOTE: A half to full syringe of the oleic acid and the blood/saline mixture is usually required. - If the PaO2/FiO2-ratio is between 100 and 200 mmHg, wait 30 min and check again. If it is consistently below 200 mmHg, start the experiment/treatment; otherwise prepare 2 more syringes as described in step 5.1 and repeat steps 5.5-5.9.
NOTE: After induction of lung injury as described, the impairment of lung function can remain stable or deteriorate or even improve within certain limits.
6. End of experiment and euthanasia
- Inject 0.5 mg of fentanyl additionally to the continuous anesthesia and wait 5 min. Inject 200 mg of propofol and 40 mmol of potassium chloride to kill the animal in deep anesthesia.
Results
The PaO2/FiO2-ratio decreases after bronchoalveolar lavage and fractionated application of oleic acid (Figure 1). Because it is unclear to predict the impact of bronchoalveolar lavage (e.g., the impact of fractionated oleic acid dose) on PaO2/FiO2-ratio, it is recommended to monitor PaO2/FiO2-ratio while inducing the lung injury. Ultrafast pO2-measurement allows to monitor PaO2 in real-time and is well established6. After the double-hit, PaO2/FiO2-ratio should persist between 100 and 200 mmHg for 30 min at a PEEP of 5 cm H2O. If it is above 200 mmHg repeat the steps 5.8 and 5.9 as described above to prevent spontaneous recovery of the animal during the time course of the experiment. Simultaneously, mean pulmonary arterial pressure (mPAP) increases during the induction of the lung injury and remains higher over the entire experiment (Figure 2). This acute pulmonary hypertension can lead to sudden hemodynamic decompensation and death of the animal. To prevent these events, hemodynamics should be measured closely and deviations treated immediately with catecholamines (e.g., norepinephrine). The functional residual capacity (FRC) of the lung also drops after induction of the lung injury and remains lower for the rest of the experiment (Figure 3). Lung injury is also histologically (Figure 4) detectable in lungs taken out after death of the animal.

Figure 1: Development of PaO2/FiO2-Ratio during 8 hours after double-hit lung injury in 4 exemplary pigs.
Representative plots showing an initial decrease in all 4 animals and stable values with little fluctuations (animal 4). Afterwards an initial rise followed by a decrease was detectable in 2 animals (animal 2 and 3). Continuously lower values remained after induction in one animal (animal 4). Please click here to view a larger version of this figure.

Figure 2: Development of mPAP (= mean pulmonary arterial pressure) during 8 h after double-hit lung injury in 4 exemplary pigs.
Representative plots showing an initial rise in all 4 animals. In two animals (animal 3 and 4) mPAP fell over 6 hours and rose finally, in two other animals (animal 1 and 2) mPAP dropped continuously. Please click here to view a larger version of this figure.
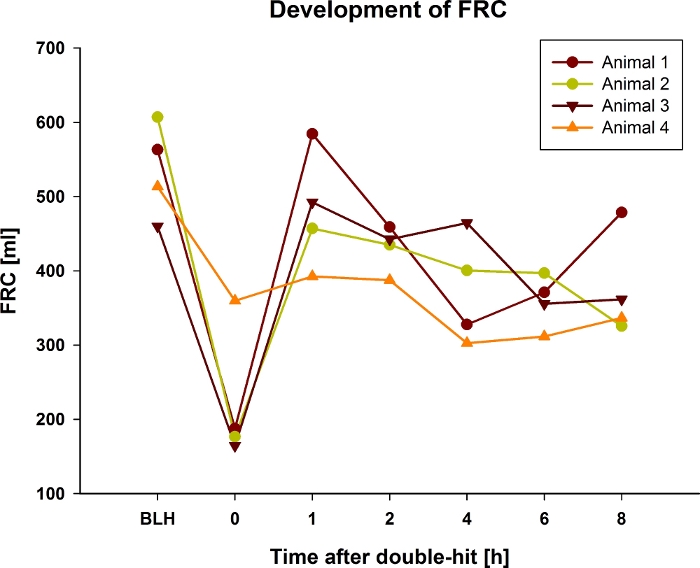
Figure 3: Development of FRC during 8 hours after double-hit lung injury in 4 exemplary pigs.
Representative plots showing an initial decrease in all 4 animals. Afterwards only one animal (animal 4) remained stable at lower values, the other animals rose shortly to drop down evenly over the experiment. Please click here to view a larger version of this figure.

Figure 4: Histologic images of lung injury after double-hit lung injury.
The lungs were fixed in formalin for paraffin sectioning and haematoxylin/eosin staining. Image magnification 10x. (A) Lymphocytic infiltration (red arrow) & atelectasis (black arrow). (B) Overdistension (red arrow) & alveolar damage (black arrow). Please click here to view a larger version of this figure.
Discussion
The described double-hit method to cause a severe lung injury in pigs is suitable to study different treatment options in ARDS. The double-hit model mimics two central elements of the pathomechanism of ARDS: loss of the alveolar-capillary unit and disruption of the endothelial barrier7. Due to the two hits, it is important to have a study protocol with predefined target values (e.g., PaO2/FiO2-ratio).
The main disadvantage of this double-hit method is the difficulty to determine the extent to which the lung injury is caused by the balanced electrolyte solution lavage, the oleic acid infusion or both. This is a common problem in other combined lung injury models as described (e.g., for a lavage and mechanical-ventilation-hit method3). Furthermore, the oleic acid method was first described to investigate the mechanism of acute lung injury caused by fat/lipid embolism8. Oleic acid is insoluble in water and must be emulsified in blood continuously. After injection of repeated doses of oleic acid, severe hemodynamic changes such as myocardial depression, systemic hypotension and pulmonary hypertension can occur immediately and result in right ventricular failure3,6,9. Right ventricular failure can lead to sudden death of the animal. During the study, two animals were lost due to the described effects. The surfactant depletion by instillation of warmed balanced electrolyte solution was first developed by Lachmann10. Human ARDS is often associated with the depletion of surfactant from the alveoli3. In animal models, repeated instillations of balanced electrolyte solution must be performed. At first, hypoxemia is reached very fast, but rapidly reversible under mechanical ventilation3. The permeability and inflammation are not significantly affected and triggered by balanced electrolyte solution3. Inflammatory changes (e.g., neutrophilic alveolitis) occur after about 4 h. The major disadvantage of using balanced electrolyte solution is that the animals require intubation, mechanical ventilation and general anesthesia3.
Overall, there are also some major advantages when using and combining both methods in pigs. Lung injury caused by oleic acid infusion is well known and has been described in detail before3,6. Kamuf et al. reported reproducible results in small and large animals and hence presented a well-suited model for studying ARDS6. Oleic acid is directly toxic to the endothelial cells3. Endothelial injury is followed by increased pulmonary microvascular permeability and intrapulmonary shunt3. Severe problems in the gas exchange can occur3. Oleic acid infusion does not only mimic the early phase of ARDS, it also reproduces the later pathophysiological changes with deposition of fibrin on the alveolar surface. Bronchoalveolar lavage is a common model to induce lung injury and is well established11. The histopathological findings in human ARDS (e.g., atelectasis and perivascular edema) can be caused by repeated lavages11. Oleic acid infusion leads to profound alveolar necrosis, congestion and edema formation, whereas bronchoalveolar lavage induces more barotrauma, atelectasis and haemorrhage9.
With this double-hit model, different ventilation strategies, specific therapeutic approaches and the investigation of pulmonary changes and lung function in ARDS can be investigated. Combining these two methods result in an ARDS model closer mimicking the pathophysiological changes occurring during human ARDS. The instrumentation and the extended monitoring of the pigs are easier to perform in large animals and are more similar to the bedside setting in the intensive care unit. In summary, this model is highly reproducible and allows further research to investigate different therapeutic approaches for the treatment of ARDS in a more realistic setting.
Disclosures
All authors disclose no financial or any other conflict of interest.
Acknowledgements
The authors want to thank Dagmar Dirvonskis for excellent technical support.
Materials
| Name | Company | Catalog Number | Comments |
| 1 M Kaliumchlorid-Lösung 7.46% 20 mL | Fresenius, Kabi Deutschland GmbH | potassium chloride | |
| Absaugkatheter Ideal CH14, 52 cm, gerade | B. Braun Melsungen AG, Germany | suction catheter | |
| Arterenol 1 mg/mL, 25 mL | Sanofi- Aventis, Seutschland GmbH | norepinephrine | |
| Atracurium Hikma, 50 mg/5 mL | Hikma Pharma GmbH , Martinsried | atracurium | |
| BD Discardit II Spritze 2, 5, 10, 20 mL | Becton Dickinson S.A. Carretera Mequinenza Fraga, Spain | syringe | |
| BD Luer Connecta | Becton Dickinson Infusion Therapy AB Helsingborg, Schweden | 3-way-stopcock | |
| BD Microlance 3 20 G | Becton Dickinson S.A. Carretera Mequinenza Fraga, Spain | canula | |
| Datex Ohmeda S5 | GE Healthcare Finland Oy, Helsinki, Finland | hemodynamic monitor | |
| Engström Carestation | GE Heathcare, Madison USA | ventilator | |
| Fentanyl-Janssen 0.05 mg/mL | Janssen-Cilag GmbH, Neuss | fentanyl | |
| Führungsstab, Durchmesser 4.3 | Rüsch | endotracheal tube introducer | |
| Incetomat-line 150 cm | Fresenius, Kabi Deutschland GmbH | perfusorline | |
| Ketamin-Hameln 50 mg/mL | Hameln Pharmaceuticals GmbH | ketamine | |
| laryngoscope | Rüsch | laryngoscope | |
| logicath 7 Fr 3-lumen 30 cm lang | Smith- Medical Deutschland GmbH | central venous catheter | |
| Masimo Radical 7 | Masimo Corporation Irvine, Ca 92618 USA | periphereal oxygen saturation | |
| Neofox Oxygen sensor 300 micron fiber | Ocean optics Largo, FL USA | ultrafast pO2-measurements | |
| Ölsäure reinst Ph. Eur NF C18H34O2 M0282.47g/mol, Dichte 0.9 | Applichem GmbH Darmstadt, Deutschland | oleic acid | |
| Original Perfusor syringe 50 mL Luer Lock | B.Braun Melsungen AG, Germany | perfusorsyringe | |
| PA-Katheter Swan Ganz 7.5 Fr, 110 cm | Edwards Lifesciences LLC, Irvine CA, USA | PAC | |
| PE-Trichter, 60 mm | Aquintos-Wasseraufbereitung GmbH, Germany | funnel | |
| Percutaneous sheath introducer set 8.5 und 9 Fr, 10 cm with integral haemostasis valve/sideport | Arrow international inc. Reading, PA, USA | introducer sheath | |
| Perfusor FM Braun | B.Braun Melsungen AG, Germany | syringe pump | |
| Propofol 2% 20 mg/mL (50 mL Flaschen) | Fresenius, Kabi Deutschland GmbH | propofol | |
| Radifocus Introducer II, Größe 5-8 Fr | Terumo Corporation Tokio, Japan | introducer sheath | |
| Rüschelit Super Safety Clear 6.5 /7.0 | Teleflex Medical Sdn. Bhd, Malaysia | endotracheal tube | |
| Seldinger Nadel mit Fixierflügel | Smith- Medical Deutschland GmbH | seldinger canula | |
| Sonosite Micromaxx Ultrasoundsystem | Sonosite Bothell, WA, USA | ultrasound | |
| Stainless Macintosh Größe 4 | Welsch Allyn69604 | blade for laryngoscope | |
| Sterofundin Infusion | B. Braun Melsungen AG, Germany | bronchoalveolar lavage | |
| Stresnil 40 mg/mL | Lilly Deutschland GmbH, Abteilung Elanco Animal Health | azaperon | |
| Vasofix Safety 22 G | B.Braun Melsungen AG, Germany | venous catheter |
References
- Rubenfeld, G. D., et al. Incidence and Outcomes of Acute Lung Injury. New England Journal of Medicine. 353 (16), 1685-1693 (2005).
- The ARDS Definition Task Force. Acute Respiratory Distress Syndrome, The Berlin Definition. Journal of the American Medical Association. 307 (23), 2526-2533 (2012).
- Matute-Bello, G., Frevert, C. W., Martin, T. R. Animal models of acute lung injury. American Journal of Physiology - Lung Cellular and Molecular Physiology. 295 (3), 379-399 (2008).
- Goncalves-de-Albuquerque, C. F., Silva, A. R., Burth, P., Castro-Faria, M. V., Castro-Faria-Neto, H. C. Acute Respiratory Distress Syndrome: Role of Oleic Acid-Triggered Lung Injury and Inflammation. Mediators of Inflammation. 2015, 260465 (2015).
- Ballard-Croft, C., Wang, D., Sumpter, L. R., Zhou, X., Zwischenberger, J. B. Large-animal models of acute respiratory distress syndrome. Annals of Thoracic Surgery. 93 (4), 1331-1339 (2012).
- Kamuf, J., et al. Oleic Acid-Injection in Pigs As a Model for Acute Respiratory Distress Syndrome. Journal of Visualized Experiments. (140), e57783 (2018).
- Ware, L. B., Matthay, M. A. The acute respiratory distress syndrome. New England Journal of Medicine. 342 (18), 1334-1349 (2000).
- Schuster, D. P. ARDS: clinical lessons from the oleic acid model of acute lung injury. American Journal of Respiratory and Critical Care Medicine. 149 (1), 245-260 (1994).
- Wang, H. M., Bodenstein, M., Markstaller, K. Overview of the pathology of three widely used animal models of acute lung injury. European Surgical Research. 40 (4), 305-316 (2008).
- Lachmann, B., Robertson, B., Vogel, J. In vivo lung lavage as an experimental model of the respiratory distress syndrome. Acta Anaesthesiologica Scandinavica. 24 (3), 231-236 (1980).
- Russ, M., et al. Lavage-induced Surfactant Depletion in Pigs as a Model of the Acute Respiratory Distress Syndrome (ARDS). Journal of Visualized Experiments. (115), e53610 (2016).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved