Method Article
Encapsulated Cell Technology for the Delivery of Biologics to the Mouse Eye
In This Article
Summary
Presented here is a protocol for the use of alginate as a polymer in microencapsulation of immortalized cells for long-term delivery of biologics to rodent eyes.
Abstract
Many current therapeutics under development for diseases of the posterior pole of the eye are biologics. These drugs need to be administered frequently, typically via intravitreal injections. Encapsulated cells expressing the biologic of choice are becoming a tool for local protein production and release (e.g., via long-term drug delivery). In addition, encapsulation systems utilize permeable materials that allow diffusion of nutrients, waste, and therapeutic factors into and out of cells. This occurs while masking the cells from the host immune response, avoiding the need for suppression of the host immune system. This protocol describes the use of alginate as a polymer in microencapsulation coupled with the electrospray method as a microencapsulation technique. ARPE-19 cells, a spontaneously arising human RPE cell line, has been used in long-term cell therapy experiments due to its lifetime functionality, and it is used here for encapsulation and delivery of the capsules to mouse eyes. The manuscript summarizes the steps for cell microencapsulation, quality control, and ocular delivery.
Introduction
Cell-based therapies represent revolutionary biological techniques that have been applied widely in medicine. Recently, they have been successfully applied in the treatment of neurodegenerative diseases, eye diseases, and cancer. Cell therapies cover a wide range of fields from cell replacement to drug delivery, and this protocol focuses on the latter. Biodegradable alginate microcapsules (MC) have shown effectiveness as a delivery system, and they are becoming widely used in the biomedical field. Alginate has been used in microencapsulation due to its simple gelling process, biodegradability, excellent biocompatibility, and stability under in vivo conditions1,2,3,4.
The electrospray method, as a microencapsulation technique, has been successfully utilized to encapsulate peptides and proteins using alginate (base polymer) and poly-l-ornithine (secondary coating polymer). Both polymers are naturally found and used for their biocompatibility5,6,7. However, the main challenge in cell-based therapies is suppression of the host immune system to avoid side effects caused by immunosuppressive drugs. The permeability of alginate microcapsules is considered a suitable property for cell encapsulation, which allows diffusion of nutrients, waste, and therapeutic factors into and out of cells while masking them from the host immune response8,9,10.
In the eye, encapsulated cells have been used in clinical trials for the constant delivery of biologics (i.e., growth factors11,12 and growth factor antagonists13) for the treatment of retinitis pigmentosa or age-related macular degeneration. Other targets such as complement inhibitors14 are also currently being explored in preclinical settings.
Protocol
All experiments were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Medical University of South Carolina Animal Care and Use Committee under protocol ID 00399.
1. Cell Culture
- Generate human retinal pigment epithelial cells (ARPE-19) cell line stably expressing the gene of choice according to published protocols14,15.
- Maintain cells in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS).
- Incubate the cells at 37 °C and 5% CO2.
- Replace the medium every 2–3 days.
- Passage the cells after reaching 70%–80% confluence using standard tissue culture procedures.
2. Cell Encapsulation
- Mix sodium alginate with deionized (DI) water for a final concentration of 2% w/v and purify by filtration with a 0.2 µm sterile syringe filter.
- Prepare the cells to be mixed with the alginate solution by trypsinizing, centrifuging, and washing them with 10 mM HEPES buffered saline solution (pH = 7.4). Using a hemocytometer, count the cells and adjust the final cell concentration to 1 x 106 in alginate solution.
NOTE: The encapsulation process should be run inside a sterilized hood. - Load ~300 µL aliquots of alginate and cells mixture into a 3 mL syringe and attach it to a syringe pump. The solution will be pumped through a 30 G blunt tip needle to a sterile gelling bath placed in a sterile 50 mL beaker below the syringe tip at 7 mm for a needle to bath spraying distance.
- The gelling bath contains a volume of 40 mL of 10 mM HEPES buffered saline containing 100 mM calcium chloride (CaCl2) and 0.5% w/v poly-L-ornithine (PLO). The PLO is a secondary polymer coating that can be omitted or changed according to the needs of the investigator.
- Adjust the voltage and flow rate and keep them both constant during the encapsulation process at 60 mm/h flow rate and 6.0 kV initial voltage to produce microcapsules size of ~150 µm.
- Connect the clip of anode wire (red) of a high voltage generator needle tip to the needle and connect the ground clip (black) to the copper wire that is halfway submerged in the gelling bath. One batch of the alginate + cell mixture (1 mL) takes approximately 30 min to prepare the encapsulated cells (approximately 25,000 microcapsules).
- Wash the formed microcapsules containing cells with washing solution (10 mM HEPES buffered saline containing 1.5 mM CaCl2, pH = 7.4) twice. Do not use PBS for washing.
- Incubate the encapsulated cells with 10% FBS supplemented DMEM media in a humidified incubator at 37 °C and 5% CO2.
NOTE: Encapsulation process instruments are depicted in Figure 1.
3. Confirmation that Encapsulation Does Not Affect Cell Viability
- After incubating the encapsulated cells for 24 h in media, prepare a small sample of 500 µL (~30 microcapsules) for staining.
- Wash the microcapsules 2x using washing solution (10 mM HEPES buffered saline containing 1.5 mM CaCl2) and stain them for live-dead viability using a live/dead assay kit.
- Prepare a staining mixture of calcein AM (acetoxymethyl) and ethidium homodimer-1 at final concentrations of 2 µM and 4 µM, respectively.
- Add 2 mL of the staining mixture to the encapsulated cells and incubate for 30–45 min in the dark at room temperature (RT).
- With careful aspiration, aspirate the staining solution and wash the microcapsules 2x with the washing solution. Use a fluorescent microscope system to observe and image the encapsulated cells.
NOTE: Documentation of encapsulation is depicted in Figure 2.
4. Confirmation That Capsules Are the Appropriate Size for Delivery and Delivery of the Biologic
- Intravitreal injections in a mouse eye are typically performed with a 27 G blunt-tip needle (inner diameter of 210 µm) attached to a 2.5 µL Hamilton syringe.
- Generate capsules ranging from 100 to 200 µm, dilute them in serum-free media, and carefully pull them up into the Hamilton syringe. PBS is not a suitable vehicle, as alginate capsules will dissolve in PBS.
- Slowly eject 1 µL drops containing capsules onto a microscope slide and determine their integrity using an upright bright-field microscope.
- Adjust the capsule size (see step 2.3) by adjusting voltage and flow rate accordingly. Smaller microcapsules are produced in a nonlinear fashion by increasing voltage and slightly decreasing flow rate8. In our hands, capsules of 150 µm in diameter proved to be most suitable. Adjustment of capsule size is also dependent on keeping the alginate concentration constant while changing the other parameters.
- Maintain a separate set of cells in capsules of the appropriate size in serum-free medium to determine the amount of secretion of the desired biologic. Use sensitive ELISAs or western blotting to determine the concentration of the biologics in the supernatant.
- Determine the required amount of capsules that need to be injected based on the known PK/PD (pharmacokinetics/pharmacodynamics) of the therapeutic. In our hands, 10 capsules per mouse eye proved to be most efficacious.
5. Capsule Delivery into Mouse Vitreous
- Perform intravitreal injections using a dissecting microscope. See Figure 3 for surgical set-up and materials used during the procedure. A detailed protocol for intravitreous injections can be found elsewhere16.
- Anesthetize mice by intraperitoneal injection of xylazine and ketamine (20 mg/kg and 80 mg/kg) or other preferred anesthetics approved by the specific institution’s Animal Care and Use Committee. Ensure the appropriate depth of anesthesia using a toe pinch17.
- Dilate the mouse pupils with phenylephrine HCL (2.5%) and atropine sulfate (1%) to allow for good visibility of the vitreous chamber and apply a lubricant eye gel to the eyes to keep them hydrated during the procedure.
- Puncture the sclera at the limbus with a 26 G needle as the guide hole, making sure that 1) the needle is at a 45° angle with the eye and table and 2) the beveled tip is pointed upwards to avoid puncturing the lens.
- Carefully inject the capsules using a 27 G blunt-tip needle attached to a Hamilton syringe at a 45° angle under visual inspection, making sure to avoid touching the lens with the needle. Injury of the lens will lead to cataract formation. The capsules should be visible in the vitreous using the dissecting microscope (Figure 4A).
- After retraction of the needle, treat the injection site with antibiotic ointments neomycin and polymyxin B sulfates in addition to dexamethasone ophthalmic antibiotic ointment.
- Apply goniotaire hypromellose demulcent ophthalmic solution (2.5%) to both eyes to prevent the corneas from drying out during the recovery period.
- Place the mouse on a heating pad held at 37°C and monitor until fully awake.
- A successful injection of encapsulated ARPE-19 cells should reveal the presence of intact capsules in the vitreous chamber of the mouse with only minor amounts of debris when imaging the eye by optical coherence tomography or other methods (Figure 4B).
- The injected mouse eye, after a few days of recovery due to the surgery, is now ready for the experimental paradigm at hand.
NOTE: The surgical set-up and documentation of capsules in the eye are depicted in Figure 3 and Figure 4, respectively.
Results
ARPE-19 cells are a spontaneously immortalized human RPE cell line that has been shown to be amenable to encapsulation and long-term survival upon implantation of capsules into the eye. The tools for alginate encapsulation are shown in Figure 1. In this study, it was demonstrated that upon encapsulation in alginate, the cells in alginate capsules were confirmed by bright-field imaging (Figure 2A). Live-dead assays were performed on the cells inside the capsules, demonstrating 90% viability post-encapsulation (Figure 2B). To ensure long-term viability of the cells inside capsules, capsules were dissolved in sodium citrate and cells were then re-plated (Figure 2C). After confirming in independent experiments that the stable transfected ARPE-19 cells expressed and secreted the biologic that was desired14, and after establishing the parameters for safely encapsulating cells, capsules were produced for intraocular injection.
Intravitreal injections into the mouse eye requires the use of a 27 G blunt-tip needle (inner diameter of 210 µm) and accurate delivery system to consistently inject the required small volume of 1 µL. The size of capsules that were not damaged during ejection through the 27 G needle was confirmed by microscopy (Figure 2A). An optimal size of 150 µm was identified. Intravitreal injection was performed under visual inspection, which allowed the visualization of capsules in the vitreous (Figure 4A). Likewise, OCT imaging was performed, which confirmed that a majority of capsules in the vitreous chamber of the mouse were intact with relatively minor amounts of debris (Figure 4B). In follow-up published experiments, it was confirmed that the desired biologic was present in the eye at therapeutically relevant doses, inhibiting the disease process under investigation14. These results confirmed that encapsulated cells can safely be injected into mouse eyes for the long-term delivery of biologics.
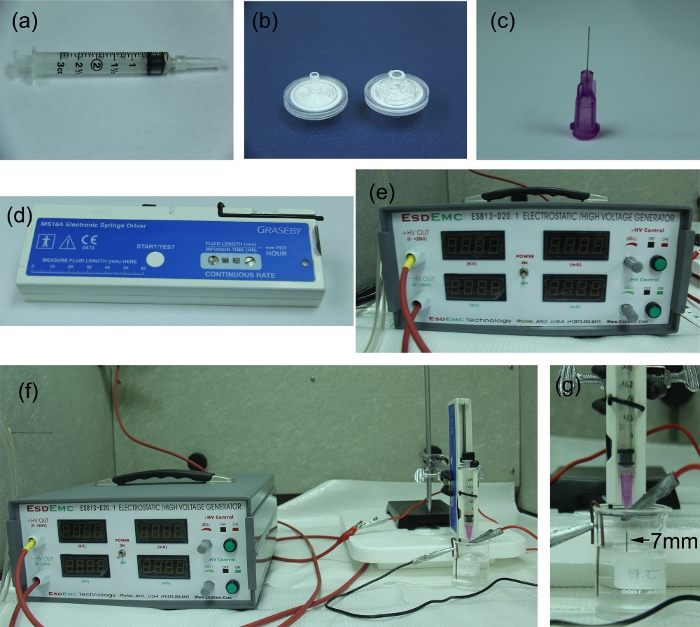
Figure 1: Encapsulation process instruments. The set-up includes (A) a 3 mL syringe, (B) 0.2 µm filters, (C) a 30 G, ½ inch blunt-tip needle, (D) an electronic syringe driver, (E) a high voltage generator, (F) the system set-up, and (G) a 7 mm fixed distance between the needle tip and gelling solution surface. Please click here to view a larger version of this figure.
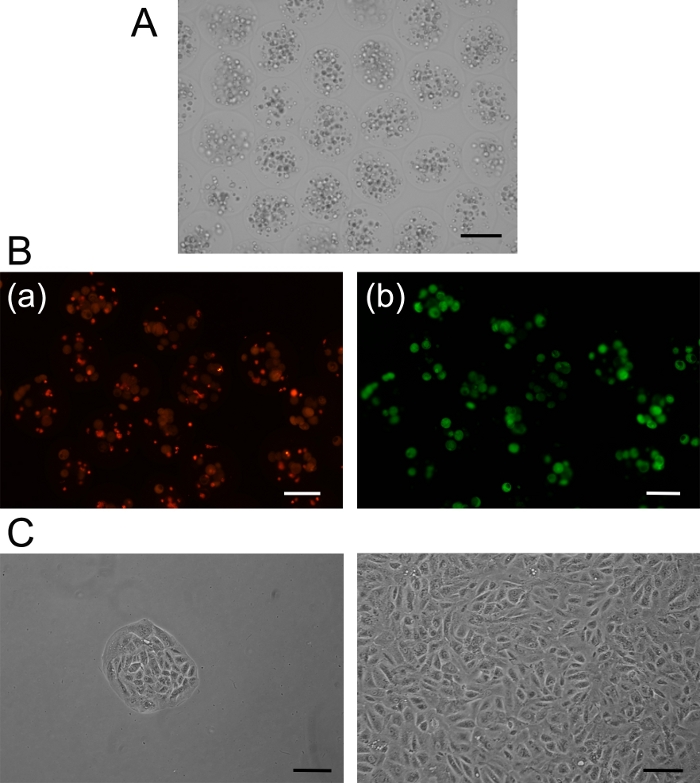
Figure 2: Cell encapsulation. (A) Microcapsules filled with ARPE 19 cells. (B) Live/ dead assay: [a] red fluorescent color indicates dead cells, and [b] green fluorescent color indicates live cells. (C) Cultured cells after recovery from microcapsules beginning with a small cluster growing to confluency. Scale bars = 100 µm. Please click here to view a larger version of this figure.

Figure 3: Surgical set-up. A dissecting microscope (1) with a video camera (2) is used to visualize the procedure. The mouse is weighed (3) to administer the appropriate amount of anesthetic and placed on a small platform (4) to ease handling and injection. Eyes are dilated with mydriatics atropine and phenylepinephrin (5) and are kept hydrated with lubricant (6). A guide hole is punctured just outside the limbus using a 26 G needle (7). The glass syringe (8), loaded with appropriately diluted capsules (9), is placed at a 45° angle to the mouse eye and advanced through the guide hole, using a micromanipulator (10). The needle track as well as the capsules being released can be visualized [(11); see Figure 4]. Upon retraction of the needle, the cornea is moistened with hypromellose (12) and antibiotic ointment (13) applied to the injection site to avoid infection. Following the procedure, the mouse will be placed on a 37 °C heating pad and monitored until fully awake. Please click here to view a larger version of this figure.
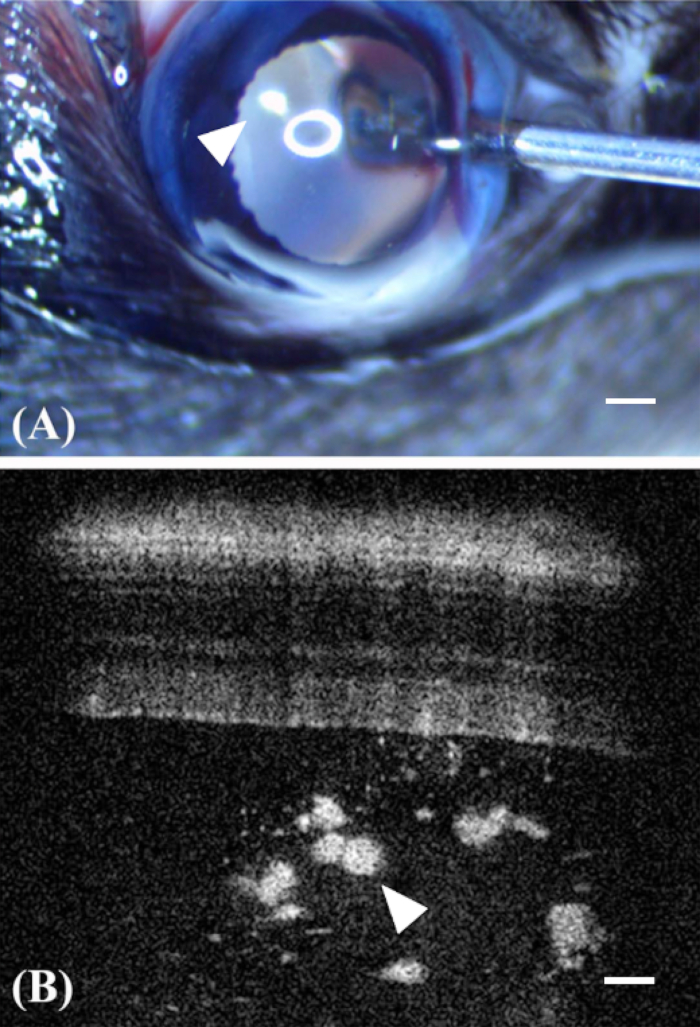
Figure 4: Encapsulated cell technology to deliver ARPE-19 into the eye. (A) Injections are performed using a blunt 27 G needle attached to a Hamilton syringe. The needle track can be followed as the tip of the needle enters the eye, avoiding the lens and the capsules (arrowhead) can been visualized, magnified by the optical system of the mouse eye. It should be noted that the circular reflection of the light source. (B) Capsules (arrowhead) can be imaged in the vitreous using optical coherence tomography. Scale bars = 200 µm. Panel B was reprinted with permission from Annamalai et al.14 Please click here to view a larger version of this figure.
Discussion
This cell encapsulation technique is relatively quick and easy to perform; however, certain points must be kept in mind to obtain accurate downstream results. Cells should be maintained in culture in a Petri dish prior to encapsulation and held at proper confluency. Encapsulation should be performed in a proper ventilation hood with regulated air flow, if possible. Too strong of an air current can affect capsule formation, especially in long-term experiments. Sterile utensils and solutions are critical for long-term maintenance of cells within the capsule.
At present, live-dead staining is used as a confirmatory tool to determine the viability of cells within the capsules. The number of cells per capsule and under the current condition (i.e., normally 12–20 cells per capsule) are also visually determined. The viability of each batch of encapsulated cell batches are monitored by this method. To further determine the viability of the cells, encapsulated cells are dissolved and re-cultured. This further demonstrates the viability and integrity of cells within the capsules, validating successful cellular encapsulation.
The parameters used for cellular encapsulation have been established for this particular cell type. The parameters stated above are those used for the encapsulation of ARPE 19 cells for these experiments. Flow rate, alginate concentration, voltage applied, and secondary coating of the capsules are all variables that can be adjusted for appropriate use of the capsules. Likewise, the amount of capsules required for a given experiment needs to be determined either empirically or based on the known PK/PD of the biologics. It is important to always perform appropriate control experiments, adding empty capsules to control for the presence of the capsules and capsules loaded with untransfected ARPE-19 cells to control for the presence of secreted factors. ARPE-19 cells can also be stably transfected with a control plasmid, as the presence of even a small number of capsules in the small vitreous of the mouse (<10 µL) appears to alter the normal physiology of the eye. Within this context, it is important to know the secretome of APRE-19 cells under encapsulation conditions (as well as in the presence of vitreous in a particular disease condition), as the secreted proteins may interfere with the efficacy of the biologic under investigation.
Finally, this technique was implemented to provide proof-of-principle for long-term delivery of complement inhibitors for the treatment of AMD and to improve the current method of intravitreal injections14. At the current stage of development, complement inhibitors are injected into the vitreous, typically using monthly injections. This includes injection of the complement factor D blocking antibody lampalizumab18, which failed in a phase III clinical trial to reduce the progression of geographic atrophy, or the complement factor 3 inhibitor APL-219, which is currently in a phase III clinical trial.
Intravitreal injection is hampered by side effects of the injection itself (i.e., risk of retinal detachment, rise in intraocular pressure, endophthalmitis, etc.). In addition, drug levels will vary significantly over the course of the month upon monthly intravitreal injections, and rebound reactions may be expected. As an alternative, gene therapy strategies are being developed, such as the soluble CD59 complement inhibitor20, which is currently in a phase I clinical trial. Encapsulated cells also allow for the continuous production of a biologic for extended periods of time and can be terminated (i.e., explanting of the capsule), if required.
To date, we have only tested for production of a biologic over the course of ~6 weeks14. It should be noted that the method described here should only be used for proof-of-principle in animal models and not for use in patients, as the alginate capsules are not sufficiently stable to completely prevent shredding during the injection and are not expected to last more than a few weeks. In contrast, a solid device such as that developed by Neurotech can last for years21 to deliver the required factors22,23,24. In addition, this new technique can also be combined with encapsulated drug delivery. Overall, it is expected that this emerging field will rapidly develop as an alternative strategy for repeated injections of therapeutics of gene therapy.
Disclosures
The authors declare no competing financial interests.
Acknowledgements
The study was supported in part by grants awarded to B. R. by the National Institutes of Health (R01EY019320), the Department of Veterans Affairs (RX000444 and BX003050), and the South Carolina SmartState Endowment.
Materials
| Name | Company | Catalog Number | Comments |
| 3 mL Syringe | BD | 309656 | |
| 30 G 1" Blunt needle | SAI Infusion technology | B30-100 | |
| Alginic acid sodium salt, from brown algae | Sigma | A0682 | |
| Atropine Sulfate Ophthalmolic solution (1%) | Akorn | NDC 17478-215-15 | for pupil dilation |
| BD 1 mL Syringe 26 G x 3/8 (0.45 mm x 10 mm) | Becton, Dickinson and Company | DG518105 500029609 REF 309625 | to generate the guide hole |
| Calcium chloride, Anhydrous, granular | Sigma | C1016 | |
| GenTeal Tears | Alcon | NDC 0078-0429-47 | to lubricate the eyes during anesthesia |
| Goniotaire: Hypromellose (2.5%) Ophthalmolic Demulcent Solution (Sterile) | Altaire Pharmaceuticals Inc. | NDC 59390-182-13 | to lubricate the eyes during anesthesia |
| Hamilton Needle/syringe Tip: 27 Gauge, Small Hub RN NDL, custum length (12mm), point style 3, 6/PK | Hamilton | 7803-01 | for intravitreal delivery of capsules |
| Hamilton Syringe: 2.5 µL, Model 62 RN SYR, NDL Sold Separately | Hamilton | 7632-01 | for intravitreal delivery of capsules |
| HEPES buffer, 1M | Fisher Bioreagents | BP299100 | |
| High voltage generator | ESD EMC Technology | ES813-D20 | |
| LIVE/DEAD Viability/Cytotoxicity Kit | Thermofisher Scientific | L3224 | |
| L-Ornithine hydrochloride, 99% | Alfa Aesar | A12111 | |
| Neomycin and Polymyxin B Sulfates and Dexamethasone Ophthalmolic Ointment | SANDOZ | NDC 61314-631-36 | antibiotic to prevent infection after intravitreal injection |
| Phenolephrine Hydrochloride Ophthalmolic Solution (2.5%) | Akorn | NDC 17478-201-15 | for pupil dilation |
| Sodium Chloride | Sigma | S-5886 | |
| Sterile syringe filters, 0.2 um | VWR | 28143-312 | |
| Syringe pump | GRASEBY | MS16A |
References
- Allen, T. M., Cullis, P. R. Drug delivery systems: entering the mainstream. Science. 303 (5665), 1818-1822 (2004).
- Tonnesen, H. H., Karlsen, J. Alginate in drug delivery systems. Drug Development and Industrial Pharmacy. 28 (6), 621-630 (2002).
- Vilos, C., Velasquez, L. A. Therapeutic strategies based on polymeric microparticles. Journal of Biomedical Biotechnology. 672760, (2012).
- Gasperini, L., Mano, J. F., Reis, R. L. Natural polymers for the microencapsulation of cells. Journal of the Royal Society Interface. 11 (100), 20140817 (2014).
- Gasper, D. P. R. . Novel strategy to produce a drug delivery system for skin regeneration. Uma nova estratégia para produzir um dispositivo para entrega de fármacos que será usado na regeneração da pele. , (2012).
- Huang, S., Fu, X. Naturally derived materials-based cell and drug delivery systems in skin regeneration. Journal of Controlled Release. 142 (2), 149-159 (2010).
- Nograles, N., Abdullah, S., Shamsudin, M. N., Billa, N., Rosli, R. Formation and characterization of pDNA-loaded alginate microspheres for oral administration in mice. Journal of Bioscience and Bioengineering. 113 (2), 133-140 (2012).
- Moore, K., Amos, J., Davis, J., Gourdie, R., Potts, J. D. Characterization of polymeric microcapsules containing a low molecular weight peptide for controlled release. Microscopy and Microanalysis. 19 (1), 213-226 (2013).
- Xu, Y., Skotak, M., Hanna, M. Electrospray encapsulation of water-soluble protein with polylactide. I. Effects of formulations and process on morphology and particle size. Journal of Microencapsulation. 23 (1), 69-78 (2006).
- Gryshkov, O., et al. Process engineering of high voltage alginate encapsulation of mesenchymal stem cells. Materials Science and Engineering: C. 36, 77-83 (2014).
- Thanos, C. G., et al. Sustained secretion of ciliary neurotrophic factor to the vitreous, using the encapsulated cell therapy-based NT-501 intraocular device. Tissue Engineering. (11-12), 1617-1622 (2004).
- Kauper, K., et al. Two-year intraocular delivery of ciliary neurotrophic factor by encapsulated cell technology implants in patients with chronic retinal degenerative diseases. Investigative Ophthalmology & Visual Science. 53 (12), 7484-7491 (2012).
- Kauper, K., et al. Long term, sustained intraocular delivery of a VEGF antagonist using encapsulated cell technology implant for the treatment of choroidal neovascular diseases. Investigative Ophthalmology & Visual Science. 53, 455 (2012).
- Annamalai, B., et al. Encapsulated Cell Technology-Based Delivery of a Complement Inhibitor Reduces Choroidal Neovascularization in a Mouse Model. Translational Visual Science Technology. 7 (2), 3 (2018).
- Alge, C. S., et al. Retinal Pigment Epithelium Is Protected Against Apoptosis by αB-Crystallin. Investigative Ophthalmology & Visual Science. 43 (11), 3575-3582 (2002).
- Chiu, K., Chang, R. C., So, K. F. Intravitreous injection for establishing ocular diseases model. Journal of Visualized Experiments. (8), 313 (2007).
- Jove Science Education Database. Lab Animal Research. Anesthesia Induction and Maintenance. Journal of Visualized Experiments. , (2019).
- Holz, F. G., et al. Efficacy and Safety of Lampalizumab for Geographic Atrophy Due to Age-Related Macular Degeneration: Chroma and Spectri Phase 3 Randomized Clinical Trials. JAMA Ophthalmology. 136 (6), 666-677 (2018).
- Kassa, E., Ciulla, T. A., Hussain, R. M., Dugel, P. U. Complement inhibition as a therapeutic strategy in retinal disorders. Expert Opinion in Biological Therapy. 19 (4), 335-342 (2019).
- Cashman, S. M., Ramo, K., Kumar-Singh, R. A Non Membrane-Targeted Human Soluble CD59 Attenuates Choroidal Neovascularization in a Model of Age Related Macular Degeneration. PLoS ONE. 6 (4), e19078 (2011).
- Vincent, L., et al. Generation of combination PDGF / VEGF-antagonist ECT devices. Investigative Ophthalmology & Visual Science. 54, 3290 (2013).
- Zhang, K., et al. Ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for treatment of geographic atrophy in age-related macular degeneration. Proc Natl Acad Sci USA. 108 (15), 6241-6245 (2011).
- Chew, E. Y., et al. Ciliary neurotrophic factor for macular telangiectasia type 2: results from a phase 1 safety trial. American Journal of Ophthalmology. 159 (4), 659-666 (2015).
- Birch, D. G., et al. Randomized trial of ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for retinitis pigmentosa. American Journal of Ophthalmology. 156 (2), 283-292 (2013).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved