Method Article
A Chronic Immobilization Stress Protocol for Inducing Depression-Like Behavior in Mice
* These authors contributed equally
In This Article
Summary
This article provides a simplified and standardized protocol for induction of depressive-like behavior in chronically immobilized mice by using a restrainer. In addition, behavior and physiological techniques to verify induction of depression are explained.
Abstract
Depression is not yet fully understood, but various causative factors have been reported. Recently, the prevalence of depression has increased. However, therapeutic treatments for depression or research on depression is scarce. Thus, in the present paper, we propose a mouse model of depression induced by movement restriction. Chronic mild stress (CMS) is a well-known technique to induce depressive-like behavior. However, it necessitates a complex procedure consisting of a combination of various mild stresses. In contrast, chronic immobilization stress (CIS) is a readily accessible chronic stress model, modified from a restraint model that induces depressive behavior by restricting movement using a restrainer for a certain period. To evaluate the depressive-like behaviors, the sucrose preference test (SPT), the tail suspension test (TST), and the ELISA assay to measure stress marker corticosterone levels are combined in the present experiment. The described protocols illustrate the induction of CIS and evaluation of the changes in behavior and physiological factors for the validation of depression.
Introduction
Major depressive disorder (MDD) is the leading cause of mental disability worldwide, with an incidence that is increasing faster than anticipated. In 2001, the World Health Organization predicted that MDD would be the second most common disease in the world by 2020. However, it was already the second most common in 20131. In addition, current antidepressants have many limitations, including delayed effectivity, drug resistance, relapse, and various side effects2,3. Researchers must therefore develop more effective antidepressants. However, the ambiguous pathophysiology of MDD presents an obstacle to the development of novel antidepressants.
Long-term stress is the main risk factor for MDD. It can induce dysfunction in the hypothalamic-pituitary-adrenal (HPA) axis, which is also related to MDD etiology4,5. As described previously, the HPA axis plays a critical role in stress-induced psychiatric pathophysiology including depression and anxiety disorders by increasing corticosterone levels6,7,8,9. Many animal models have been based on sustained activation of the HPA axis, which is observed in patients with MDD4. Moreover, high glucocorticoids induced by chronic stress and subcutaneously injected glucocorticoids cause depressive behaviors along with neural cell death, atrophy of neuronal processes, and reduced adult neurogenesis in the brain of rodents10,11. Another important brain area associated with depression is the medial prefrontal cortex (mPFC). The mPFC plays a crucial role in controlling brain subregions, such as the hypothalamus and amygdala, that control emotional behavior and stress responses8,9. For instance, lesions in the dorsal mPFC induced HPA axis dysfunction and enhanced corticosterone secretion due to restraint stress12,13. A recent study also showed that repeated restraint stress increased corticosterone levels, which could be decreased by glutamine supplementation via glutamate-glutamine cycle between neurons and astrocyte in the mPFC9.
The first chronic stress paradigm used to study the etiology of MDD was suggested by Katz14. Willner et al. then proposed a chronic mild stress (CMS) model based on the findings of Katz. They confirmed that the model had predictive validity by observing that antidepressants restored CMS-induced anhedonic-like behavior15,16. Typically, the CMS model consists of a combination of various mild stresses, such as mild noise, cage tilting, wet bedding, altered light-dark cycles, cage shaking, forced swimming, and social defeat. The CMS model is widely utilized by researchers; however, this model is of poor replicability, and time- and energy-inefficient. Therefore, there is a growing demand for a standardized and simplified protocol for induction of depressive-like behavior and physiological analysis to evaluate depression. Compared to the CMS model, the chronic immobilization stress (CIS; also known as chronic restraint stress) model is simpler and more efficient; therefore, the CIS model can be widely used in chronic stress studies17,18,19,20,21,22,23,24. In addition, CIS can be used in both male and female mice to develop depressive behaviors25,26. During CIS, animals are placed in a body-fit sized cylinder for 1-8 hours per day for 2 or 4 weeks9,27,28. Of these, restraint stress condition for 2 hours per day for 2 weeks is sufficient to cause depressive behaviors with minimal pain in mice9,28. Under restraint conditions, blood corticosterone levels were rapidly increased9,28,29. Several studies have shown that the CIS model has predictive validity, confirming that CIS-induced depressive-like symptoms are restored by antidepressants19,20,30,31. Herein, we report the detailed procedures of CIS, as well as some behavioral and physiological outcomes after CIS in mice.
Protocol
All experimental protocols and animal care were conducted according to the guidelines of the University Animal Care Committee for Animal Research of Gyeongsang National University (GLA-100917-M0093).
1. Materials
- Mice
- Use males of C57BL/6 strain background weighing 22–24 g at postnatal week 7. Habituate in the breeding room for 1 week before the experiments.
NOTE: All mice were purchased from a laboratory animal company. - House mice individually in a temperature-controlled vivarium (22–24 °C) under a 12-hour light/dark cycle (lights on at 6:00 A.M.), with normal laboratory chow and water available ad libitum.
- Use males of C57BL/6 strain background weighing 22–24 g at postnatal week 7. Habituate in the breeding room for 1 week before the experiments.
- Restrainer
- Use a cylindrical, transparent, acrylic tank (height = 8.5 cm, diameter = 2.5 cm) fixed on a square pedestal to restrain and to produce depressive behavior (Figure 1A). The diameter of this cylinder was made to fit the body so that the mouse could not turn and move forwards or backwards. The restrainer can be purchased commercially or made in the lab.
- Tail suspension apparatus
- Use a reasonable size tail suspension box made of translucent acrylic (height = 30 cm, width = 20 cm, length = 20, Figure 1B). To prevent interactions between the animals, use rectangular partitions within the box so that the floor and three of the four walls are blocked by acrylic plates. Leave the remaining two sides of the box open to allow video recording and to fix the horizontal bar. The box can be purchased commercially or made in the lab.
- Video recording device and video tracking software
- Use a black and white-display closed-circuit television camera (see Table of Materials) connected to a computer and a tripod (or other support products) to allow recording of the behavioral experiment. Video recording is essential to allow behavioral scoring in this experiment, because at least two mice are tested at the same time.
- Ensure that the camera resolution is high enough to allow the video data to be analyzed using the video tracking software (see Table of Materials) installed in the connected computer.
2. Induction of depression by CIS restraint
NOTE: Handle the mouse gently, but firmly with confidence. Both rough and tentative handling is another stress factor in the experiment and it is an important reason for the mouse struggling, biting, and scratching.
- Set the room light to light (200 Lux) conditions using a digital lux meter.
- House the mouse in a separate cage at least a week before testing and place the mouse in the testing room for at least 30 min before the experiment.
NOTE: Handle the mice at least once a day for at least 3 consecutive days before the experiment so that the mice become familiar with the experimenter. An adaptational period before the experiment is necessary to ensure that the mice acclimatize to the circumstance, such as the testing room. - Gently hold the mouse tail to avoid tensing the mouse, and then carefully place it on a rough surface (top of wire bar of the cage or cage lid).
- Cover the restrainer with a small white towel, and then gently place the mouse at the opening of the restrainer so that the mouse enters the restrainer voluntarily.
NOTE: In this case, the mouse is positioned in the opposite direction to that which it enters the restrainer with. To lead the mouse to enter the restrainer voluntarily, the restrainer is covered with a small towel to make the inside darker. - Place the closure to restrain the mouse as tight as possible, being careful to avoid damage to the body, such as tail, feet, and testicles.
- Restrain the mouse for 2 h/day (9:00 A.M. to 11:00 A.M.) for 15 consecutive days.
- Measure body weight and food intake every 48 h during exposure to the restrainer (i.e., food intake quantity during the 48 h before the initiation of the movement restraint).
NOTE: When measuring body weight and food intake, place control mice in their home cages in the testing room during CIS. Ensure that other environmental factors are the same as for the CIS mice. - Confirm the induction of depression by performing behavioral tests such as the sucrose preference test (SPT) and the tail suspension test (TST) (refer to steps 4 and 5).
- Confirm the induction of depression by measuring the stress marker corticosterone using ELISA assay (refer to section 6).
3. The sucrose preference test
- Before testing, habituate the mice to the presence of two drinking bottles (one containing 0.1 M sucrose and the other containing plain water) for 48 h. Switch the positions of the two bottles after 24 h to reduce any confounding produced by a side bias.
- On the 3rd day, deprive the mice of water for 24 h.
- On the day of the SPT experiment, expose the mice to two drinking bottles for 6 h. After 3 h, switch the position of the water bottles.
- Record the volume (mL) of sucrose solution and water consumed and then calculate the animals’ affinity to sucrose.
- Generally, calculate sucrose preference as a percentage of the volume of sucrose consumption over the total fluid consumption during the test.
4. The tail suspension test
- Bring the CIS-induced mice into the testing room at least 30 min before beginning the TST.
- Set the room light to dim (50 Lux) conditions.
- To obtain the highest resolution video file, place the camera as close to the mouse as possible (around 40 cm from the mouse).
- Suspend the mouse firmly from the horizontal bar (30 cm from the bottom line) using cellophane adhesive tape (the distance from the tip of the tail is 1 cm). Complete the process of applying tape to the mouse as soon as possible to minimize other sources of stress.
- Once the mouse is positioned in the middle of suspension box, start recording and observe the behavioral alterations continuously for 6 min.
NOTE: If the mouse attempts to climb its tail, use a stick or climb stopper to prevent it from doing so. - At the end of the experiment, move the mouse to its home cage and carefully remove the tape from its tail.
- Analyze the accumulated time of immobile periods using the video tracking software.
NOTE: The duration of immobility is the most important CIS parameter. This can be calculated as the accumulated time of immobile periods, defined in terms of a motion threshold contained within the level-filtering device of the software.
5. Measuring corticosterone levels in blood by ELISA
NOTE: A day after the behavioral test, the mice are sacrificed for blood collecting.
- Anesthetize the mouse with 5% isoflurane in an induction chamber until anesthesia. Ensure the mouse has sufficient time in the induction chamber (at least 2 min) to prevent waking up during surgery.
- Collect blood from the heart using a 1 mL syringe, and store the blood in vacutainers containing K3EDTA on the ice (at 9 A.M.)
- Separate plasma by centrifugation at 1,000 × g for 15 min at 4 °C.
- Quantify plasma corticosterone levels using the corticosterone ELISA kit (see Table of Materials) according to the manufacturer's protocol.
Results
In the representative experiment, all data were acquired from 6 - 8 mice per group. Representative materials and the method to insert the mouse voluntarily into the restrainer are shown in Figure 1.
To perform the behavioral test and blood sampling after CIS induction, mice were subjected to the experimental procedure as summarized in Figure 2A. As shown in Figure 2 and Figure 3, CIS induces depressive-like behaviors well and releases the stress marker corticosterone. In addition, these indexes were recovered by glutamine supplementation (mice were fed glutamine-supplemented diets during the entire experimental period, 150 mg/kg) as shown in Figure 3.
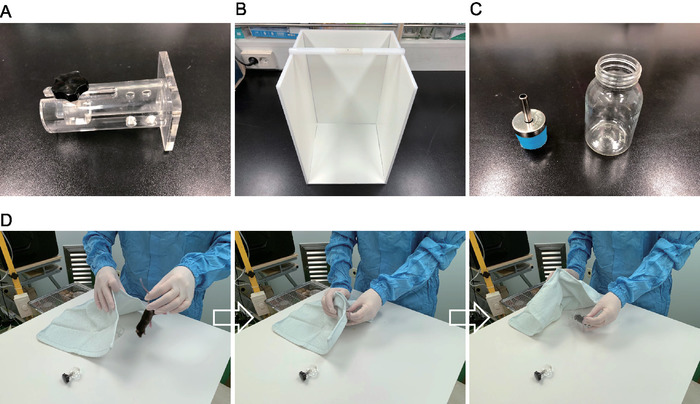
Figure 1: Restrainer setup. (A) Restrainer, (B) tail suspension box, and (C) water bottle and ball nozzle. (D) The process of inserting the mouse into the restrainer to induce CIS. From the left panel, mouse voluntarily enters the restrainer after the restrainer is covered with a small towel. The right panel shows that the mouse has completely entered the restrainer. This figure was modified from Son et al.9 Copyright permission has been obtained from the journal for all reused figures. Please click here to view a larger version of this figure.
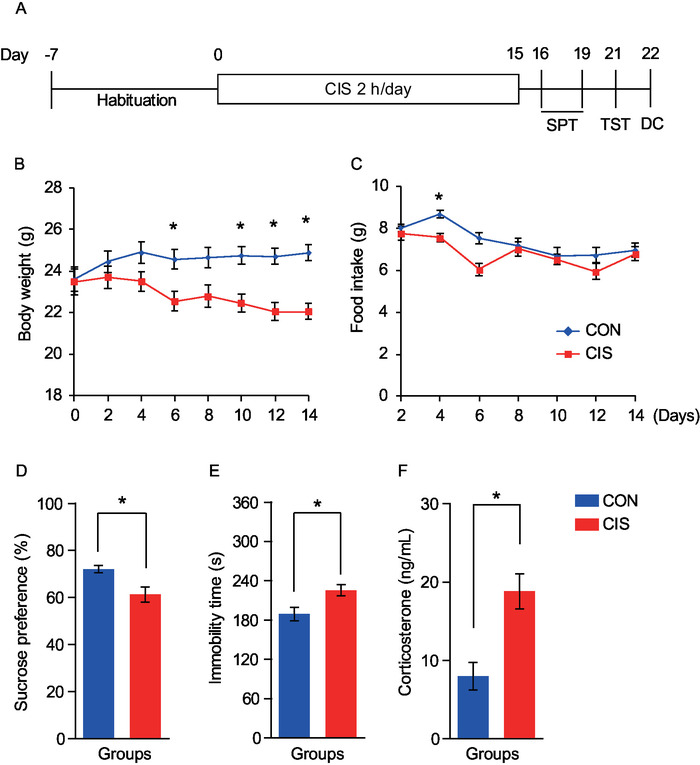
Figure 2. Induction of the chronic immobilization stress and evaluation of depressive-like behaviors in mice. (A) Experimental procedure. Body weight (B) and food intake (C) in the control group (blue line, n = 8) and in the CIS group (red line, n = 8). (D and E) Sucrose preference and immobility time (n = 8 in both tests). (F) Blood corticosterone levels (n = 7/group). Data are shown as mean ± SEM. *p < 0.05 as determined by (B and C) two-way ANOVA with Bonferroni post-hoc test or (D–F) unpaired Student's t-test. CIS = chronic immobilization stress, SPT = sucrose preference test, TST = tail suspension test, DC = decapitation. This figure was modified from Son et al.9 Copyright permission has been obtained from the journal for all reused figures. Please click here to view a larger version of this figure.

Figure 3. A glutamine-supplemented diet ameliorates depressive-like behaviors. Body weight (A) and food intake (B) in the control group (blue line, n = 7), CIS group (red line, n = 7) and CIS + glutamine supplemented group (green line, n = 7). Sucrose preference (C), immobility time (D) and blood corticosterone levels (E) (n = 6-7/group). Data are shown as mean ± SEM. *p < 0.05 as determined by (A and B) two-way ANOVA with Bonferroni post-hoc test or (C-E) unpaired Student's t-test. Gln = glutamine. This figure was modified from Son et al.9 Copyright permission has been obtained from the journal for all reused figures. Please click here to view a larger version of this figure.
Discussion
The complexity of the brain and heterogeneity of MDD make it challenging to create animal models that completely reproduce the condition. Many researchers have overcome this difficulty using an endophenotype-based approach32, in which anhedonia (lack of interest in rewarding stimuli) and despair are considered evolutionarily conserved and quantifiable behaviors in animal models, which are also seen in patients with depression33. In the present paper, we have presented a method in which CIS was sufficient to induce anhedonia and despair, demonstrating translational relevance between CIS and MDD. Moreover, many studies have used CIS to identify the mechanism eliciting depressive-like behaviors and to assess antidepressants capable of restoring normal behavior9,19,20,30,31,34. Thus, the CIS may be appropriate for studying the etiology of MDD and may therefore be useful in the development of new antidepressants.
Several factors affect the development of depressive-like behavior during CIS. The first is animal strain because the extent of stress response to CIS may vary depending on the animal strain. Indeed, several strain-related differences in response to depressive behavioral tests and antidepressants are known35,36. In this regard, particular attention should be paid to the tail-climbing behavior of the commonly used C57BL strain37,38,39. Second, environmental stress factors, such as light, noise, and housing, should be minimized. Although social isolation stress may influence the findings40,41, we conducted CIS on single-housed mice because isolation has more advantages than disadvantages. For example, it can minimize social defeat stress because CIS often causes group-housed mice to attack each other. Indeed, the control mice also attack their housemates, affecting the baseline behavior in the TST and SPT. Another factor to consider before starting the experiment is sex. In this article, we performed all experiments with male mice, as emotional and cognitive behaviors are affected by the menstrual cycle in female mice42,43,44. Moreover, female rodents are relatively more susceptible to stress-related disorders, such as depression. Therefore, if the experimenter wants to use female mice, the time point of depressive-like behavior induction should be confirmed and the CIS protocol should be modified. In addition, all mice should be allowed a period of habituation to the new circumstance, and the experimenter should avoid adding new animals to the testing room during the experiment, because the mice may sense new olfactory and ultrasonic cues during the experiment. When the experimenter is moving the mice to another floor or a long distance, it is necessary to cover the breeding cage with a piece of black cloth. Lastly, age is an important factor in determining the extent of response and recovery to stress45. We focused on the etiology of MDD in adolescence—8-week-old mice were used throughout the experiment. Experimenters should consider whether the abovementioned factors may influence the results when designing the CIS.
To validate CIS induction, tests that indicate depression, such as body weight and food intake measurement, TST, and SPT, should be performed and a physiological stress indicator, such as changes in corticosterone, should be investigated9,46,47. However, the TST method applied in this experiment is not recommended in rats because rats are too heavy to be supported by their tails. In such cases, the TST should be replaced with forced swimming or open field tests39,48. In this experiment, the primary consideration was the size of the suspension box. By using adhesive cellophane tape, the tail of the mouse was suspended on a horizontal bar located in the middle of the box on the ceiling. Therefore, the box should be large enough to prevent the mouse from contacting the wall during the experiment. The SPT, an indicator of anhedonia, suggests an emotional disorder such as depression. In the present experiment, the interest of the mice in a sweet drink was evaluated by using sucrose.
In order to induce depressive-like behavior, we modified the restraint technique of the CMS model to establish CIS, which is a simplified and highly reproducible technique to perform experiments in depression. However, in using CIS as a repetitive restraint model, there is a possibility that experimental animals could adapt to CIS and become insensitive to it. In addition, locomotion tests may not be appropriate as prolonged restraint could affect the movement of animals. Therefore, the establishment of a set point of restraint time in a day and consecutive days is important to minimize other factors except depression. In addition, it is necessary to perform the behavioral and the physiological test to verify the induction of depression after exposure to CIS.
In conclusion, despite the increasing interest of researchers in depression, it remains challenging to systematically define the pathological mechanism, which can be ascribed to the diverse and complex pathophysiology of depression. Hence, simplified animal models to induce depression, such as CIS, may provide important evidence to establish the mechanism of depression induction and suggest a good experimental platform to obtain therapeutic answers for such a complex mental problem.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2015R1A5A2008833 and NRF-2016R1D1A3B03934279) and the grant of lnstitute of Health Sciences (IHS GNU-2016-02) at Gyeongsang National University.
Materials
| Name | Company | Catalog Number | Comments |
| 1 ml disposable syringes | Sungshim Medical | P000CFDO | |
| Balance | A&D Company | FX-2000i | |
| Ball nozzle | Jeung Do B&P | JD-C-88 | |
| CCTV camera | KOCOM | KCB-381 | |
| Corticosterone ELISA kits | Cayman Chemical | ||
| Digital lux meter | TES | TES-1330A | |
| Ethovision XT 7.1 | Noldus Information Technology | ||
| Isoflurane | HANA PHARM CO., LTD. | Ifran solution | |
| Mice | Koatech | C57BL/6 strain | |
| Restrainer | Dae-jong Instrument Industry | DJ-428 | |
| Saccharose (sucrose) | DAEJUNG | 7501-4400 | |
| Small animal isoflurane anaesthetic system | Summit | ||
| Acrylic bar | The apparatus was made in the lab for TST test | ||
| Tail suspension box | The apparatus was made in the lab | ||
| Timer | Electronics Tomorrow | TL-2530 | |
| Water bottle | Jeung Do B&P | JD-C-79 |
References
- Ferrari, A. J., et al. Burden of Depressive Disorders by Country, Sex, Age, and Year: Findings from the Global Burden of Disease Study 2010. PLoS Medicine. 10 (11), (2013).
- Trivedi, M. H., et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. The American Journal of Psychiatry. 163 (1), 28-40 (2006).
- Gartlehner, G., et al. Second-Generation Antidepressants in the Pharmacologic Treatment of Adult Depression: An Update of the 2007 Comparative Effectiveness Review. Second-Generation Antidepressants in the Pharmacologic Treatment of Adult Depression: An Update of the 2007 Comparative Effectiveness Review. [Internet]. , (2011).
- Checkley, S. The neuroendocrinology of depression and chronic stress. British Medical Bulletin. 52 (3), 597-617 (1996).
- Parker, K. J., Schatzberg, A. F., Lyons, D. M. Neuroendocrine aspects of hypercortisolism in major depression. Hormones and Behavior. 43 (1), 60-66 (2003).
- de Kloet, E. R., Joels, M., Holsboer, F. Stress and the brain: from adaptation to disease. Nature Reviews Neuroscience. 6 (6), 463-475 (2005).
- McEwen, B. S. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European Journal of Pharmacology. 583 (2-3), 174-185 (2008).
- Chiba, S., et al. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 39 (1), 112-119 (2012).
- Son, H., et al. Glutamine has antidepressive effects through increments of glutamate and glutamine levels and glutamatergic activity in the medial prefrontal cortex. Neuropharmacology. 143, 143-152 (2018).
- Gregus, A., Wintink, A. J., Davis, A. C., Kalynchuk, L. E. Effect of repeated corticosterone injections and restraint stress on anxiety and depression-like behavior in male rats. Behavioural Brain Research. 156 (1), 105-114 (2005).
- Woolley, C. S., Gould, E., McEwen, B. S. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Research. 531 (1-2), 225-231 (1990).
- Diorio, D., Viau, V., Meaney, M. J. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. The Journal of Neuroscience. 13 (9), 3839-3847 (1993).
- Figueiredo, H. F., Bruestle, A., Bodie, B., Dolgas, C. M., Herman, J. P. The medial prefrontal cortex differentially regulates stress-induced c-fos expression in the forebrain depending on type of stressor. European Journal of Neuroscience. 18 (8), 2357-2364 (2003).
- Katz, R. J. Animal model of depression: Effects of electroconvulsive shock therapy. Neuroscience and Biobehavioral Reviews. 5 (2), 273-277 (1981).
- Willner, P., Towell, A., Sampson, D., Sophokleous, S., Muscat, R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology. 93 (3), 358-364 (1987).
- Slattery, D. A., Cryan, J. F. Modelling depression in animals: at the interface of reward and stress pathways. Psychopharmacology. 234 (9-10), 1451-1465 (2017).
- Joo, Y., et al. Chronic immobilization stress induces anxiety- and depression-like behaviors and decreases transthyretin in the mouse cortex. Neuroscience Letters. 461 (2), 121-125 (2009).
- Jung, S., et al. Decreased expression of extracellular matrix proteins and trophic factors in the amygdala complex of depressed mice after chronic immobilization stress. BMC Neuroscience. 13 (1), (2012).
- Seo, J. S., et al. NADPH Oxidase Mediates Depressive Behavior Induced by Chronic Stress in Mice. Journal of Neuroscience. 32 (28), 9690-9699 (2012).
- Seo, J. S., et al. Cellular and molecular basis for stress-induced depression. Molecular Psychiatry. 22 (10), 1440-1447 (2016).
- Bowman, R. E., Zrull, M. C., Luine, V. N. Chronic restraint stress enhances radial arm maze performance in female rats. Brain Research. 904 (2), 279-289 (2001).
- McLaughlin, K. J., Baran, S. E., Wright, R. L., Conrad, C. D. Chronic stress enhances spatial memory in ovariectomized female rats despite CA3 dendritic retraction: Possible involvement of CA1 neurons. Neuroscience. 135 (4), 1045-1054 (2005).
- Qin, M., Xia, Z., Huang, T., Smith, C. B. Effects of chronic immobilization stress on anxiety-like behavior and basolateral amygdala morphology in Fmr1 knockout mice. Neuroscience. 194, 282-290 (2011).
- Popoli, M., Yan, Z., McEwen, B. S., Sanacora, G. The stressed synapse: The impact of stress and glucocorticoids on glutamate transmission. Nature Reviews Neuroscience. 13 (1), 22-37 (2012).
- Bourke, C. H., Neigh, G. N. Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Hormones and Behavior. 60 (1), 112-120 (2011).
- Eiland, L., Ramroop, J., Hill, M. N., Manley, J., McEwen, B. S. Chronic juvenile stress produces corticolimbic dendritic architectural remodeling and modulates emotional behavior in male and female rats. Psychoneuroendocrinology. 37 (1), 39-47 (2012).
- Sun, L., et al. Effects of Hint1 deficiency on emotional-like behaviors in mice under chronic immobilization stress. Brain and Behavior. 7 (10), 1-11 (2017).
- Kim, K. S., Han, P. L. Optimization of chronic stress paradigms using anxiety-and depression-like behavioral parameters. Journal of Neuroscience Research. 83 (3), 497-507 (2006).
- Kim, G., et al. The GABAB receptor associates with regulators of G-protein signaling 4 protein in the mouse prefrontal cortex and hypothalamus. BMB Reports. 47 (6), (2014).
- Jangra, A., et al. Honokiol abrogates chronic restraint stress-induced cognitive impairment and depressive-like behaviour by blocking endoplasmic reticulum stress in the hippocampus of mice. European Journal of Pharmacology. 770, 25-32 (2016).
- Hurley, L. L., Akinfiresoye, L., Kalejaiye, O., Tizabi, Y. Antidepressant effects of resveratrol in an animal model of depression. Behavioural Brain Research. 268 (5), 1-7 (2014).
- Gottesman, I. I., Gould, T. D. The endophenotype concept in psychiatry: etymology and strategic intentions. The American Journal of Psychiatry. 160 (4), 636-645 (2003).
- Cryan, J. F., Mombereau, C. In search of a depressed mouse: Utility of models for studying depression-related behavior in genetically modified mice. Molecular Psychiatry. 9 (4), 326-357 (2004).
- Son, H., Jung, S., Shin, J., Kang, M., Kim, H. Anti-Stress and Anti-Depressive Effects of Spinach Extracts on a Chronic Stress-Induced Depression Mouse Model through Lowering Blood Corticosterone and Increasing Brain Glutamate and Glutamine Levels. Journal of Clinical Medicine. 7 (11), 406 (2018).
- Crowley, J. J., Blendy, J. A., Lucki, I. Strain-dependent antidepressant-like effects of citalopram in the mouse tail suspension test. Psychopharmacology. 183 (2), 257-264 (2005).
- Ripoll, N., David, D. J. P., Dailly, E., Hascoët, M., Bourin, M. Antidepressant-like effects in various mice strains in the tail suspension test. Behavioural Brain Research. 143 (2), 193-200 (2003).
- Mayorga, A. J., Lucki, I. Limitations on the use of the C57BL/6 mouse in the tail suspension test. Psychopharmacology. 155 (1), 110-112 (2001).
- Cryan, J. F., Mombereau, C., Vassout, A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 29 (4-5), 571-625 (2005).
- Can, A., Dao, D. T., Terrillion, C. E., Piantadosi, S. C., Bhat, S., Gould, T. D. The Tail Suspension Test. Journal of Visualized Experiments. (58), 2-7 (2011).
- Weiss, I. C., Pryce, C. R., Jongen-Rêlo, A. L., Nanz-Bahr, N. I., Feldon, J. Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behavioural Brain Research. 152 (2), 279-295 (2004).
- Hilakivi, L. A., Ota, M., Lister, R. Effect of isolation on brain monoamines and the behavior of mice in tests of exploration, locomotion, anxiety and behavioral “despair.”. Pharmacology, Biochemistry and Behavior. 33 (2), 371-374 (1989).
- Dalla, C., Pitychoutis, P. M., Kokras, N., Papadopoulou-Daifoti, Z. Sex differences in response to stress and expression of depressive-like behaviours in the rat. Current Topics In Behavioral Neurosciences. 8 (2), 97-118 (2011).
- Bangasser, D. A., Valentino, R. J. Sex differences in stress-related psychiatric disorders: Neurobiological perspectives. Frontiers in Neuroendocrinology. 35 (3), 303-319 (2014).
- Palanza, P. Animal models of anxiety and depression: How are females different?. Neuroscience and Biobehavioral Reviews. 25 (3), 219-233 (2001).
- Novais, A., Monteiro, S., Roque, S., Correia-Neves, M., Sousa, N. How age, sex and genotype shape the stress response. Neurobiology of Stress. 6, 44-56 (2017).
- Kim, J. G., Jung, H. S., Kim, K. J., Min, S. S., Yoon, B. J. Basal blood corticosterone level is correlated with susceptibility to chronic restraint stress in mice. Neuroscience Letters. 555, 137-142 (2013).
- Jeong, J. Y., Lee, D. H., Kang, S. S. Effects of Chronic Restraint Stress on Body Weight, Food Intake, and Hypothalamic Gene Expressions in Mice. Endocrinology and Metabolism. 28 (4), 288 (2013).
- Gould, T. D., Dao, D. T., Kovacsics, C. E. . Mood and anxiety related phenotypes in mice: characterization using behavioral tests. , (2009).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved