Method Article
Design and Construction of Artificial Extracellular Matrix (aECM) Proteins from Escherichia coli for Skin Tissue Engineering
In This Article
Summary
Recombinant technologies have enabled material designers to create novel artificial proteins with customized functionalities for tissue engineering applications. For example, artificial extracellular matrix proteins can be designed to incorporate structural and biological domains derived from native ECMs. Here, we describe the construction and purification of aECM proteins containing elastin-like repeats.
Abstract
Recombinant technology is a versatile platform to create novel artificial proteins with tunable properties. For the last decade, many artificial proteins that have incorporated functional domains derived from nature (or created de novo) have been reported. In particular, artificial extracellular matrix (aECM) proteins have been developed; these aECM proteins consist of biological domains taken from fibronectin, laminins and collagens and are combined with structural domains including elastin-like repeats, silk and collagen repeats. To date, aECM proteins have been widely investigated for applications in tissue engineering and wound repair. Recently, Tjin and coworkers developed integrin-specific aECM proteins designed for promoting human skin keratinocyte attachment and propagation. In their work, the aECM proteins incorporate cell binding domains taken from fibronectin, laminin-5 and collagen IV, as well as flanking elastin-like repeats. They demonstrated that the aECM proteins developed in their work were promising candidates for use as substrates in artificial skin. Here, we outline the design and construction of such aECM proteins as well as their purification process using the thermo-responsive characteristics of elastin.
Introduction
For several decades, both synthetic and natural materials have been explored for use as scaffolds in tissue engineering1,2. While synthetic materials such as polymers offer excellent structural integrity and tunable mechanical properties, they often have insufficient bioactivity to promote growth and infiltration of tissues. On the other hand, natural materials such as extracellular matrix (ECM) proteins have excellent biological activity, but have limitations such as batch-to-batch variability, rapid degradation and immunogenicity issues. As such, recombinant proteins are desired, since they can be designed to mimic only the desirable properties of native proteins3,4.
Recombinant protein engineering has garnered widespread interests as a versatile platform for the design and production of novel artificial protein biopolymers. By controlling the genetic sequence, the functionalities of the artificial proteins can be tailored for a wide variety of applications5,6. In particular, artificial extracellular matrix (aECM) proteins can be tailored to have multiple functionalities for applications in tissue engineering, regeneration and wound repair2,7. More importantly, advances in cloning and purification technologies have increased scalability and reduced the cost of manufacturing recombinant proteins tremendously. It is possible to produce large quantities of recombinant proteins at low production costs which are economic for use in the clinic5.
Artificial extracellular matrix proteins have been developed for tissue engineering applications8-11. For instance, Tirrell et al. designed a small diameter vascular graft using artificial proteins containing fibronectin CS5 sequence and elastin-like repeats (ELP-CS5). They showed that human umbilical vein endothelial cells (HUVECs) were able to adhere and grow on these materials12. Others have also incorporated short bioactive sequences taken from fibronectin, collagen, laminin, fibrinogen and vitronectin as well as structural domains that mimic elastin, spider silk and collagens to create a variety of fusion proteins10. Bulk cross-linked films made out of elastin-based aECM proteins also exhibited mechanical properties similar to that of native elastin (elastic moduli ranges between 0.3-0.6 MPa)13. Subsequently, aECM proteins containing longer fibronectin fragments were also reported to accelerate wound healing in vitro due to increased integrin binding affinities8.
Recently, integrin-specific artificial ECM proteins have been developed by Tjin and coworkers14. Each aECM protein contains a bioactive cell-binding domain taken from ECM components of native human skin2,7,15, such as laminin-5, collagen-IV and fibronectin. For example, the integrin α3Β1 has been shown to bind the PPFLMLLKGSTR sequence found in the laminin-5 alpha-3 chain globular domain 3 (LG3)16,17. In their report, they showed that primary human skin epidermal keratinocytes preferentially engage different integrins for binding to each of the aECM proteins, depending on the type of cell binding domain present.
The aECM proteins discussed in the work by Tjin et al. contain flanking elastin-like domains {(VPGIG)2VPGKG(VPGIG)2}8 that confer elasticity which mimics the mechanical properties of human skin. In addition, the incorporation of lysine residues within the elastin-like repeats also increases the overall protein solubility in aqueous solvents. In addition, the lysine residues also serve as crosslinking sites to facilitate the formation of crosslinked aECM films12. Inclusion of elastin-like repeats within the aECM protein sequence allow the proteins to be readily purified via Inverse Transition Cycling (ITC)14. Elastins undergo a sharp and reversible phase transition at a specific temperature known as the lower critical solution temperature (LCST) or the inverse transition temperature (Tt)18-20. Elastins and elastin-like repeats adopt hydrophilic random coil conformations below their LCST and become soluble in water, whereas above their LCST, elastins aggregate rapidly into micron-size particles. Such phase transitions are reversible and hence, can be exploited to allow elastin-based aECM proteins to be readily purified via the ITC technique21.
In this work, we report a generalized procedure to design, construct and purify artificial ECM proteins containing bioactive cell-binding domains, fused to elastin-like repeats. The process to design and clone the plasmids that encode for the amino acid sequences for the aECM proteins is described. The steps involved to purify the aECM proteins using ITC are outlined. Finally, the methods to determine the purity of the aECM proteins obtained using SDS-PAGE electrophoresis and Western Blotting are discussed.
Protocol
1. Cloning of Recombinant Plasmids Encoding for aECM Proteins
- Design the amino acid sequence of the functional domain (e.g., cell-binding domain and elastin-like repeats). Design restriction sites flanking the ends of the functional domains to facilitate sub-cloning using free software according to software instructions (e.g., http://biologylabs.utah.edu/jorgensen/wayned/ape/). Here, select unique restriction sites that are not present in the functional domain to confine digestion to the intended sites. Choose restriction sites that appear in the multiple cloning site (MCS) of the host vector (e.g., pET22b(+)) for sub-cloning.
- Reverse translate the amino acid sequence into the nucleotide sequence using free websites according to software instructions. (e.g., http://www.bioinformatics.org/sms2/rev_trans.html). Ensure codons are optimized for E. coli hosts.
- Gene of interest can be purchased commercially as single stranded oligonucleotides (i.e., if the oligonucleotides < 100 base pairs (bp)) and perform DNA annealing (see step 1.4) to reduce the cost. Otherwise, for genes larger than 100 bp, they could be purchased through commercial companies.
- DNA annealing of the oligonucleotides
- Anneal the DNA oligomers to obtain the desired gene sequence. Dissolve DNA oligonucleotides in a DNA oligomer buffer (10 mM Tris: 2-amino-2-(hydroxymethyl)-propan-1,3-diol, pH 8.0, filtered) to a final concentration of 1 µg/µl.
- Add 4 µl of each oligomer to 32 µl of DNA annealing buffer (10 mM Tris, 100 mM NaCl and 100 nM MgCl2) to achieve a total 40 µl mixture.
- Boil a beaker of water using a hotplate and immerse the mixture for 5 min, 95 °C. Remove the beaker and gradually cool the entire set up in a Styrofoam box O/N. The oligomers have been annealed and are ready for digestion.
- Add the corresponding restriction enzymes to digest the annealed oligomers (i.e., referred to as insert) and host vector (i.e., pET22b(+)) separately. Use the following recipe (1-2 µg of DNA, 2 µl of each restriction enzyme, 5 µl of 10x restriction enzyme buffer, and water up to a total 50 µl mixture) for 3-4 hr at 37 °C.
NOTE: The pET22b(+) plasmid vector is ampicillin antibiotic-resistant and contains a 6x-His tag at the C-terminus. The 6x-His tag present in pET22b(+) allows us to use His-tagged antibodies to identify the target protein via western blotting in step 7. - Add 6x loading dye to each digestion mixture. Run the digestion mixtures separately including a DNA ladder on 1.2% DNA agarose gels containing a UV-fluorescent DNA stain for 1 hr at 100 V. Visualize the 1.2% DNA agarose gel with a UV light illuminator.
- Slice the gel to extract digested DNA products using commercial gel purification kits. Elute using the minimum volume for the column to achieve a minimum DNA concentration of 50-100 ng/µl.
- Combine the gene of interest sequentially by ligating the digested DNA insert (i.e., elastin repeats or cell binding domains obtained by DNA annealing) into the plasmid vector using T4 ligase using the following recipe: (2 µl of vector, 1 µl of T4 ligase, 1.5 µl of T4 ligation buffer, x µl of insert, 10.5-x µl water for a total 15 µl mixture). Incubate the ligation mixture at RT for 2 hr.
NOTE: Molar concentration of vector to insert should be varied to optimize ligation efficiency. Volume of insert described as x in the recipe depends on the eluted DNA concentration from step 1.7. - Thaw E. coli DH5α chemically competent cells (or any cloning strain) on ice. Warm 2xYT agar plates (Table 1) containing ampicillin (25 µg/ml) to 37 °C.
- Transform the cells using heat shock:
- Aliquot 50 µl of competent cells into clean, pre-chilled micro-centrifuge tubes. Pipette 5 µl (between 100 pg to 100 ng) of the ligation mixture into the cells, pipetting gently up and down to mix. Leave the mixture on ice for 20 min.
- Immerse the micro-centrifuge tube containing the cell mixture in a 42 °C water bath for 2 min and returned to on ice for 2 min. Time the immersion duration to minimize heat damage to the cells.
- Add 500 µl of SOC media (Table 1) into the micro-centrifuge tube and incubate at 37 °C with shaking for 1 hr.
- Spread 50 500 µl of the cell/ligation mixture onto a 2xYT agar plate that has been pre-warmed to RT, containing ampicillin (25 µg/ml) and incubate plates upside-down at 37 °C O/N (12-16 hr).
- The next day, pick DNA colonies from the agar plate using clean pipette tips. Grow the colonies in 5 ml of 2YT media (Table 1) containing ampicillin (25 µg/ml) O/N at 37 °C O/N (12-16 hr) with shaking (225 rpm).
- The following day, use a plasmid isolation kit to extract the DNA plasmids for each colony picked according to manufacturer’s protocol. Elute the DNA with 50 µl of water.
- Perform a test digest with restriction enzymes using the following recipe: (5 µl DNA, 0.2 µl of each restriction enzyme, 1 µl 10x restriction enzyme buffer and top up water for a total 10 µl mixture), incubate for 2 hr at 37 °C to screen for colonies that likely contain the insert and run on 1.2% DNA agarose gel as in step 1.6.
NOTE: Upon digestion, a successful ligation should results in two bands, one each for vector and insert which correspond to their respective molecular weight (Figure 1). - Send the likely colonies for DNA sequencing using T7 promoter forward and reverse primers to commercial sequencers.
2. Transformation of Recombinant Plasmid into Bacterial Expression Host
- From the sequencing result, select a colony that has been successfully ligated and use the DNA plasmid for transformation into an E. coli expression host.
- Thaw E. coli expression strain (BL21(DE3)pLysS) on ice. Meanwhile, warm agar plates containing ampicillin (25 µg/ml) and chloramphenicol (34 µg/ml) to RT.
NOTE: The pLysS plasmid present in bacteria strain BL21(DE3)pLysS contains a chloramphenicol resistance gene. The pLysS plasmid contains a T7 repressor gene which is constitutively expressed to limit leaky expression of the aECM protein. Chloramphenicol is necessary to select for bacteria cells that contains pLysS during culture. - Repeat step 1.10 to obtain transformed E. coli cells that are ready to express the artificial proteins. Wrap agar plates in Parafilm and store upside-down in 4 °C for up to one month.
3. Bacterial Expression of aECM Proteins
- Refer to Figure 2, pick a colony from the transformed agar plate with a pipette tip and inoculate into 10 ml of sterile Terrific Broth (TB) media (Table 1) containing both ampicillin and chloramphenicol antibiotics in a test tube. Incubate this starter culture at 37 °C O/N (12-16 hr) with shaking at 225 rpm.
- Transfer 10 ml of the starter culture into 1 L fresh and sterile TB media supplemented with the same antibiotics in a 3 L Erlenmeyer flask. Incubate the culture at 37 °C with shaking at 225 rpm for 2-3 hr and observe the optical density of the culture (OD600) reached 0.6-0.8 by pipetting 1 ml of culture into an empty cuvette for reading. Save 1 ml of culture prior to induction for SDS-PAGE characterization.
- To measure OD600 of the cell culture, prepare 1 vial of TB media in a cuvette for a blank measurement. Separately, transfer 1 ml of culture from the culture flask into a new empty cuvette. Measure the optical density of the culture using a spectrophotometer against the blank control at an absorbance of 600 nm.
- Save the sample (for subsequent SDS-PAGE analysis) by transferring the 1 ml of culture sample into a 1.5 ml microcentrifuge tube, centrifuge at 12,000 x g for 2 min, and decant supernatant.
- Induce the culture with isopropyl β-D-1-thiogalactopyranoside (IPTG) to a final concentration of 1 mM and incubate at 37 °C with shaking at 225 rpm for another 4 hr. Save 1 ml of culture at the end of induction at 4 hr for SDS-PAGE characterization.
- Harvest the cells by transferring the culture to 1 L centrifuge bottles and centrifuge at 12,000 x g for 30 min at 4 °C. Discard the supernatant, weigh the cell pellets and resuspend in TEN buffer (1 M Tris, 0.01 M EDTA, 0.1 M NaCl, pH = 8.0) at 0.5 g/ml.
4. Lysis of Bacterial Cultures
- Freeze the re-suspended cell culture at -80 °C O/N. Thaw the frozen cell culture in a water bath at RT or on ice to lyse the cells. Add 10 μg/ml Deoxyribonuclease I (DNase I), 10 μg/ml Ribonuclease A (RNase I), and 50 μg/ml phenylmethylsulfonyl fluoride (PMSF) while thawing and homogenize the solution with slow stirring.
- After all resuspended cells are thawed, adjust the solution to pH 9.0 to increase the protein solubility in water12. Add 6 N NaOH drop wise with stirring on ice to achieve a homogenous consistency. Lyse by ultrasonic disruption for 20 min on ice using a 2 mm diameter flat tip, 5 sec pulse.
- Centrifuge the cell solution at 12,000 x g for 30 min at 4 °C. Transfer the supernatant to a clean, empty bottle and store at 4 °C for purification later.
- Meanwhile, resuspend the cell pellet again with TEN buffer and re-freeze at -80 °C. To complete cell lysis, repeat freeze/thaw and sonication process up to three times. Save 20 µl cell lysate for SDS-PAGE characterization.
5. Purification of aECM Proteins Using Inverse Transition Cycling
- Collate the cell lysate from step 4.3 and proceed to purify the aECM proteins using ITC, similar to that used for purification of elastin-based proteins21. Cycles will be done with the different temperatures which are 4 °C (referred to as “cold”) and 37 °C (referred to as “warm”) cycles respectively.
- Split the cell lysate into 50 ml centrifuge bottles, and centrifuge at 40,000 x g for 2 hr at 4 °C. The appearance of the cell pellet should be dark brown and look runny.
- Remove the supernatant by pipetting to get a clean separation from the pellet. Collect the supernatant in clean centrifuge bottles and add NaCl to a final concentration of 1 M (to a maximum of 3 M). Warm the solution to 37 °C for 2 hr with shaking at 225 rpm.
NOTE: Addition of NaCl will trigger the transition of the elastin component of the aECM causing aggregation. The supernatant will turn turbid. If the protein concentration is high enough, white foamy protein can be seen at the sides of the centrifuge bottle. - Centrifuge the supernatant from step 5.3 at 40,000 x g for 2 hr at 37 °C. Decant the supernatant. Crush the pellet using a metal spatula and resuspend the pellet bits in ice-cold autoclaved distilled water (50 mg/ml) with vigorous stirring O/N at 4 °C using a magnetic stir bar and plate. The pellet should be completely dissolved.
- Repeat steps 5.2 to 5.4 for another three to five times to obtain a higher purity of the protein.
- After the final cycle of purification, desalt the protein solution by dialyzing it against distilled water at 4 °C. Dialyze protein solution against water for 2-3 days with changes in water every 4 hr or 8 hr for O/N. Save 20 µl of the purified protein for SDS-PAGE and lyophilize the rest of the purified protein and store at -80 °C until further use.
6. Characterization of aECM Proteins Using SDS-PAGE Electrophoresis
- Prepare a 12% SDS-PAGE gel (if the molecular weight is large, use 8% SDS-PAGE gels for better separation). Add 2x SDS loading buffer to each sample, heat the samples for 10 min at 100 °C and run samples on the gel for 1 hr at 100 V or until the protein ladder corresponding to the molecular weight of the aECM protein reaches the middle of the gel.
- Retrieve the gel from the SDS-PAGE set up and add Coomassie Brilliant Blue stain (Table 2) with a volume to submerge the gel for 1 hr in a Petri dish. Rinse gel in a destaining solution (Table 2) for 5 min on a rocker. Change the destaining solution, and continue to destain the gel with rocking until the background of the gel becomes clear. Protein bands should be clearly visible.
- Compare the position of the target protein against the protein ladder to determine the molecular weight of the target protein.
- To further confirm the presence of the target protein, perform western blotting as in step 7.
7. Characterization of aECM Proteins Using Western Blotting
- Run the samples on a 12% SDS-PAGE gel as in section 6 with no staining.
- Cut nitrocellulose membrane to a size slightly larger than the SDS-PAGE gel. Cut 4 pieces of filter paper to the same size.
- Wet one piece of filter paper with Western Transfer Buffer (20% v/v methanol, 25 mM Tris, 190 mM Glycine, pH 8.3), place the SDS-PAGE gel on top of the filter paper, followed by another piece of filter paper, then the nitrocellulose membrane and a final other two pieces of filter paper. Ensure the whole set-up is submerged with sufficient western transfer buffer.
NOTE: The gel and membrane should not be in contact at this point of time as proteins could bind to the membrane upon contact. - Transfer the proteins onto the nitrocellulose membrane by placing two filter papers into a western semi-dry transfer unit, followed by the nitrocellulose membrane, and carefully place the SDS-PAGE gel within and on the membrane, lastly place the last two wet filter paper on the SDS-PAGE gel. Run the western transfer at 45 mA, 30 min. Add buffer if voltage is higher than 30 V.
NOTE: Preferably place the SDS-PAGE gel on the membrane with one attempt and avoid shifting the gel unnecessarily on the membrane as proteins could bind to the membrane upon contact. - Retrieve and block the nitrocellulose membrane with blocking solution (5% non-fat milk in PBS pH 7.4, filtered with filter paper) for 2 hr at RT. Dispose of blocking buffer and add PBS to rinse.
NOTE: Change to new gloves to avoid contaminating the membrane with unwanted proteins. - Incubate primary anti-His antibody with dilution in PBS at 1:1,000 at RT for 1 hr with rocking. Prepare a mixture in a volume which could submerge the whole membrane, for example, with dilution ratio 1:1,000, add 1 µl of antibody (stock concentration: 1 mg/ml) to 999 µl PBS for a 1 ml total mixture.
- Rinse the membrane with 5 ml of PBST (PBS with 0.1% Tween20).
- Incubate with secondary antibody conjugated to horse-radish peroxidase (HRP) with dilution in PBS at 1:5,000 at RT for 1 hr with rocking. Prepare a mixture in a volume which could submerge the whole membrane, for example, with dilution ratio 1:5,000, add 1 µl of antibody (stock concentration: 1 mg/ml) to 4,999 µl PBS for a 5 ml total mixture.
- Wash the membrane with 5 ml of PBST and repeat twice.
- Apply the chemiluminescent substrate to the membrane with a volume to cover the membrane and incubate by following the instructions for the substrate used.
NOTE: The substrate’s sensitivity to protein detection may vary, we recommend a substrate with maximum sensitivity for very low signals in the event increasing antibody concentration does not increase signals. - Capture the chemiluminescent signals using a CCD camera based imager. Adjust the primary and secondary antibody dilution ratio if signals are weak and non-specific. Incubate longer in blocking solution if background signal is high.
Results
In designing fusion proteins containing elastin-like repeats, it is important to maintain an overall elastin content, large enough fraction of the fusion protein18. This is to ensure that the fusion protein construct retains its elastin-like characteristics, in order to use ITC for purification. The aECM proteins design and sequences described in this section were specifically taken from the work by Tjin et al.14. In this work, three aECM proteins were successfully cloned into the pET22b(+) expression vector. Sequential ligation first began with ligating the elastin repeats insert into the pET vector, followed by inserting the cell binding domain. Finally the last set of elastin repeats were inserted at the terminal end of the cell binding domain by using the same method. To verify if the recombinant genes has been successfully constructed, each recombinant plasmid was test digested with XhoI and SalI for 3 hr at 37 °C. Figure 1 shows the DNA insert and vector bands after the test digestion. For each of the aECM proteins, the size of the inserts corresponded to the size of the gene, confirming that the cloning was indeed successful. Further verification through DNA sequencing also confirmed the test digestion results.
We were able to purify the aECM proteins through ITC given sufficient elastin content present in each aECM protein. Figure 2 shows the schematic of the overall purification process. The O/N culture was inoculated in 1 L shake flask with a typical starting OD600 of 0.01. The culture grew to OD600 of 0.6-0.8 after 3-4 hr. Protein expression was induced using 1 mM of IPTG. After 4 hr, the typical OD of 1.5 is achieved. The bacterial culture was harvested and subjected to centrifugation. The supernatant was discarded and the cell pellet was weighed. The average weight of the cell pellet was about 3 g/L of culture. The pellet was re-suspended in TEN buffer and frozen at -80 °C O/N.
A series of freeze/thaw cycles were used to lyse the cells. The freeze-thaw step causes the cell to swell and shrink, ultimately breaking due to ice crystals formed during the freezing process. Subsequently, DNase I and RNase I were added to the cell lysate to digest all DNA and RNA. PMSF is a protease inhibitor, which was also added to the cell lysate to minimize protein degradation. To further ensure complete cell lysis, the lysate was further sonicated while kept on ice.
The cell lysate was then subjected to 3 cycles of ITC purification. At the end of the 3 cycles, the pellet at the end of the warm cycle resembled honey in consistency and had a slight yellow coloration. The pellet turned transparent upon contact with water, and readily dissolved in pre-chilled autoclaved water with shaking. The purified protein is dialyzed to remove any residual salt. The protein solution obtained at the end of the final warm cycle was transferred to a short length of dialysis tubing (with 12 kDa molecular weight cut off). At the end of the dialysis, the protein solution was transferred to a clean 50 ml centrifuge tube and frozen at -20 °C. The next day, the frozen protein solution was lyophilized to remove the water content. Figure 3 shows an image of the dialyzed protein, having a typical wool-like appearance.
To determine if the protein expression was indeed induced, samples collected before and after induction were analyzed by SDS-PAGE electrophoresis. Here, 12% SDS-PAGE gel was sufficient to separate proteins with molecular weights of 20-150 kDa. In the event that the molecular weight is large, i.e., > 100 kDa, 8% SDS-PAGE gels could be used for better protein separation. Figure 4 shows an intense protein band at the predicted molecular weight in the induced sample (lane 2). Typically, SDS-PAGE analysis was performed immediately after protein expression before purification commences. Subsequently, samples taken through the purification process could also be analyzed using SDS-PAGE electrophoresis to ensure efficient purification of the target protein. Figure 4 also shows a SDS-PAGE gel containing a purified protein sample with a molecular weight of 22 kDa (lane 4), corresponding to the LN-5 aECM14. The estimated purity of the aECM proteins was about 90%-95%; this value was derived by comparing ratios of the band intensities of the target protein and other protein bands present on the SDS gel (Figure 4, lane 4). Finally, to determine if the purified protein was indeed the aECM proteins, western blotting was performed. Subsequently, MALDI-TOF was also performed to determine accurately the impurity content of the purified material14. Figure 5 shows the protein purity of the three aECM proteins with a SDS-PAGE gel in Figure 5A and to compare against an image of the nitrocellulose membrane for western blotting in Figure 5B, showing the presence of the target proteins Col-IV aECM and LN-5 aECM, tagged using anti-6x His-tag antibodies conjugated with Horse-radish Peroxidase (HRP). The final yields of the aECM proteins ranged between 60 mg/L to 120 mg/L.

Figure 1. DNA agarose gel electrophoresis of aECM proteins. Verification of final aECM protein DNA constructs through restriction enzymes digestion ran on 1.2% DNA agarose gel. Recombinant plasmids encoding for each of the aECM proteins were double digested with XhoI and SalI for 3 hr at 37 °C. The sizes of the inserts correspond to the size of each aECM protein gene (FN910: 1.8 kbp, Col-IV: 696 bp, LN-5: 699 bp) and the size of the vector pET22b(+) used is 5.5 kbp.
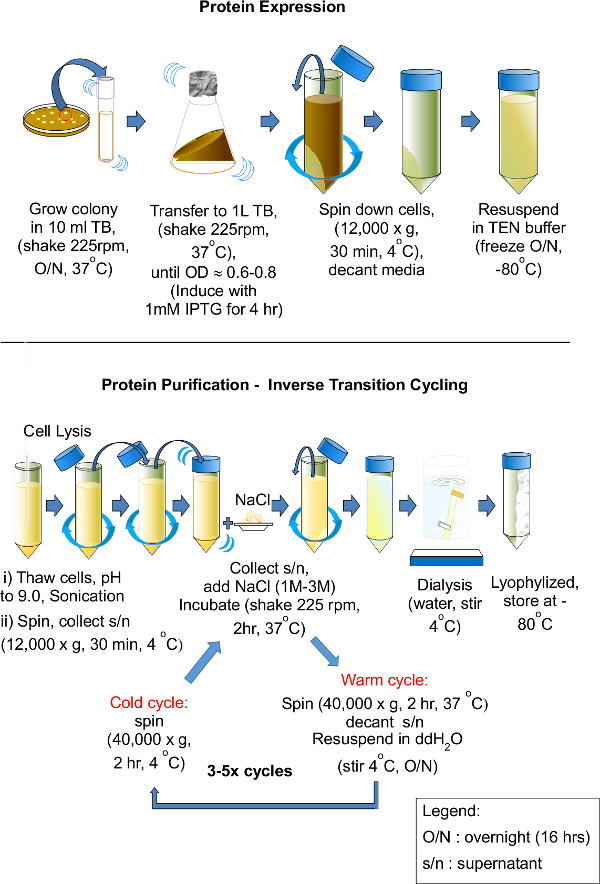
Figure 2. Schematic flow for the protein expression, lysis and purification of aECM proteins. The basic guide for recombinant protein expression, starting from the inoculation of a single colony to small culture and scale up to 1 L culture, harvesting and resuspending of cells, followed by protein lysis. Protein purification was carried out by using LCST behavior of elastin-like domains present in the artificial ECM proteins. The aECM proteins are purified via ITC. Multiple cold and warm cycles were performed to achieve a high purity of target protein. Finally, the target protein were dialyzed with water and lyophilized until further use. “O/N” = overnight. “s/n” = supernatant.

Figure 3. Image of lyophilized aECM protein purified by Inverse Transition Cycling. Lyophilized LN5-aECM has a wool-like appearance.

Figure 4. SDS-PAGE gel electrophoresis of aECM protein (LN-5 aECM). Lane 1 and 2 shows culture before and after protein induction. Presence of a thick band near 22 kDa shows that the LN-5 aECM was successfully expressed. Lane 3 shows the full cell lysate while lane 4 shows the purified LN-5 aECM protein after 3 cycles of ITC.

Figure 5. (A) SDS-PAGE gel of the aECM proteins. (B) Western blot probed for 6x His-tag for Col-IV and LN-5 aECM proteins. Both Col-IV and LN-5 aECM proteins contain C-terminus 6xHis-tag. Positive chemiluminescence observed at the predicted molecular weight at 22 kDa for sample LN-5 aECM and 24 kDa for sample Col-IV aECM representing 6x His-tag present in the purified protein.
| Media / L | Components and instructions |
| SOC media | 20 g Tryptone |
| 5 g Yeast extract | |
| 0.6 g NaCl | |
| 0.2 g KCl | |
| Add and autoclave in 940 ml water, | |
| then add sterile (filter sterilized) | |
| 10 ml 1 M MgCl2 | |
| 10 ml 1 M MgSO4 | |
| 40 ml 20% glucose | |
| 2xYT media | 16 g Tryptone |
| 10 g Yeast extract | |
| 5 g NaCl | |
| 15 g Bacto Agar (addition only for agar plate) | |
| Dissolve in water and top up to 1 L and autoclave. | |
| To prepare agar plates, cool to 55 °C before the addition of antibiotics. | |
| Mix well prior to pouring in Petri dish. | |
| Cool the plates until agar is solidified. | |
| Wrap plates in Parafilm and store upside-down in 4 °C. | |
| Terrific Broth (TB) | 12 g Tryptone |
| 24 g Yeast extract | |
| 4 ml Glycerol | |
| Dissolve in 700 ml water and autoclave. | |
| 16.42 g K2HPO4 (Merck) | |
| 2.31 g KH2PO4 (Merck) | |
| Dissolve in 300 ml water and autoclave separately, to ensure salts are fully dissolved. | |
| Combine both medium and salts after cooling. |
Table 1. Recipes for SOC media, 2xYT media/agar and Terrific Broth. Media for E. coli culture used in molecular cloning and protein expression.
| Solution / L | Components and instructions |
| Coomassie Brilliant Blue stain | 0.25 g Coomassie blueR250 |
| 100 ml Glacial acetic acid | |
| 450 ml Methyl alcohol | |
| 450 ml water | |
| Destaining solution | 100 ml Glacial acetic acid |
| 400 ml Methyl alcohol | |
| 500 ml water |
Table 2. Recipes for solutions to stain and destain SDS-PAGE gel. Coomassie Brilliant Blue R250 for staining and a destaining solution for characterization using SDS-PAGE.
Discussion
Recombinant protein engineering is a versatile technique to create novel protein materials using a bottom-up approach. The protein-based materials can be designed to have multiple functionalities, tailored according to the application of interest. Due to increasing advancement in cloning and protein expression technologies, it has become relatively simple (and cost effective) to create a variety of artificial proteins in a reproducible and scalable manner. The elastin-like domain has been incorporated in a number of artificial proteins, to serve as a purification tag, as well as to confer mechanical properties. Artificial proteins that contain an elastin-like sequence can be readily purified using ITC, which eliminates the need for expensive purification columns and antibodies.
This protocol describes the steps to construct recombinant plasmids encoding for aECM proteins. Genes encoding for the elastin-like repeats were purchased. For highly repetitive peptide sequence like elastin like polypeptides, it is recommended to design the cloning strategy such that recursive directional ligation (RDL) strategies can be used22. By using RDL, repetitive polypeptides with a specific chain length could be synthesized and therefore genes of interest can be purchased as short monomers.
In our work, the cell-binding domains were designed to contain flanking NheI restriction sites on both ends to allow modular swapping of the central cell binding domain. It is important to select restriction sites that are unique and are not present in the functional domain or elsewhere outside of the multiple cloning site (MCS) of the host vector. This is to confine the digestion of the insert or vector to the intended sites. In some cases, it may be necessary to introduce new restriction sites in the host vector using mutagenesis prior to insertion of the functional domain.
Our cloning strategy allowed us to readily change the central cell binding domain to obtain three variants of aECM proteins. We also noted that inserting a lysine residue after the starting Methionine increased protein yield by more than 10-fold. This dramatic increase in protein yield was most apparent with LN-5 aECM protein, where the final protein yield increased from 5 mg/L to 60 mg/L.
Various E. coli expression hosts were compared, and the BL21(DE3)pLysS E. coli strain gave the best protein yields. The improvement in protein yield was minimal when protein expression was induced with IPTG for longer than 4 hr. There was no significant difference in protein expression at IPTG concentrations greater than 1 mM. However, protein expression was the highest when induced at O.D. closer to 0.6.
For effective recovery of aECM proteins using ITC, it is important to note the temperature of the centrifuge apparatus. For example, most of the purification steps were performed in a cold room (4 °C) to ensure maximal solubility of the aECM proteins. It may also be necessary to pre-chill to (4 °C) the centrifuge rotor O/N prior to the cold cycle. Likewise, the centrifuge rotor was pre-heated to (37 °C) prior to the warm cycle to ensure that the temperature of the protein solution was maintained above its Tt.
In summary, the procedures to design and clone recombinant plasmids encoding artificial ECM proteins are described. In particular, the expression and purification of the aECM proteins using ITC are outlined. Finally, characterization of the aECM proteins using SDS-PAGE electrophoresis and Western Blotting were described.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgements
The authors would like to acknowledge funding from Ministry of Education AcRF Tier 1 (RG41) and start up grant from Nanyang Technological University. Low and Tjin are funded by the Research Student Scholarship (RSS) from Nanyang Technological University, Singapore.
Materials
| Name | Company | Catalog Number | Comments |
| pET22b (+) | Novagen | 69744 | T7 expression vectors with resistance to ampicillin |
| BL21(DE3)pLysS | Invitrogen | C6060-03 | additional antibiotics - chloramphenicol |
| Isopropyl-beta-D-thiogalactoside (IPTG) | Gold Biotechnology | I2481C | 1M stock solution with autoclaved water, make fresh prior to induction. |
| QIAprep Spin Miniprep Kit | Qiagen | 27106 | plasmid isolation kit |
| T4 ligase | New England Biolabs | M0202S | |
| Ampicillin | Affymetrix | 11259 | |
| Chloramphenicol | Affymetrix | 23660 | |
| Zymoclean™ gel DNA recovery kit | Zymo Research | D4001 | |
| XL10-gold strain | Agilent Technologies | 200315 |
References
- Chen, Q., Liang, S., Thouas, G. A. Elastomeric biomaterials for tissue engineering. Prog Polym Sci. 38 (3-4), 584-671 (2013).
- Groeber, F., Holeiter, M., Hampel, M., Hinderer, S., Schenke-Layland, K. Skin tissue engineering - In vivo and in vitro applications. Adv Drug Deliv Rev. 63 (4-5), 352-366 (2011).
- Kushner, A. M., Guan, Z. Modular Design in Natural and Biomimetic Soft Materials. Angewandte Chemie International Edition. 50 (39), 9026-9057 (2011).
- Gagner, J. E., Kim, W., Chaikof, E. L. Designing protein-based biomaterials for medical applications. Acta Biomater. 10 (4), 1542-1557 (2014).
- Gomes, S., Leonor, I. B., Mano, J. F., Reis, R. L., Kaplan, D. L. Natural and genetically engineered proteins for tissue engineering. Prog Polym Sci. 37 (1), 1-17 (2012).
- Werkmeister, J. A., Ramshaw, J. A. M. Recombinant protein scaffolds for tissue engineering. Biomedical Materials. 7 (1), 012002 (2012).
- MacNeil, S. Biomaterials for tissue engineering of skin. Materials Today. 11 (5), 26-35 (2008).
- Fong, E., Tirrell, D. A. Collective Cell Migration on Artificial Extracellular Matrix Proteins Containing Full-Length Fibronectin Domains. Advanced Materials. 22 (46), 5271-5275 (2010).
- Ratner, B. D., Bryant, S. J. BIOMATERIALS: Where We Have Been and Where We are Going. Annual Review of Biomedical Engineering. 6 (1), 41-75 (2004).
- Cai, L., Heilshorn, S. C. Designing ECM-mimetic materials using protein engineering. Acta Biomater. 10 (4), 1751-1760 (2014).
- Annabi, N., et al. Elastomeric recombinant protein-based biomaterials. Biochemical Engineering Journal. 77, 110-118 (2013).
- Heilshorn, S. C., DiZio, K. A., Welsh, E. R., Tirrell, D. A. Endothelial cell adhesion to the fibronectin CS5 domain in artificial extracellular matrix proteins. Biomaterials. 24 (23), 4245-4252 (2003).
- Simnick, A. J., Lim, D. W., Chow, D., Chilkoti, A. Biomedical and Biotechnological Applications of Elastin-Like Polypeptides. Polymer Reviews. 47 (1), 121-154 (2007).
- Tjin, M. S., Chua, A. W. C., Ma, D. R., Lee, S. T., Fong, E. Human Epidermal Keratinocyte Cell Response on Integrin-Specific Artificial Extracellular Matrix Proteins. Macromolecular Bioscience. 14 (8), 1125-1134 (2014).
- MacNeil, S. Progress and opportunities for tissue-engineered skin. Nature. 445 (7130), 874-880 (2007).
- Kariya, Y., et al. Differential regulation of cellular adhesion and migration by recombinant laminin-5 forms with partial deletion or mutation within the G3 domain of α3 chain. J Cell Biochem. 88 (3), 506-520 (2003).
- Shang, M., Koshikawa, N., Schenk, S., Quaranta, V. The LG3 module of laminin-5 harbors a binding site for integrin α3Β1 that promotes cell adhesion, spreading, and migration. J Biol Chem. 276 (35), 33045-33053 (2001).
- Hassouneh, W., Christensen, T., Chilkoti, A. Elastin-like polypeptides as a purification tag for recombinant proteins. Current Protocols in Protein Science. , 6.11.1-6.11.16 (2010).
- Keeley, F., Mecham, R., Keeley, F. Ch. 4. Evolution of Extracellular Matrix.Biology of Extracellular Matrix. , 73-119 (2013).
- Le, D. H. T., et al. Self-Assembly of Elastin-Mimetic Double Hydrophobic Polypeptides. Biomacromolecules. 14 (4), 1028-1034 (2013).
- MacEwan, S. R., Hassouneh, W., Chilkoti, A. Non-chromatographic Purification of Recombinant Elastin-like Polypeptides and their Fusions with Peptides and Proteins from Escherichia coli. Journal of visualized experiments: JoVE. (88), e51583 (2014).
- Meyer, D. E., Chilkoti, A. Genetically encoded synthesis of protein-based polymers with precisely specified molecular weight and sequence by recursive directional ligation: examples from the elastin-like polypeptide system. Biomacromolecules. 3 (2), 357-367 (2002).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved