Method Article
Construction of Modular Hydrogel Sheets for Micropatterned Macro-scaled 3D Cellular Architecture
In This Article
Summary
We describe the fabrication of micropatterned hydrogel sheets using a simple process, which can be assembled and manipulated in a freestanding form. Using these modular hydrogel sheets, a simple macro-scaled 3D cell culture system can be generated with a controlled cellular microenvironment.
Abstract
Hydrogels can be patterned at the micro-scale using microfluidic or micropatterning technologies to provide an in vivo-like three-dimensional (3D) tissue geometry. The resulting 3D hydrogel-based cellular constructs have been introduced as an alternative to animal experiments for advanced biological studies, pharmacological assays and organ transplant applications. Although hydrogel-based particles and fibers can be easily fabricated, it is difficult to manipulate them for tissue reconstruction. In this video, we describe a fabrication method for micropatterned alginate hydrogel sheets, together with their assembly to form a macro-scale 3D cell culture system with a controlled cellular microenvironment. Using a mist form of the calcium gelling agent, thin hydrogel sheets are easily generated with a thickness in the range of 100 - 200 µm, and with precise micropatterns. Cells can then be cultured with the geometric guidance of the hydrogel sheets in freestanding conditions. Furthermore, the hydrogel sheets can be readily manipulated using a micropipette with an end-cut tip, and can be assembled into multi-layered structures by stacking them using a patterned polydimethylsiloxane (PDMS) frame. These modular hydrogel sheets, which can be fabricated using a facile process, have potential applications of in vitro drug assays and biological studies, including functional studies of micro- and macrostructure and tissue reconstruction.
Introduction
Hydrogels are particularly promising biomaterials, and are expected to be important in basic biology, pharmacological assays and medicine.1 Biofabrication of hydrogel-based cellular constructs has been suggested to reduce the use of animal experiments,2,3 replace transplantable tissues,4 and improve cell-based assays.5,6 Water-containing (hydro-) viscoelastic materials (gels) allow a large number of cells to be encapsulated and maintained in a scaffold structure to control the 3D cellular microenvironment. In combination with the guidance of microfluidic or micropatterning technologies, the geometry of the hydrogel constructs can be precisely controlled at the cellular scale. To date, a variety of shapes of hydrogels, including particles,7-9 fibers,10-12 and sheets,13-15 have been used as building units in bottom-up approaches to the fabrication of macro-scale multi-cellular architectures.
Both hydrogel-based particles and fibers have been readily and rapidly fabricated for applications as micro-scale cellular environments, with fluidic controls using microfluidic devices. However, as the basic units of engineered tissues, it would be complicated to rearrange them and to enlarge their volume as macro-scale constructs.16 It is more difficult to achieve macro-scaled constructs than to produce micron-sized basic modules. Sheet-like units of hydrogel-based constructs can be used to increase the volume of scaffolds via a simple assembly process. Consequentially, stacked layers of hydrogel sheets provide not only a volumetric increase but also a geometric extension in a 3D space.
We have previously reported a method of fabricating micropatterned hydrogel sheets,13-15 together with their assembly into multi-layered cellular architectures. The technique enables complex micropatterning and modular design of cellular constructs via a stacking process of multi-layered structures. Through the fabrication of stacked modular hydrogel sheets, which are micropatterned, a 3D cell culture system with a controlled macro-scale cellular microenvironment can be realized. This video protocol describes a simple yet powerful fabrication method that can be used to construct modular hydrogel sheets, based on the human liver carcinoma cell line (HepG2). We demonstrate herein simple manipulation of these patterned modular hydrogel sheets, and their assembly into a multi-layered structure.
Protocol
1. Preparation of the Micropatterned Molds and Hydrogels
- Produce the desired micro-scale patterns using SU-8 photoresist on the surface of a silicon wafer via a standard two-step photolithography technique15,17 for casting PDMS molds. The example shown uses a liver lobule-like mesh pattern (Figure 1).
- Weigh out PDMS and a curing agent solution with a ratio of 1:5 (i.e., 12.5 g of PDMS and 2.5 g of curing agent).
- Mix the 15 g of the solution thoroughly, degas the bubbles in a vacuum chamber, and then spread the mixed solution onto a micropatterned surface of the silicon wafer evenly within a foil casting dish.
- Place the silicon wafer onto a 65 °C heated plate for 90 min on a flat surface to cure the PDMS.
- Remove the cured PDMS from the casting dish and the silicon wafer.
- Cut the edges of the PDMS and place it onto a 100-mm-diameter petri dish with the micropatterned side up.
- Wash the micropatterned cured PDMS on the petri dish using 70% ethanol and distilled water for primary sterilization. Then, dry them completely for 10 min in an oven at 65 °C.
- Dissolve and mix O/N 3 g of a powdered nonionic surfactant in 100 ml of distilled water, creating a 3% (w/v) coating solution.
- Place the micropatterned PDMS molds in a plasma cleaner and clean them for 1 min (85 W, 0.73 mbar) to create a hydrophilic surface, to facilitate the addition of aqueous liquids. Then, coat the surface of the PDMS with the 100 ml surfactant solution for at least 3 hr (orO/N) using a laboratory rocker.
- Wash the surfactant solution from the PDMS molds and dry them completely in an oven at 65 °C for 10 min. Then, sterilize each micropatterned mold by exposure to ultraviolet (UV) radiation over 30 min.
2. Prepare the Cell Suspension in a Hydrogel Precursor
- To prepare hydrogel precursor, dissolve 0.1 g of sodium alginate powder in 10 ml of phosphate buffered saline (PBS), creating a 1% (w/v) alginate precursor. To dissolve the powder completely, incubate and mix them O/N.
- Filter the solution through a 0.22 µm filter connected to a 1 ml syringe.
- Culture the HepG2 cells in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin on a conventional tissue culture dish until 70% - 80% confluence in a 5% CO2 humidified incubator at 37 °C.
- Wash the cells once using PBS, and then trypsinize them by adding 1 ml of 0.05% trypsin-EDTA for 4 min in a 5% CO2 humidified incubator at 37 °C. Centrifuge the cells harvested from the culture dish at 250 x g for 3 min and resuspend using 1 ml of PBS following removal of the supernatant.
- Count the number of single distributed cells in PBS using an automated cell counter.
- Following centrifugation at 250 x g for 3 min and PBS removal, add 1 ml of the 1% (w/v) of sodium alginate solution to the remaining cell pellet and mix them gently using pipetting. The final seeding density of the cells should be 5 x 106 - 107 cells/ml. Incubate the cell/hydrogel suspension in a 5% CO2 humidified incubator at 37 °C.
3. Loading and Cross-linking of Cell/Hydrogel Suspension
- Dissolve 1.47 g of calcium chloride dehydrate in 100 ml of ddH2O to produce a cross-linking reagent (i.e., 100 mM CaCl2·2H2O in ddH2O).
- Rinse out the interior of a humidifier with ultrasonic transducer using 70% ethanol, and fill it with 200 ml of cross-linking reagent. The humidifier is 110 mm wide, 300 mm long and 170 mm deep (i.e., 110 mm x 300 mm x 170 mm (W x H x D)).
- Place the micropatterned PDMS molds in a plasma cleaner and clean them for 1 min at 85 W to create a hydrophilic surface.
- Steadily load 7.2 µl of the well-mixed cell/hydrogel suspension at the edge of the micropattern in the mold. The example shown uses a liver lobule-like mesh pattern (Figure 1). The volume of the suspension depends on the topographic pattern.
- To achieve gelation of the cell/hydrogel suspension, turn on the humidifier and verify that the humidifier produces a mist of the cross-linking reagent. Spray the cross-linking reagent onto the hydrogel precursor for 5 min, covering the topographic surface of the PDMS molds within a range of 5 cm.
Note: Distances longer and shorter than 5 cm could cause incomplete and uneven gelation, respectively. Ensure that the humidifier has a spraying rate of 250 ml/hr, producing 20 ml of mist of the cross-linking reagent in 5 min. - Following the cross-linking process, turn off the humidifier and fill the PDMS molds with PBS.
4. Handling of Single Modular Hydrogel Sheets
- Detach each hardened hydrogel sheet from the micropatterned molds via pipetting PBS gently around the hydrogel sheet using a 200 µl pipette tip.
- Pick up each floating hydrogel sheet using an end-cut 1,000 µl pipette tip end. Each liver lobule-like mesh hydrogel sheet has dimensions of 8 mm x 8.7 mm, and be 100 - 200 µm thick.
- Transfer a single layer of the hydrogel sheet into 1 ml of DMEM in a 12-well plate, and culture the cells in vitro in a floating manner using the hydrogel construct as a unit component in the 12-well plate over a week in a 5% CO2 humidified incubator at 37 °C. Exchange the culture medium every other day.
5. Assembly of Multi-layered Hydrogel Sheets
- Repeat steps 1.1 to 1.5 to produce a PDMS frame with dimensions of 18 mm x 18 mm x 4 mm (W x H x D), and which contains 170-µm-high pillar structures at the lower surface. Use 42 g of the mixture of PDMS and curing agent, with the same ratio as in step 1.2. Place a specialized polycarbonate mold on the silicon wafer for the PDMS frame to create an interior frame with dimensions of 8 mm x 9 mm x 2 mm (W x H x D).
- Sterilize the PDMS frame and 180-µm-pore nylon filter papers submerged in distilled water and tweezers in an autoclave for 15 min at 121 °C.
- Place the sterilized PDMS frame onto a quarter of a piece of nylon filter paper (with a diameter of 5 cm) in a 60-mm diameter petri dish.
- Transfer a modular hydrogel sheet into the interior of the PDMS frame using an end-cut 1,000 µl pipette tip. The hydrogel sheets used for assembly should be cultured for at least a day after they were fabricated.
- Align the edge of each modular hydrogel sheet with the PDMS frame using an empty 200 µl pipette tip.
- Repeat steps 5.4 and 5.5 using modular hydrogel sheets to form a stack of 4 - 6 layers.
- Remove the culture medium by flowing it out through the pillar structures at the bottom of the PDMS frame. Then add 2 µl of alginate solution (2% w/v) at a corner of the multi-layer construct.
- Spray a mist of the cross-linking reagent for 30 sec onto the multi-layer construct to attach the edges of each layer with those of another. Use 2 ml of mist of the cross-linking reagent (at a spraying rate of 250 ml/hr).
- Rinse the multi-layered construct gently with 400 µl DMEM and remove the PDMS frame using tweezers.
- Detach the multi-layered construct from the filter paper by gently wiping with a cell lifter following the addition of 4 ml of DMEM.
- Transfer the multi-layered construct to a 6-well plate containing 3 ml of DMEM using filter paper, and culture the cells in vitro in a floating manner, with the hydrogel construct as a multi-scale cellular scaffold in the 6-well plate over a week in a 5% CO2 humidified incubator at 37 °C. Exchange the culture medium every other day.
Results
We have described the fabrication and manipulation of freestanding cellular hydrogel sheets. As shown in Figure 1, we fabricated micropatterned PDMS molds, and cell-containing hydrogel was loaded onto the hydrophilic surface of these molds and cross-linked using a humidifier to generate an aerosolized mist of gelling agent. Following release from the molds, HepG2 cells were cultured in freestanding hydrogel sheets with various patterns (Figure 2). Thus, the thin hydrogel sheets provided a 3D culture environment. Furthermore, modular hydrogel sheets may be assembled by layering the sheets using a micropipette with an end-cut tip, and using a PDMS frame, which enables 3D macro-scale cell culture (Figure 3).
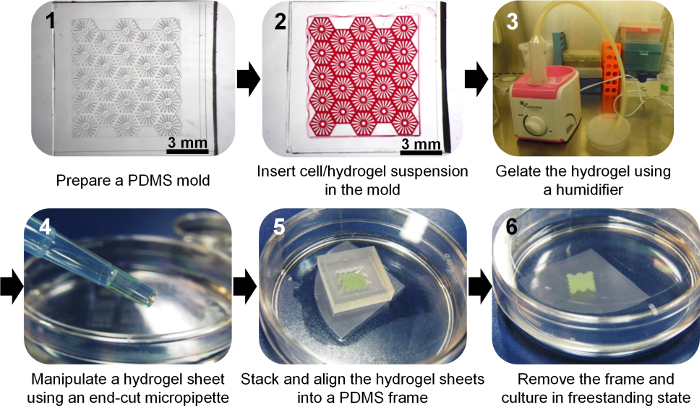
Figure 1. Flow Chart Showing the Fabrication of a Hydrogel Sheet and Multi-layer Hydrogel Sheets. Please click here to view a larger version of this figure.

Figure 2. Freestanding 3D Cell Culture in Various Hydrogel Sheets. The fabrication method of spraying cross-linking reagent enabled precise patterning of alginate hydrogel at the micro-scale. Furthermore, the fabricated hydrogel sheets could be harvested from the mold and manipulated easily in freestanding conditions. HepG2 cells were patterned and cultured in a flat hydrogel sheet (A), hydrogel sheets with hexagonal pillars (B), mesh (C), liver lobule-like mesh (D), an array of holes (E), and multiple microcomb-like microfibers (F). The scale bars are as follows: 500 µm (A, D, F), 100 µm (B, C), and 2 mm (E). Please click here to view a larger version of this figure.
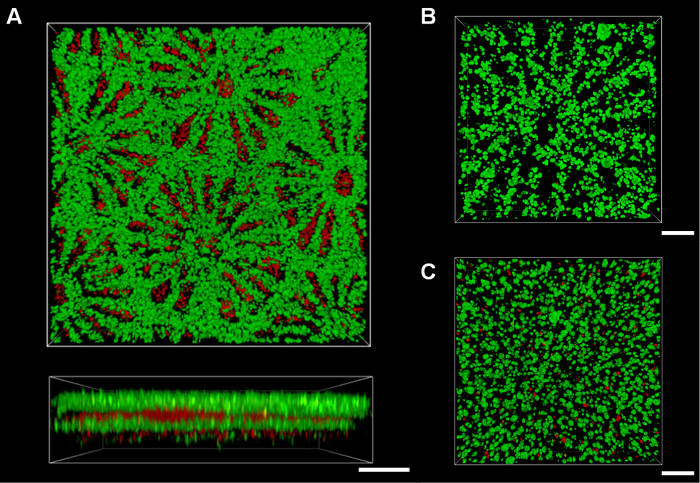
Figure 3. Assembly of Micropatterned Hydrogel Sheets for Macro-scale Cellular Constructs. Various types of hydrogel sheet could be assembled via layer-by-layer stacking with alignment, including the assembly of five hydrogel sheets containing HepG2 cells stained with green fluorescence and NIH3T3 cells stained with red fluorescence (A). An assembly of micropatterned hydrogel sheets with expanded pipeline structure (B) provided higher cell viability than that of non-patterned hydrogel sheets (C) after 7 days. The viability was assessed by staining HepG2 cells with calcein-AM (live cells shown as green) and ethidium homodimer-1 (dead cells shown as red). Scale bars: 500 µm (A) and 200 µm (B, C). Please click here to view a larger version of this figure.
Discussion
This protocol provides a simple method of fabricating modular hydrogel sheets, and assembling them to form 3D cellular scaffolds.
To construct clear-cut patterned alginate structures in a short time, we should identify a cross-linking process that can create sufficiently rigid structures to maintain the complex micropatterns from the mold, as well as maintain cell viability and metabolism. We have developed a cross-linking process, including a sol–gel transition, to spray a cross-linking reagent using a humidifier in the form of a mist. Without this cross-linking process, the micro-scale patterns could not be generated in the form of a thin hydrogel construct with the limited diffusive depth (100 - 200 µm), which has high diffusive permeability to metabolites and nutrients. Furthermore, compared with existing diffusion-based methods, which require 30 - 60 min,18−20 the cross-linking time was relatively short (3 - 5 min).
Moreover, it was also challenging to manipulate the thin hydrogel sheets, which were mechanically stable only in a liquid medium. As shown in Figure 1, the thin hydrogel structure was simply and stably handled and manipulated using an end-cut conventional tip for transferal between different culturing conditions. Using generation and manipulation techniques, we could provide not only a single-layer sheet in a floating manner for potential applications in drug assays but also stacked multi-layer structures, with potential applications as scaffolds for tissue-like constructs.
Multi-layered cellular architectures can be generated by stacking the modular single-layer hydrogel sheets (Figure 3A). Each modular hydrogel sheet may contain differing cell types, and may be exposed to different culture conditions. These multi-layer constructs may then be selectively assembled. However, simply stacking hydrogel sheets may lead to cell necrosis inside the assembled structure when the total depth exceeds the limited diffusive depth (Figure 3C). To overcome this problem, it would be necessary to align micromesh patterns, such as liver lobule-like meshes, when the multi-layer hydrogel sheets are assembled. We therefore require a PDMS frame with the same interior pattern as the border of the hydrogel sheet to align the stacked hydrogel sheets. Thus, aligned hole-array micropatterns in the assembled mesh hydrogel sheets could be used to form expanded pipeline structures, facilitating more efficient nutrient transport for improved cell viability (Figure 3B).
One limitation of this technique is the absence of cell–matrix interactions in the alginate hydrogel. Alginate cannot provide cell adhesion ligands for in vivo-like cell–matrix interactions;21 however, it does offer mechanical and geometrical effects as a 3D cell-encapsulating scaffold with cell–cell interactions. For primary cells and stem cells, alginate could be used following modification with RGD-containing peptides,21 or in combination with collagen or gelatin.14,18 Another limitation is that, once the cell-embedded hydrogel sheets are fabricated with low cell density below 5 x 106 cells/ml, construction of multi-layered structure could cause weak attachments between the hydrogel sheets because only the edge of the structure is fixed by use of additional alginate. However, the layers can be stacked compactly as a single scaffold via drainage structure under the PDMS frame. In addition, higher density of cells in the hydrogel sheets could proliferate on the surface as well as within the layer after one day culture, which would help attachments between layers as shown in Figure 3A.
The hydrogel sheet fabrication and manipulation technique described here may be adapted to the in vitro culture systems and tissue-like cellular models to provide an in vivo-like microenvironment for applications, including cellular assays and engineering artificial organs. This technique could be applied to cell-based drug assays and biological studies that require geometrically different 3D cellular micro- and macroenvironments that incorporate various cells or hydrogel types.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This research was supported by a National Leading Research Laboratory Program (Grant NRF-2013R1A2A1A05006378) through the National Research Foundation of Korea funded by the Ministry of Science, ICT and Future Planning. The authors also acknowledge a KAIST Systems Healthcare Program.
Materials
| Name | Company | Catalog Number | Comments |
| Sylgard 184 Silicone Elastomer Kit | Dow Corning Corporation | 000000000001064291 | |
| Pluronic F-127 | Sigma-Aldrich | P2443 | Powdered nonionic surfactant |
| Alginic acid sodium salt, low viscosity | Alfa Aesar | B25266 | |
| Calcium chloride dihydrate | Sigma-Aldrich | C7902 | |
| Ultrasonic humidifier | MediHeim | MH-2800 | Modified equipment, Maximum sprayed rate: 250 ml/hr |
| Nylon net filter hydrofilic, 180 μm | EMD Millipore | NY8H04700 | |
| Polycarbonate mold | Customized mold for fabrication of a PDMS frame pattern |
References
- Hoffman, A. S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 64 (Supplement), 18-23 (2012).
- Lan, S., Starly, B. Alginate based 3D hydrogels as an in vitro co-culture model platform for the toxicity screening of new chemical entities. Toxicol. Appl. Pharm. 256 (1), 62-72 (2011).
- Szot, C. S., Buchanan, C. F., Freeman, J. W., Rylander, M. N. 3D in vitro bioengineered tumors based on collagen I hydrogels. Biomaterials. 32 (31), 7905-7912 (2011).
- Lim, F., Sun, A. M. Microencapsulated islets as bioartificial endocrine pancreas. Science. 210 (4472), 908-910 (1980).
- Koh, W. G., Itle, L. J., Pishko, M. V. Molding of hydrogel microstructures to create multiphenotype cell microarrays. Anal. Chem. 75 (21), 5783-5789 (2003).
- Xu, Y., et al. A Microfluidic Hydrogel Capable of Cell Preservation without Perfusion Culture under Cell-Based Assay Conditions. Adv Mater. 22 (28), 3017-3021 (2010).
- Um, E., Lee, D. S., Pyo, H. S., Park, J. K. Continuous generation of hydrogel beads and encapsulation of biological materials using a microfluidic droplet-merging channel. Microfluid. Nanofluid. 5 (4), 541-549 (2008).
- Lee, D. H., Lee, W., E, U. m., Park, J. K. Microbridge structures for uniform interval control of flowing droplets in microfluidic networks. Biomicrofluidics. 5 (3), 034117 (2011).
- Lee, D. H., Bae , C. Y., Han, J. I., Park, J. K. In situ analysis of heterogeneity in the lipid content of single green microalgae in alginate hydrogel microcapsules. Anal. Chem. 85 (18), 8749-8756 (2013).
- Yamada, M., Sugaya, S., Naganuma, Y., Seki, M. Microfluidic synthesis of chemically and physically anisotropic hydrogel microfibers for guided cell growth and networking. Soft Matter. 8 (11), 3122-3130 (2012).
- Yamada, M., et al. Controlled formation of heterotypic hepatic micro-organoids in anisotropic hydrogel microfibers for long-term preservation of liver-specific functions. Biomaterials. 33 (33), 8304-8315 (2012).
- Onoe, H., et al. Metre-long cell-laden microfibres exhibit tissue morphologies and functions. Nat. Mater. 12 (6), 584-590 (2013).
- Lee, W., Son, J., Yoo, S. S., Park, J. K. Facile and Biocompatible Fabrication of Chemically Sol−Gel Transitional Hydrogel Free-Standing Microarchitectures. 12 (1), 14-18 (2011).
- Lee, W., et al. Cellular hydrogel biopaper for patterned 3D cell culture and modular tissue reconstruction. Adv. Healthcare Mater. 1 (5), 635-639 (2012).
- Bae, C. Y., Min, M. K., Kim, H., Park, J. K. Geometric effect of the hydrogel grid structure on in vitro formation of homogeneous MIN6 cell clusters. Lab Chip. 14 (13), 2183-2190 (2014).
- Bruzewicz, D. A., McGuigan, A. P., Whitesides, G. M. Fabrication of a modular tissue construct in a microfluidic chip. Lab Chip. 8 (5), 663-671 (2008).
- Choi, S., Park, J. K. Two-step photolithography to fabricate multilevel microchannels. Biomicrofluidics. 4 (4), 046503 (2010).
- Lee, B. R., et al. In situ formation and collagen-alginate composite encapsulation of pancreatic islet spheroids. Biomaterials. 33 (3), 837-845 (2012).
- Cabodi, M., Choi, N. W., Gleghorn, J. P., Lee, C. S., Bonassar, L. J., Stroock, A. D. A microfluidic biomaterial. J. Am. Chem. Soc. 127 (40), 13788-13789 (2005).
- Choi, N. W., Cabodi, M., Held, B., Gleghorn, J. P., Bonassar, L. J., Stroock, A. D. Microfluidic scaffolds for tissue engineering. Nat. Mater. 6 (11), 908-915 (2007).
- Rowley, J. A., Madlambayan, G., Mooney, D. J. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 20 (1), 45-53 (1999).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved