Method Article
Autonomous and Rechargeable Microneurostimulator Endoscopically Implantable into the Submucosa
* These authors contributed equally
In This Article
Summary
The application of high-frequency low-energetic stimulation can alleviate the symptoms of gastric dysmotility. In this research, a miniature, endoscopically implantable and wirelessly rechargeable device which is implanted into a submucosal pocket is presented. Successful both-way communication and stimulation control were achieved during an experiment on live pig.
Abstract
Gastric dysmotility can be a sign of common diseases such as longstanding diabetes mellitus. It is known that the application of high-frequency low-energetic stimulation can help to effectively moderate and alleviate the symptoms of gastric dysmotility. The goal of the research was the development of a miniature, endoscopically implantable device to a submucosal pocket. The implantable device is a fully customized electronic package which was specifically designed for the purpose of experiments in the submucosa. The device is equipped with a lithium-ion battery which can be recharged wirelessly by receiving an incident magnetic field from the charging/transmitting coil. The uplink communication is achieved in a MedRadio band at 432 MHz. The device was endoscopically inserted into the submucosal pocket of a live domestic pig used as an in vivo model, specifically in the stomach antrum. The experiment confirmed that the designed device can be implanted into the submucosa and is capable of bidirectional communication. The device can perform bipolar stimulation of muscle tissue.
Introduction
Gastric dysmotility can be a sign of several relatively common diseases such as gastroparesis, which is usually characterized by a chronic progression and imposes rather severe consequences on the social, work-related, and physical status of the patient. Most cases of gastroparesis are usually diabetic or idiopathic in origin and are often resistant to available medication1. Patients afflicted with this condition most commonly present with nausea and repeated vomiting. Based on previous research, it is known that the application of high-frequency low-energetic electrical stimulation can help to effectively moderate and alleviate the symptoms of gastric dysmotility1,2.
Based on previous studies, it is proven that high-frequency gastric electrical stimulation can significantly improve the symptoms and gastric emptying3. It has also been shown that lower esophageal sphincter neurostimulator therapy is safe and effective for the treatment of gastroesophageal reflux disease (GERD), reducing the acid exposure and eliminating daily proton-pump inhibitor (PPI) usage without stimulation related adverse effects4. Before human trials, first studies were performed in animal models (canine models5). Based on these studies, electrical stimulation of the lower esophageal sphincter (LES, 20 Hz, pulse width of 3 ms) caused a prolonged contraction of the LES5. Similar effects of high (20 Hz, pulse width of 200 μs) and low (6 cycles/min, pulse width of 375 ms) frequency electrical stimulation on LES in GERD patients were investigated. Both high and low frequency stimulation were effective6. However, currently, there are only two neurostimulation devices for gastric or esophageal stimulation available on the market7,8. In those devices, the electrodes can be implanted surgically, laparoscopically or robotically. The device itself is implanted subcutaneously. This requires general anaesthesia and have a bulky device fitted, using intramuscular catheters which allow for the stimulation of the gastric or esophageal muscle tissue. So, the option of using a wirelessly communicating device implanted into the gastric submucosal layer would represent a definite advantage and improvement in patient comfort. As stated in the previous research9,10, it was proven that an implantation of a miniature neurostimulator into submucosa is possible. For the endoscopic submucosal implantation, we use a technique called endoscopic submucosal pocketing (ESP), based on endoscopic submucosal tunnel dissection10. The goal of this research is to further improve this concept of an implantable neurostimulator, primarily in the scope of power management (specifically the wireless recharging capability), conformity with respective laws and regulations for wireless communication links in medical implantable devices and possibility of bipolar neurostimulation. Next, the presented microneurostimulator is capable of bidirectional communication and the stimulation parameters can be changed in real-time, even while the device is implanted.
This technique is suitable for teams with a therapeutic endoscopist experienced in endoscopic pocketing or tunnel dissections. Next, a hardware and embedded software designer with experience in building hardware prototypes with microcontrollers and radio frequency circuits using surface mount technology is needed. For building the hardware prototypes, a lab equipped with a reflow soldering station and basic equipment for electrical measurements (at least a digital multimeter, an oscilloscope, a spectrum analyzer and PICkit3 programmer) is required.
Protocol
All endoscopic procedures including animal subjects have been approved at the Institute of Animal Physiology and Genetics, Academy of Science Czech Republic (Biomedical Center PIGMOD), Libechov, Czech Republic (project Experiments in implantation of battery-less and battery devices into submucosa of the esophagus and stomach — experimental study). All experiments are done in compliance with Czech law 246/1992 Sb. "On the protection of animals against maltreatment, as amended". Transmitter device is not required to be sterilized, because it is an external device that is not in direct contact with the animal.
1. Implantable Device Design
- Prepare the PCB using a third-party PCB manufacturing service. The complete printed circuit board design is provided in the supplementary file "gerber_implant.7z". The schematic diagram is provided in Figure 1.
- Place the PCBs on a flat surface (Figure 2a). Use a solder paste dispenser with 0.6 mm needle and 60 psi pressure to manually dispense the soldering paste onto every metallic pad on the PCB. Begin with the top side of the PCB (Figure 2b). The total amount of soldering paste for both sides of the PCB should not exceed 15 μL.
- With a pair of antistatic tweezers, place all components on the top layer of the PCB (Figure 2e). Use Figure 3 for component position and supplementary file "bom_implantabledevice.csv" for the assignment of components to their numbers.
- Use a PCB hot air gun station at 260 °C to solder all components (Figure 4a). Wait until all the solder paste melts, then put away the hot air gun and allow the board cool to room temperature.
- Turn the PCB over and dispense solder paste on the other side. Use the same needle and pressure as stated in 1.2 (Figure 2d).
- As in step 1.3., place all components of the bottom layer of the PCB. Refer to Figure 3 for component position and the supplementary file "bom_implantabledevice.csv" for the assignment of components to their numbers.
- Repeat the heating of the PCB with a hot air gun to solder all components on the bottom side. Use the same process as in step 1.4.
- Check the PCB for any short circuits visually. If any short circuit is found, remove it with a soldering iron.
- Manufacture the wireless charging/communication coil. Use 17 turns of AWG42 wire. The size of the coil is 26 x 13.5 mm2 (Figure 4d). Twist the two output wires.
- Design and manufacture the electrode. The electrode design is provided in the supplementary file "gerber_electrodes.7z". Use the same manufacturing process as in step 1.1. This PCB is fully completed after manufacturing, and no components are required to be soldered onto it. Solder two AWG42 wires to the small rectangular contacts (Figure 4f)
- Prepare the antenna by using 7 cm of enameled wire and scraping off 3 mm of the enamel from one end (Figure 4e)
- Connect the PICkit 3 programmer to the PCB (Figure 4b-c)
- Connect pads 6 and 7, according to Figure 5, to pins 2 and 3 of the PICkit programmer, respectively.
- Connect pads TP1, TP2 and TP3 (refer to Figure 3) to pins 1, 5 and 4 of the PICkit programmer, respectively
- Plug in the PICkit 3 programmer into the USB port of a computer with MPLAB IPE software installed.
- Run the MPLAB IPE software and program the firmware into the microcontroller.
- Run the MPLAB IPE v3.61. Select "Settings | Advanced Mode"
- Into the Password field, enter the default password which is 'microchip'. Click "Log on". A tab with different panels on the left will appear.
- On the top left, click "Operate", then in top middle part of the screen, click "Device field" and type in "PIC16LF1783". Click "Apply".
- Select panel "Power" on the left (Figure 6).
- Change the VDD voltage value to 2.55. This step is critical.
Caution: Setting this value above 2.8 V will damage the board (Figure 7). - Click the checkbox "Power Target Circuit" from "Tool" (Figure 7).
- Click the "Operate" tab on the left (Figure 6).
- Click "Connect".
- Download the supplementary file "IMPLANTABLE_V2.X.production.hex" and note its location on the hard drive. In the IPE software, find the Source line and click the "Browse" button near it (Figure 8).
- Click Program. Wait till the software says that the software has been successfully downloaded to the microcontroller (Figure 9).
- Desolder the wires soldered to pads TP1, TP2 and TP3 (Figure 3) as well as wires soldered to pads 6 and 7 (Figure 5).
- Connect the PCB to all other electrical components except for the battery (Figure 10a).
- Solder the wireless charging/communication coil to pads 2 and 3 according to Figure 8. The polarity is not important.
- Connect the antenna to pad 1 according to Figure 5. Connect the PCB electrodes to pads number 4 and 5 according to Figure 5. The polarity is not important.
- Solder the CG-320 battery to pads 6 and 7 (Figure 5). The negative terminal of the battery must be soldered onto pad 7. Be careful while performing the next steps. The device is now powered and is sensitive to short circuits and contact with metallic objects.
- To test the functionality of the wireless charging circuitry, all steps in part 2 need to be completed. After that, place the wireless charger/transmitter in close proximity of the device. Use a multimeter to measure the voltage of the battery. If the battery voltage is slowly rising (several mV per min), the charging function is working.
- Wind the antenna around the device in a spiral (Figure 10b)
- Cut a 32 mm long piece of a heat-shrinkable tubing with an internal diameter of 9.5 mm.
- Place the coil on the PCB. Refer to Figure 7b for the correct placement.
- Put the heat-shrinkable tubing over the device, coil and antenna. Only the electrodes should protrude from the tubing. Refer to Figure 7c for correct placement.
- Heat the tubing with a hot air gun to 150 °C to shrink and then allow it to cool (Figure 10d).
- Apply epoxy glue to the left end to seal one side of the tubing (Figure 10e).
- Glue the electrode to the back side of the PCB with tubing. Also glue the other end of the tubing. Refer to Figure 10f for correct placement.
- Wait for at least 24 h for the glue to harden and fully cure.
- After the completion of the wireless charger/transmitter device, test the implantable device for water leaks by placing it into a 30 cm high column of saturated saline solution for 1 h. Any major leak can be spotted as a sudden drop of the battery voltage or the malfunction of the device caused by the saline solution shorting the electronics. After the test, the device is fully prepared to be implanted.
- Test the stimulation function of the implant using an oscilloscope. Connect two measurement electrodes of the oscilloscope to the tin metal plated contact pads of the electrode on the implantable device. Observe the stimulation pattern on the oscilloscope screen. The correct stimulation pattern is given in Figure 11.
2. Wireless Charger/Transmitter Design
- The PCB design is provided in the supplementary file "gerber_transmitter.7z". Use the same manufacturing process as for the implantable device. The schematic diagram is provided in Figure 12.
- Place the PCBs on a flat surface. Use a solder paste dispenser with 0.6 mm needle and 60 psi pressure to manually dispense the soldering paste onto every metallic pad on the PCB. The total amount of solder paste dispensed on the PCB should not exceed 50 μL.
- With a pair of antistatic tweezers, place all components on the top layer of the PCB. Consult Figure 13 for the component position and the supplementary file "bom_transmitterdevice.csv" for the assignment of components to their numbers.
- Use a PCB hot air gun station preset to 260 °C to solder all components. Wait until all the solder paste melts, put away the hot air gun and allow the board to cool to room temperature.
- Repeat the steps 2.3–2.4 for the bottom side of the device. Follow a similar procedure as during the manufacturing of the implantable device.
- Create a coil with 3 turns of AWG18 enameled wire (Figure 14c) and connect it to pads COIL1 and COIL2 (Figure 13).
- Make an aluminum heatsink for the power transistors (Figure 13, Q1 and Q2). The exact shape of the heatsink is not critical. One of the possible embodiments is shown in Figure 9d. In this case, the heatsink also forms an enclosure for the device.
- Connect the PICkit 3 programmer to the assembled PCB. Connect pads TP1 to TP5 (Figure 13) with pins 1 to 5 of the PICkit programmer, respectively.
- Plug in the PICkit 3 programmer into the USB port of a computer with MPLAB IPE software installed.
- Run the MPLAB IPE software and program the firmware into the microcontroller. The process is the same as for the implantable device, except for the VDD voltage and file uploaded.
- Run the MPLAB IPE v3.61. Select "Settings | Advanced Mode".
- Into password box, enter the default password which is 'microchip'. Click "Log on". A tab with different panels on the left will appear.
- On the top left, click "Operate", then in the top middle part of the screen, click the "Device" and type in "PIC16LF1783". Click "Apply".
- Select panel "Power" on the left
- Change the VDD voltage value to 3.3.
- Click the checkbox "Power Target Circuit" from "Tool".
- Click the "Operate" tab on the left.
- Click "Connect".
- Download the supplementary file "IMPLANTABLE_V2_TRANSMITTER.X.production.hex" and note its location on the hard drive. In the IPE software, find the Source line and click the "Browse" button near to it.
- Click "Program". Wait till the software says that the software was downloaded to the microcontroller successfully.
- Desolder the wires soldered to pads TP1 to TP5
- Connect a 12 V power supply to the V- and V+ pads (Figure 5). The negative terminal has to be connected to the V- pad.
- Plug in a mini-USB to USB-A cable to the X1 connector (Figure 5) and connect to a computer with PuTTy software pre-installed.
- Open the PuTTY software and set it up (Figure 15).
- Open the PuTTY software. Select "Serial" as connection type.
- Enter COMx as a Serial line, where x is the number of the COM port of the device. If no other COM port device was installed, this number will be 1.
- Enter "38400" as Speed. Click "Open". The charger/transmitter device is now ready to be used. Press H key for help.
3. Endoscopic Implantation
- Use a live mini pig as an in vivo model, adult (8-36 months), 20-30 kg weight.
- Let the pig fast for 24 h prior to the procedure.
- Allow clear liquids ad libitum.
- Administer intramuscular tiletamine (2 mg/kg), zolazepam (2 mg/kg) and ketamine (11 mg/kg) as a premedication.
- Apply intravenous thiopental ad effectum (5% solution) and inhalation anesthesia with isoflurane, N2O and propofol injection. Proper anesthesia is confirmed by reflexes and muscle tone, eye position, palpebral reflex and pupillary reflex. Circulation, oxygenation, ventilation and body temperature are continuously monitored.
- In order to perform the implantation and visualization, use an animal model dedicated endoscope. Insert it using the standard way into the in vivo model.
- Grasp the device externally with a snare. After that, insert it into the stomach, then release it.
- Extract the endoscope, equip it with a dissection cap (15.5 mm), and then re-insert it to the stomach.
- In order to implant the device to submucosa, apply saline solution mixed with methylene blue into the submucosal layer using an injection therapy needle catheter (25 G).
- Make a horizontal incision to create an opening in the submucosa using an electrosurgical knife with a knob-shaped tip.
- Using the affixed cap, insert the cap into the newly created space, and with the use of an electrosurgical knife, continue disrupting, dilating, and dissecting the submucosal layer, creating a sufficiently large-enough pocket to insert the stimulation device.
- Grasp the device which is lying freely inside the stomach with insertion and extraction loops and, using grasping forceps, navigate it into the submucosal pocket. Place the stimulation electrodes in contact with the muscularis propria using grasp forceps.
- Use an over the scope clip to secure the device in place inside the submucosal pocket and prevent any migration or dislodging.
4. Experiment — After Implantation
- After successful implantation, place the charger/transmitter coil in proximity of the implanted device.
- Plug in the RTL2832 dongle in the PC.
- Run the HDSDR software and set the center frequency to 432 MHz.
- Open the HDSDR software (Figure 15) for correct settings and PuTTY software (Figure 16). In the HDSDR software, click "Options | Select Input | ExtIO".
- Select Bandwidth — "960000". Select the LO frequency to 431.95 MHz. Select the Tune frequency to 432.00 MHz.
- Transmit a Manchester coded sequence from the charger/transmitter by pressing the R key in the PuTTY terminal and receive the OOK modulated reply from the implant by observation of the main HDSDR window ( Figure 17e-f).
5. Euthanasia after the Experiment
- Use an anesthetic overdose for euthanasia (lethal dose of thiopental and KCl).
Results
Figure 17 shows that an endoscopic placement of the gastric neurostimulator into a pocket in submucosa as well as proper placement of the electrodes to the muscular layer was successful. The dimensions of the device (Figure 10) are 35 x 15 x 5 mm3 while the weight is 2.15 g. Figure 17 shows the circuit diagram of the device showing that the device comprises of 6 different modules which are connected together. Figure 3 shows the PCB layout and component placement in the device. Figure 18 shows that in order to implant the device into the submucosal layer, a technique called an endoscopic submucosal pocket9,10 (ESP) was used. The stimulator was attached near the muscular layer (muscularis propria) where it is theoretically the optimal stimulation depth. Creating the submucosal pocket and implanting of the gastric neurostimulator endoscopically took 20–30 min. During this procedure, there is no intraprocedural complication such as perforation or severe bleeding. Migration of the device in the stomach could not be determined because the experiment was non-survival. After the implantation, bidirectional communication link with the implantable device was established with an external device shown in Figure 14. The approximate distance between the charger/programmer coil and the implant was 10 cm. The achieved signal-to-noise (SNR) ratio with RTL2832 based software-defined-radio (SDR) receiver was over 40 dB.

Figure 1: Schematic diagram of the implantable device. The Figure shows how different components and circuit parts are connected in the implantable device. Please click here to view a larger version of this figure.

Figure 2: Fabrication of the implantable device - PCB assembly. (a) PCB, top view. (b) Solder paste applied to top layer. (c) An example of hand placement of 0402 capacitor. (d) Solder paste applied to bottom layer. (e) Fully populated top side of the PCB. (f) Fully populated bottom side of the PCB Please click here to view a larger version of this figure.

Figure 3: Design of the implantable device. (a) Top copper layer of the PCB. (b) Component names on the top layer. (c) Bottom copper layer of the PCB. (d) Component names on the bottom layer. (e) Composite picture of all PCB layers Please click here to view a larger version of this figure.
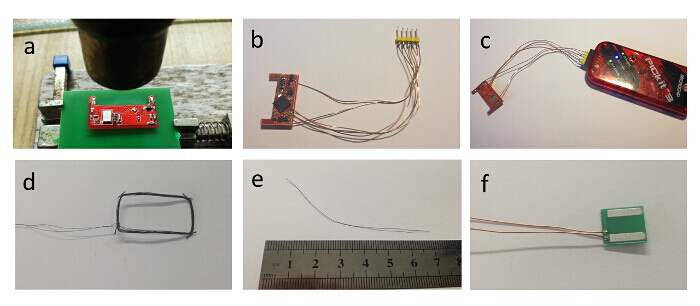
Figure 4: Fabrication of the implantable device — preparation of other parts. (a) Hot air flow of the bottom side of the PCB. (b) Programming wires soldered to the PCB. (c) PCB connected to the programmer. (d) Wireless charging coil. (e) 432 MHz antenna. (f) Stimulation electrodes with two wires attached Please click here to view a larger version of this figure.

Figure 5: Recommended solder joint placement for the external components of the implantable device. The picture shows where the coil, antenna, battery and electrodes should be soldered. Please click here to view a larger version of this figure.
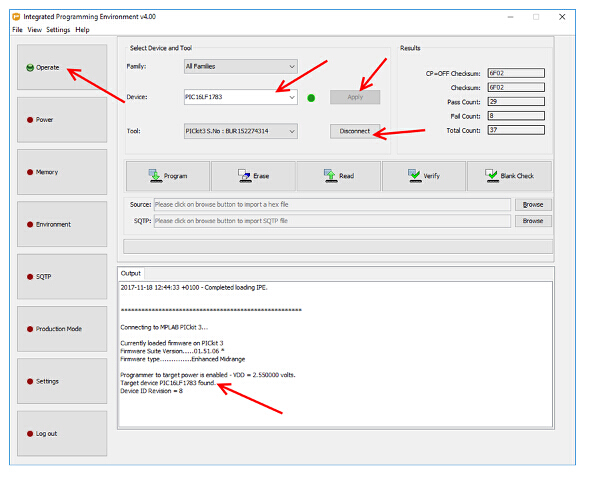
Figure 6: Establishing a connection with the implantable device — important settings mentioned in the text are marked with red arrows. This picture is from the MPLAB IPE software, a screen which shows how to determine that the microcontroller inside the implantable device is correctly communicating with the PICkit programmer is provided. Please click here to view a larger version of this figure.

Figure 7: Power settings of the software used for programming — important settings mentioned in the text are marked with red arrows. This is picture from the MPLAB IPE software. It shows how to properly power the implantable device for programming Please click here to view a larger version of this figure.

Figure 8: Choosing a correct programming file for the implantable device. The picture shows which button to click in order to load the supplementary .hex file correctly. Please click here to view a larger version of this figure.

Figure 9: Process of programming the firmware into the implantable device. The picture shows which button to press to program the software into the implantable device. Please click here to view a larger version of this figure.

Figure 10: Fabrication of the implantable device — final assembly. (a) Wireless charging coil, stimulation electrodes and antenna soldered to the PCB, together with battery. (b) Stacked implant. (c) Transparent heat shrinkable tubing put over the PCB. (d) Shrinking of the tubing with hot air. (e) Tubing fully shrinked and ends glued. (f) Finalized implantable device Please click here to view a larger version of this figure.

Figure 11: Typical output stimulation pattern of the device as displayed on the DSOX1102G oscilloscope. After programming of the implantable device, soldering of the electrodes and the battery, output stimulation pattern similar to the one displayed in the figure should appear at the electrodes. Please click here to view a larger version of this figure.

Figure 12: Schematic diagram of the wireless charger/transmitter device. The figure is analogical to Figure 1. Shown here is the internal workings of the wireless charger/transmitter device Please click here to view a larger version of this figure.

Figure 13: Design of the charger/transmitter device. (a) Top copper layer of the PCB. (b) Component names on the top layer. (c) Bottom copper layer of the PCB. (d) Component names on the bottom layer. (e) Composite picture of all PCB layers Please click here to view a larger version of this figure.
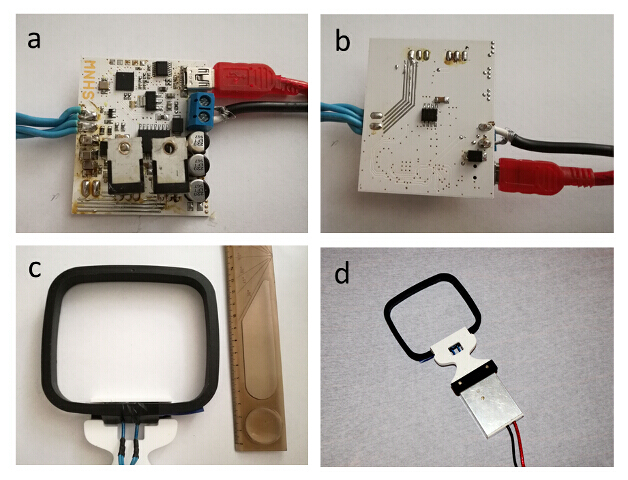
Figure 14: Fabrication of the wireless charger/transmitter device. (a) Completed PCB, top side (b) Completed bottom side of the PCB (c) Mechanical design of the wireless transmitter/charger coil (d) One possible embodiment of the finalized charger/transmitter device Please click here to view a larger version of this figure.

Figure 15: Correct settings of the HDSDR software. The HDSDR software is used together with the RTL2832U based USB receiving dongle as a spectrum analyzer to display the radio spectrum. In this case, it is used to receive the answer from the implant transmitted at approximately 432 MHz. Please click here to view a larger version of this figure.

Figure 16: Correct settings of the PuTTY software. The PuTTY software is used for communication with the charger/transmitter device. It must be correctly configured in order to display correct data to the user. Please click here to view a larger version of this figure.

Figure 17: Endoscopical implantation of the implantable device and checking if it is working. (a) In vivo model in animal endoscopic unit. (b) Insertion of the endoscope by the standard way into the in vivo model. (c) Implantable device prototype grasped with a snare. (d) Process of establishing bidirectional wireless link with the implantable device. (e) HDSDR software. (f) Detail of OOK modulated data transmitted by the implant. (g) X-ray — device position check. (h) X-ray scan of the implant area, the device as well as over the scope clip is clearly visible. (i) Detailed device view. Please click here to view a larger version of this figure.
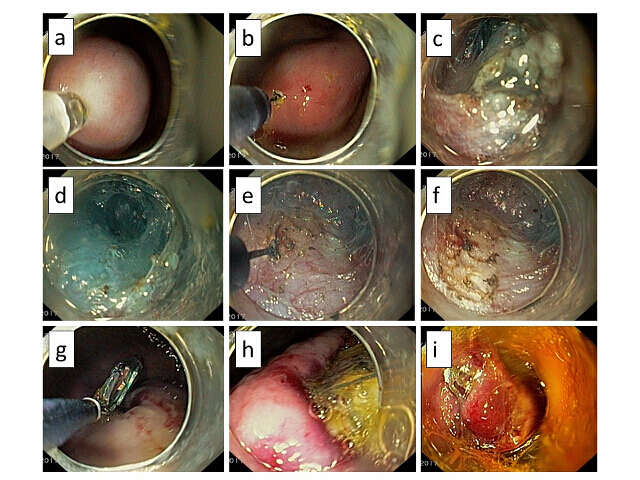
Figure 18: View of device implantation and endoscopic technique. (a) Submucosal injection with methylene blue. (b) Submucosal incision (an entrance for the submucosal pocket formation). (c) Tunnelisation of the submucosal pocket. (d-f) Disrupting, dilating, and dissecting the submucosal layer. (g, h) Device implantation. (i) Closing the entry with over the scope clip. Please click here to view a larger version of this figure.
Supplementary File 1: gerber_implant.7z. Zip archive with files required to manufacture the printed circuit board of the implantable device. Please click here to download this file
Supplementary File 2: gerber_transmitter.7z. Zip archive with files required to manufacture the printed circuit board of the charger/transmitter device. Please click here to download this file
Supplementary File 3: gerber_electrodes.7z. Zip archive with files required to manufacture the electrodes. Please click here to download this file
Supplementary File 4: IMPLANTABLE_V2.X.production.hex. Firmware for the implantable device. Please click here to download this file
Supplementary File 5: IMPLANTABLE_V2_TRANSMITTER.X.production.hex. Firmware for the charger/transmitter device. Please click here to download this file
Supplementary File 6: bom_implantabledevice.csv. Bill of material (BOM) file describing the assignment of component values to specific components on the PCB of the implantable device. Please click here to download this file
Supplementary File 7: bom_transmitterdevice.csv. BOM file describing the assignment of component values to specific components on the PCB of the charger/transmitter device. Please click here to download this file
Discussion
The design of the implantable device should primarily focus on the overall size of the device, achievable stimulation profiles (maximum voltage, maximum deliverable current, length of pulses and pulse frequency). Main limitation from the hardware perspective is the size and availability of suitable components. To minimize the overall size, surface mount components are preferred because of their compact packaging. The best solution would be to integrate bare chip dies on the substrate. However, this is limited by both the availability of bare die packaging option for components and the accessibility of the wire bonding technology. Second important parameter is the battery. Lithium batteries are preferred because of its high energy density. Also, the nominal voltage of 3.7 V is beneficial. The major benefit of the presented hardware topology is its small size and minimum invasiveness. Compared to the current solutions7,8, the presented solution is a magnitude smaller and can be implanted directly to the submucosa, without need for external leads and subcutaneous implantation of the neurostimulator.
Except for the hardware itself, in future, additional attention needs to be given to the device enclosure. The first point is the biocompatibility and hermeticity11 to avoid possible rejection of the implant. The other is the fixation of the device in the submucosa to avoid unwanted migration of the implant.
The most critical steps during endoscopic implantation is the capturing of the device and its placement into the submucosal pocket. The limitation is the size of the pocket, which must be, from the observations, approximately at least twice as large as the device to be implanted. Next issue is the correct orientation of the implant inside the pocket. With the respect to the technical difficulty of the endoscopic procedure, this method is dedicated to experts with experience with tunnel dissection or peroral endoscopic myotomy (POEM).
The next problematic part is the closure of the pocket which is relatively difficult using the over the scope clip. However, the use of this type of clip prevents the migration and rejection of the device. Limitations of this technique from hardware point of view include the hardware development equipment to solder with required accuracy. The device is designed to withstand during the surgery and a short time afterwards. Thus, with current enclosure, it is not designed to stay for prolonged periods of time inside the body. Also, the material of the enclosure is not biocompatible which represents a high risk of rejection of the implant in case of a survival experiment. This technique can be further developed, especially in terms of the development of biocompatible and hermetic enclosure which is essential for survival model experiments. Next, the functionality of multiple integrated circuits can be concentrated into a single application specific integrated circuit. Similarly, smaller surface mount components can be used to make the device more compact. The next possible direction of this research may lead to development of novel endoscopical methods for the treatment of other gastrointestinal diseases such as GERD, incontinence or sphincter dysfunctions12.
Disclosures
This work was supported by the Research Project PROGRES-Q28, and awarded by Charles University in Prague. The authors thank to Ass. Prof. Jan Martínek, Ph.D. and PIGMOD centre.
Acknowledgements
The authors declare that they have no competing financial interests.
Materials
| Name | Company | Catalog Number | Comments |
| EIA 0402 ceramic capacitor 1.8 pF | AVX | 04025U1R8BAT2A | 1 pc |
| EIA 0402 ceramic capacitor 100 nF | TDK | CGA2B3X7R1H104K050BB | 7 pcs |
| EIA 0402 ceramic capacitor 100 pF | Murata Electronics | GRM1555C1H101JA01D | 1 pc |
| EIA 0402 thick film resistor 10 kΩ | Vishay | CRCW040210K7FKED | 1 pc |
| EIA 0402 ceramic capacitor 10 nF | Murata Electronics | GRM155R71C103KA01D | 3 pcs |
| EIA 0402 ceramic capacitor 10 pF | Murata Electronics | GJM1555C1H100JB01D | 3 pc |
| EIA 0402 ceramic capacitor 12 pF | Murata Electronics | GJM1555C1H120JB01D | 2 pcs |
| EIA 0402 ceramic capacitor 18 pF | KEMET | C0402C180J3GACAUTO | 2 pcs |
| EIA 0402 resistor 1 mΩ | Vishay | MCS04020C1004FE000 | 2 pcs |
| EIA 0402 resistor 1 kΩ | Yageo | RC0402FR-071KL | 1 pc |
| EIA 0402 ceramic capacitor 1 nF | Murata Electronics | GRM1555C1H102JA01D | 3 pcs |
| EIA 0603 ceramic capacitor 2.2 uF | Murata Electronics | GCM188R70J225KE22D | 2 pcs |
| EIA 0402 resistor 220 kΩ | Vishay | CRCW0402220KJNED | 5 pcs |
| 0805 22 uH inductor | TDK | MLZ2012N220LT000 | 1 pc |
| EIA 0402 resistor 330 kΩ | Vishay | CRCW0402330KFKED | 1 pc |
| EIA 0603 ceramic capacitor 4.7 uF | TDK | C1608X6S1C475K080AC | 1 pc |
| EIA 0402 resistor 470 Ω | Vishay | RCG0402470RJNED | 1 pc |
| EIA 0402 resistor 470 kΩ | Vishay | CRCW0402470KJNED | 1 pc |
| EIA 0603 inductor 470 nH | Murata Electronics | LQW18ANR47G00D | 1 pc |
| EIA 0402 resistor 47 kΩ | Murata Electronics | CRCW040247K0JNED | 2 pcs |
| 27.0000 MHz crystal 5032 | AVX / Kyocera | KC5032A27.0000CMGE00 | 1 pc |
| EIA 0402 capacitor 6.8 pF | Murata Electronics | GJM1555C1H6R8CB01D | 1 pc |
| EIA 0402 inductor 82 nH | EPCOS / TDK | B82498F3471J | 1 pc |
| ABS05 32.768 kHz crystal | ABRACON | ABS05-32.768KHZ-T | 1 pc |
| CDBU00340-HF schottky diode | COMCHIP technology | CDBU00340-HF | 2 pcs |
| CG-320S Li-Ion pinpoint battery | Panasonic | CG-320S | 1 pc |
| HSMS282P schottky diode rectifier | Broadcom / Avago | HSMS-282P-TR1G | 1 pc |
| MAX8570 step-up converter | Maxim Integrated | MAX8570EUT+T | 1 pc |
| MICRF113 RF transmitter | Microchip Technology | MICRF113YM6-TR | 1 pc |
| 4.3 V Zener diode | ON Semiconductor | MM3Z4V3ST1G | 1 pc |
| OPA237 operational amplifier | Texas Instruments | OPA237N | 1 pc |
| PIC16LF1783 8-bit microcontroller | Microchip Technology | PIC16LF1783-I/ML | 1 pc |
| TPS70628 low-drop regulator | Texas Instruments | TPS70628DBVT | 1 pc |
| EIA 1206 thick film resistor 0 Ω | Yageo | RC1206JR-070RL | 2 pcs |
| EIA 0603 thick film resistor 0 Ω | Yageo | RC0603JR-070RL | 1 pc |
| EIA 0402 thick film resistor 100 kΩ | Yageo | RC0402FR-07100KL | 1 pc |
| EIA 0603 thick film resistor 100 kΩ | Yageo | RC0603FR-07100KL | 1 pc |
| EIA 0805 ceramic capacitor 100 nF | KEMET | C0805C104K5RAC7210 | 2 pcs |
| EIA 0402 thick film resistor 10 kΩ | Yageo | RC0402JR-0710KL | 1 pc |
| EIA 1206 ceramic capacitor 10 nF | Samsung | CL31B103KHFSW6E | 2 pcs |
| EIA 0402 thick film resistor 1 kΩ | Yageo | RC0402JR-071KL | 2 pcs |
| EIA 0402 thick film resistor 220 Ω | Yageo | RC0402JR-07220RL | 2 pcs |
| EIA 0402 ceramic capacitor 220 nF | TDK | C1005X5R1C224K050BB | 1 pc |
| EIA 1206 ceramic capacitor 22 nF | TDK | C3216X7R2J223K130AA | 2 pcs |
| SMC B tantalum capacitor 22 uF | AVX | TPSB226K010T0700 | 1 pc |
| EIA 0402 thick film resistor 27 Ω | Yageo | RC0402FR-0727RL | 2 pcs |
| EIA 1206 thick film resistor 3.3 Ω | Yageo | RC1206JR-073K3L | 3 pcs |
| SOT23 3.3V zener diode | ON Semiconductor | BZX84C3V3LT1G | 1 pc |
| SMC A tantalum capacitor 4.7uF | KEMET | T491A475M016AT | 2 pcs |
| EIA 0603 thick film resistor 470 Ω | Yageo | RC0603JR-07470RL | 2 pcs |
| EIA 1206 ceramic capacitor 470 nF | KEMET | C1206C471J5GACTU | 3 pcs |
| Electrolytic capacitor 470 uF | Panasonic | EEE-1CA471UP | 3 pcs |
| EIA 0402 ceramic capacitor 47 pF | AVX | 04025A470JAT2A | 2 pcs |
| 0603 GREEN LED | Lite-On Inc. | LTST-C191KGKT | 1 pc |
| 0603 RED LED | Lite-On Inc. | LTST-C191KRKT | 1 pc |
| 16 MHz CX3225 crystal | EPSON | FA-238 16.0000MB-C3 | 1 pc |
| 0805 ferrite bead | Wurth Electronics Inc. | 742792040 | 1 pc |
| IR2110SO FET driver | Infineon Technologies | IR2110SPBF | 1 pc |
| FT230XS USB to seriál converter | FTDI Ltd. | FT230XS-R | 1 pc |
| Mini USB connector | EDAC Inc. | 690-005-299-043 | 1 pc |
| PIC16F1783 8-bit microcontroller | Microchip Technology | PIC16F1783-I/ML | 1 pc |
| REG1117 3.3 V regulator SOT223 | Texas Instruments | REG1117-3.3/2K5 | 1 pc |
| Schottky SMB diode rectifier | STMicroelectronics | STPS3H100UF | 1 pc |
| SMB package TVS diode | Littelfuse Inc. | 1KSMBJ6V8 | 1 pc |
| IRLZ44NPBF N-channel MOSFET | Infineon Technologies | IRLZ44NPBF | 2 pcs |
| RTL2832U receiver dongle | EVOLVEO | Mars | 1 pc |
| PICkit 3 | Microchip Technology | PICkit 3 | 1 pc |
| Mini USB to USB A cable | OEM | Mini USB to USB-A | 1 pc |
| Printed circuit board, implantable device | --- | Manufacture with the provided supplementary file | 1 pc |
| Printed circuit board, transmitter/receiver device | --- | Manufacture with the provided supplementary file | 1 pc |
| Printed circuit board, implantable device | --- | Manufacture with the provided supplementary file | 1 pc |
| AWG18 wire | Alpha Wire | 3055 BK001 | 2 m |
| AWG42 wire | Daburn Electronics | 2420/42 BK-100 | 1 m |
| Olympus GIFQ-160 | Olympus | N/A (part is obsoleted) | 1 pc |
| Single-use electrosurgical knife with knob-shaped tip and integrated jet function | Olympus | KD-655L | 1 pc |
| Single-use oval electrosurgical snare | Olympus | SD-210U-15 | 1 pc |
| 15.5 mm lens hood | FujiFilm | DH-28GR | 1 pc |
| Injection therapy needle catheter | Boston Scientific | 25G | 1 pc |
| Alligator law grasping forceps | Olympus | FG-6L-1 | 1 pc |
| Instant Mix 5 min epoxy | Loctite | N/A | 1 pc |
| Heat shrinkable tubing, inside diameter 9.5 mm | TE Connectivity | RNF-100-3/8-X-STK | 1 pc |
| ChipQuik solder paste | Chip Quik | SMD4300AX10 | 1 pc |
References
- Abell, T., et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 125 (2), 421-428 (2003).
- O'Grady, G., Egbuji, J., Du, P., Cheng, L. K., Pullan, A. J., Windsor, J. A. High-frequency gastric electrical stimulation for the treatment of gastroparesis: a meta-analysis. World J Surg. 33 (8), 1693-1701 (2009).
- Chu, H., Lin, Y., Zhong, L., McCallum, R. W., Hou, X. Treatment of high-frequency gastric electrical stimulation for gastroparesis. J Gastroenterol Hepatol. 27 (6), 1017-1026 (2012).
- Rodríguez, L., et al. Electrical stimulation therapy of the lower esophageal sphincter is successful in treating GERD: final results of open-label prospective trial. Surg Endosc. 27 (4), 1083-1092 (2013).
- Ellis, F., Berne, T. V., Settevig, K. The prevention of experimentally induced reflux by electrical stimulation of the distal esophagus. Am J Surg. 115, 482-487 (1968).
- Rinsma, N. F., Bouvy, N. D., Masclee, A. A. M., Conchillo, J. M. Electrical Stimulation Therapy for Gastroesophageal Reflux Disease. J Neurogastroenterol. 20 (3), 287-293 (2014).
- Medtronic Inc, . . Enterra Therapy 3116 - Gastric Electrical Stimulation System. , (2016).
- Rodriguez, L., et al. Two-year results of intermittent electrical stimulation of the lower esophageal sphincter treatment of gastroesophageal reflux disease. Surgery. 157 (3), 556-567 (2015).
- Hajer, J., Novák, M. Development of an Autonomous Endoscopically Implantable Submucosal Microdevice Capable of Neurostimulation in the Gastrointestinal Tract. Gastroent Res Pract. , 8098067 (2017).
- Deb, S., et al. Development of innovative techniques for the endoscopic implantation and securing of a novel, wireless, miniature gastrostimulator (with videos). Gastrointest. Endosc. 76 (1), 179-184 (2012).
- Jiang, G., Zhou, D. D. . Technology advances and challenges in hermetic packaging for implantable medical devices. , (2017).
- Vonthein, R., Heimerl, T., Schwandner, T., Ziegler, A. Electrical stimulation and biofeedback for the treatment of fecal incontinence: a systematic review. Int J Colorectal Dis. 28 (11), 1567-1577 (2013).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved