Method Article
Exploring the Regulation of Lipid Droplet Catabolism through Lipophagy
In This Article
Summary
Lipophagy is a selective form of autophagy that involves the degradation of lipid droplets. Dysfunctions in this process are associated with cancer development. However, the precise mechanisms are not yet fully understood. This protocol describes quantitative imaging approaches to better understand the interplay between autophagy, lipid metabolism, and cancer progression.
Abstract
Macroautophagy, commonly referred to as autophagy, is a highly conserved cellular process responsible for the degradation of cellular components. This process is particularly prominent under conditions such as fasting, cellular stress, organelle damage, cellular damage, or aging of cellular components. During autophagy, a segment of the cytoplasm is enclosed within double-membrane vesicles known as autophagosomes, which then fuse with lysosomes. Following this fusion, the contents of autophagosomes undergo non-selective bulk degradation facilitated by lysosomes. However, autophagy also exhibits selective functionality, targeting specific organelles, including mitochondria, peroxisomes, lysosomes, nuclei, and lipid droplets (LDs). Lipid droplets are enclosed by a phospholipid monolayer that isolates neutral lipids from the cytoplasm, protecting cells from the harmful effects of excess sterols and free fatty acids (FFAs). Autophagy is implicated in various conditions, including neurodegenerative diseases, metabolic disorders, and cancer. Specifically, lipophagy -- the autophagy-dependent degradation of lipid droplets -- plays a crucial role in regulating intracellular FFA levels across different metabolic states. This regulation supports essential processes such as membrane synthesis, signaling molecule formation, and energy balance. Consequently, impaired lipophagy increases cellular vulnerability to death stimuli and contributes to the development of diseases such as cancer. Despite its significance, the precise mechanisms governing lipid droplet metabolism regulated by lipophagy in cancer cells remain poorly understood. This article aims to describe confocal imaging acquisition and quantitative imaging analysis protocols that enable the investigation of lipophagy associated with metabolic changes in cancer cells. The results obtained through these protocols may shed light on the intricate interplay between autophagy, lipid metabolism, and cancer progression. By elucidating these mechanisms, novel therapeutic targets may emerge for combating cancer and other metabolic-related diseases.
Introduction
Autophagy is a general term used to describe catabolic processes in which the cell transports its components to the lysosome for degradation. To date, three types of autophagy have been identified: microautophagy, macroautophagy, and chaperone-mediated autophagy1,2,3. Macroautophagy, hereafter referred to as autophagy, is an essential pathway for regulating cellular homeostasis. Disruption of this balance can lead to the development of pathological conditions4.
Autophagy is a complex process that involves multiple steps. The first step is autophagy induction, triggered by various stimuli such as the withdrawal of growth factors (insulin and insulin-like growth factors), pathogenic infections, reduced cellular energy levels (ATP), extracellular or intracellular stress (e.g., hypoxia, endoplasmic reticulum (ER) stress, oxidative stress), and nutrient deficiency (amino acids, glucose)5. The second step involves the formation of the phagophore, where membrane isolation is initiated from the ER, plasma membrane, and mitochondria. De novo formation involves conserved machinery of cytosolic proteins that are sequentially recruited6, such as the Ser/Thr kinase Unc-51-like kinase-1 complex (ULK1: ATG1 in yeast), Beclin-1, and VPS347. After the formation of the ULK1 complex, the class III phosphatidylinositol 3-kinase (PI3K) complex I is recruited to the isolated membrane (IM), which functions in the initial sequestering of cargos8. Furthermore, the ULK1 complex has the ability to recruit ATG9 to the isolation membrane (IM), an essential step since ATG9 vesicles are recognized as membrane carriers that facilitate IM expansion9.
Two ubiquitin-like (Ubl) conjugation systems are critical for the expansion process: the microtubule-associated protein 1 light chain 3 (LC3-I) system and the ATG12 system10. Prior to conjugation, the LC3 precursor undergoes cleavage. The cytosolic LC3-I is then conjugated to phosphatidylethanolamine (PE) to produce the membrane-associated LC3-PE (LC3-II), which facilitates autophagosome formation11. The cargo must be internalized into the forming double-membrane autophagosome during this process. Autophagy can internalize random targets for degradation or capture selective cargos through specific autophagy receptors such as p62/SQSTM112. The last step is the fusion of the formed autophagosome with lysosomes, leading to autolysosome formation. Although the precise mechanism for autolysosome formation remains elusive, membrane-tethering complexes, the RAS-related GTP-binding protein, and the soluble-N-ethylmaleimide-sensitive factor attachment protein receptors (SNARE) proteins are involved in this fusion process13. Furthermore, the microtubule cytoskeleton system is essential for trafficking mature autophagosomes and lysosomes from random initiation sites toward the perinuclear area for autolysosome formation14,15,16. In the autolysosome, the randomly or selectively sequestered cargos are degraded proteolytically by lysosomal proteases17.
The autophagy process is conserved across all eukaryotic organisms and is crucial in regulating intracellular conditions through cytoplasmic turnover. It removes misfolded or aggregated proteins, eliminates intracellular pathogens, and clears damaged organelles. Several organelles, including the endoplasmic reticulum, mitochondria, peroxisomes, lysosomes, nucleus, and LDs, have been reported as targets of autophagy16,18,19,20,21,22. LDs originate from the ER and are essential storage organelles central to lipid and energy homeostasis. Their distinct architecture consists of a hydrophobic core of neutral lipids enclosed by a phospholipid monolayer embedded with specific proteins. These droplets can interact with various cellular organelles through membrane contact sites23. Additionally, autophagy helps recycle primary resources to maintain optimal cellular conditions. For example, the degradation of LDs can lead to ATP production through fatty acid β-oxidation24.
Autophagy is associated with various diseases, including neurodegenerative diseases, metabolic disorders, and cancer17. Autophagy can promote or inhibit tumor growth in cancer, depending on the context25,26. For instance, Beclin 1 +/- mice exhibit a high incidence of spontaneous lymphomas and carcinomas in organs such as the lung, liver, and mammary tissue. Conversely, the loss of the autophagy-related gene Atg7 in intestinal epithelial cells attenuates tumor growth driven by the loss of the primary tumor suppressor in colorectal cancer, adenomatous polyposis coli (APC)27,28. Thus, the loss of autophagy-related genes can have opposing effects on tumor growth.
Cancer cells must produce energy to sustain their growth, division, and survival29. They have high avidity for lipids, which are used for the biosynthesis of structural components and energy production30. Cancer cells can adapt their metabolism to environmental conditions. For example, when glycolysis is suppressed in cervical cancer-derived HeLa cells, oxidative phosphorylation is increased to obtain the necessary ATP for survival31. Lipids in a cell do not exist as non-esterified FFAs due to their potential cytotoxicity at high concentrations. Instead, cells store FFAs and cholesterol as neutral, inert biomolecules such as sterol esters and triglycerides in LDs32. Consequently, lipophagy can contribute to cancer metabolism by supplying FFAs to produce energy, an emerging field in cancer research. However, the pathways that upregulate mitochondrial FA oxidation in cancer cells remain poorly understood. FFAs uptake and accumulation have been shown to enhance the aggressiveness of different cancer types33,34,35. Lipid metabolism reprogramming is a hallmark of cancer metabolic reprogramming, playing a pivotal role as an adaptive response to manage adverse physiological scenarios in the tumor microenvironment36,37. Indeed, LDs accumulation has been observed in many human cancers, including lung, breast, and prostate cancers, and is associated with aggressiveness and poor clinical prognosis38,39,40.
Given the relevance of autophagy and LDs in cancer metabolism and the poorly understood mechanisms, it is essential to establish protocols for studying their contribution to cancer development. This study describes a protocol to evaluate lipophagy through confocal imaging acquisition and quantitative imaging analysis protocols to investigate lipid metabolic changes in cancer cells.
Protocol
This study was conducted using epithelial adenocarcinoma HeLa cells (CCL2, ATCC). The protocol focuses on studying lipid droplets (LDs) during the induction of lipophagy in live cells to quantify the time course of LD number variation and LD-autophagosome interactions in cells expressing the wild-type (p62/SQSTM1-S182S) and two site-specific mutants of the autophagy receptor p62/SQSTM116. Expression of a phospho-defective form (p62/SQSTM1-S182A) increases the number of LDs, while expression of a phospho-mimicking form (p62/SQSTM1-S182E) reduces the number of LDs16. First, a method for analyzing LDs in live cells using confocal microscopy is described. Then, the protocol for unbiased, fully automated image acquisition and analysis is explained using a robotized confocal microscope. The details of the reagents and equipment used in this study are provided in the Table of Materials.
1. Confocal live cell imaging
- Cell culture
- Culture the cells at 37 °C and 5% CO2 in Dulbecco's Modified Eagle's Medium (DMEM), supplemented with 10% v/v fetal bovine serum (FBS), 1,000 U/mL penicillin, 100 µg/mL streptomycin, and Amphotericin B. Maintain the cells until they reach no more than 80% confluency.
- Wash the cells with 1 mL of 1x phosphate-buffered saline, collect using 0.25% v/v trypsin/EDTA, and centrifuge at 100 x g for 5 min at room temperature.
- Discard the supernatant using a pipette.
- Resuspend the cells in DMEM culture medium with 10% v/v FBS and seed (0.05 x 106 cells/well) into 35 mm glass-bottom dishes.
- Transfect the cells using TransIT-LT1 transfection or Lipofectamine 2000 reagent. Use 1 µg plasmid DNA of each p62/SQSTM1-mcherry phospho-variants and wild-type p62/SQSTM1-mcherry16.
- Incubate the cells at 37 °C and 5% CO2 for 48 h before proceeding with subsequent treatments.
NOTE: At 16-24 h after transfection, p62/SQSTM1 forms large condensates rather than discrete p62/SQSTM1 autophagosomes. These large condensates largely dissipate by the 48-h mark, allowing for more precise observation of autophagosomes.
- Organelle labeling
- Wash the cells in a glass-bottom dish twice with 1x PBS (preheated to 37 °C).
- Incubate the cells with BODIPY 493/503 diluted to 0.5 µM in DMEM medium supplemented with 10 mM HEPES and keep for 30 min at 37 °C, 5% CO2. BODIPY 405 and BODIYPY 633 can also be used with the same results.
- Check that expression of the p62/SQSTM1 variants induces changes in LDs number and total fluorescence intensity.
- Wash the cells with 1x PBS (room temperature) and maintain them in red-phenol-free DMEM supplemented with 10 mM ES-qualified HEPES buffer for live-cell imaging.
NOTE: BODIPY 493/503 emits bright green fluorescence, making it convenient for double fluorescence labeling. However, under certain conditions, such as repeated excitation, it can emit red fluorescence, potentially leading to erroneous interpretations (false colocalization) when combined with red markers. For more information on 550 nm excitation fluorophores, refer to the article by Ohsaki et al.41.
- Confocal microscopy image acquisition in live cell imaging
NOTE: Images of LDs are acquired using a confocal microscope with a 63x oil immersion objective (N.A. 1.4). A compatible image acquisition software was used for capturing the images. Temperature is maintained at 37 °C using automated temperature control system.
NOTE: Images of lipid droplets (LDs) were acquired using a confocal microscope with a 63x oil immersion objective (N.A. 1.4). Compatible image acquisition software was used to capture the images. The temperature was maintained at 37 °C using an automated temperature control system.- Adjust the multiline argon gas laser to 10% working power, with the 488 nm laser line at 1%-2% working potency, resulting in an overall laser power of 0.1%-0.2%.
- To minimize cell damage and probe photobleaching, adjust the 568 nm laser to 3%-5% potency.
- Adjust the image acquisition settings in the software to a resolution of 1024 x 1024 pixels. The system uses a hybrid detector operating at 600 Hz, with an average of 2.
- Adjust the spectral ranges between 478-494 nm (green emission for LDs) and 600-625 nm wavelength (red emission for mCherry).
- Adjust the pinhole size to 1 Airy Unit (AU) for 488 nm wavelength.
- Incubate the cells with 8-Br-cAMP (100 mM) to activate PKA kinase and to increase LDs degradation16.
- Capture fluorescent images at 5 min intervals, 37 °C for 60 min.
- Image analysis
- Open a stack file containing individual live image sequences using ImageJ. Use ROI manager for manual cell segmentation. Save regions for the next step.
- Open the stack file and recall saved regions for each image. Adjust LDs channel threshold.
- Measure the total fluorescence intensity by selecting multi-measure on the ROI manager.
NOTE: During autophagy, the total fluorescence intensity and the number of LDs can change significantly. Additionally, interactions between LDs and lysosomes increase during autophagy induction.
- Contacts and colocalization analysis
- Perform a multispectral capture at 1 s intervals for 5 min at 37 °C.
- Set the multiline argon gas laser to 10% potency, with the 488 nm laser line at 0.1%-0.5% working power to minimize cell damage and probe photobleaching. Adjust 568 nm laser to 3%-5% potency.
- Click on ComDet V: Plugins < ComDet V 0.5.3 < Detect Particles. Define the minimum distance that points must have to be considered colocalized.
- Choose the parameters that define the particle, such as its size (mm or pixels unit) and threshold, for both channels independently. A summary will be provided with the number of particles in each channel for each time and the corresponding colocalization percentage, which can be exported to Excel (see Supplementary File 1).
NOTE: ComDet V plugin is recommended for organelles with an oval shape, such as lipid droplets, endosomes, peroxisomes, or lysosomes. It is not recommended for colocalization with organelles like mitochondria. The "ComDet V" plugin (ImageJ FIJI distribution) allows temporal colocalization to be analyzed through particle analysis. To install it in Fiji, copy the URL http://sites.imagej.net/Ekatrukha/, go to Help, Update, select Manage update sites - Add update site and paste the URL. Apply the changes; it is recommended to use two channels. If there are more channels, separate them and rejoin using Merge.
- Dynamic movement of LDs
- Define regions of interest (ROIs) for beads and the cells and save them. Go to plugins and select Tracking and TrackMate.
- Open TrackMate. The plugin's first window provides information about the time intervals at which the images are captured and the image resolution. To confirm time intervals without making changes, click on NEXT.
- Select LoG detector and then NEXT. In the LoG Detector Configuration, filter particles according to their diameter, from 0.8-1.0 microns, and set an appropriate threshold. Perform a Preview to confirm the proper parameters, and click on NEXT.
- Set the initial Threshold, a quality threshold that limits the number of spots to be analyzed. This is required when following many spots on the plane, which is challenging. Do not use the filter; press NEXT without changing the parameters.
- Select a View: Choose HyperStack Displayer and click on NEXT.
- Select Linear motion LAP tracker for particles with constant speed in the plane.
- Define the maximum distance between two spots when starting a new track. Depending on the images, it is suggested to be between 0.5 -1 micron.
- Set the maximum distance from a predicted position for candidate spots for analysis.
- Set Max Frame Gap as the maximum time to follow a spot that may disappear from the focal plane. We recommend two time points.
- Select Display Options. The summary table, including minimum speed, maximum speed, mean speed, median speed, and track displacement, is exported to Excel (see Supplementary File 2).
- Select Plot Features. To represent the results visually, go to Tracks. Select the measure to represent (velocity and displacement) on the X-axis, and spots are placed on the Y-axis.
- Select an Action to save videos showing the movement of spots. This allows visualization of spots that show significant changes.
NOTE: Enhancing autophagy optimizes cellular flux and reduces LD number, highlighting its crucial role in metabolic regulation and cellular homeostasis16. The plugins mentioned are available for ImageJ software (FIJI distribution). Equivalent software can also be used. For all fluorescence intensity measurements, the integrated fluorescence density ("RawIntDen" in ImageJ) was used, which accounts for the total fluorescence across each image pixel, taking the area into consideration.
2. Fully automated confocal image acquisition and image analysis in fixed cells
NOTE: This method allows for assessing several conditions and developing triplicate measurements for each condition, improving the confidence in average measured values and enabling the determination of standard deviation or standard error for statistical differentiation between experiments. The workflow of the method is depicted in the flowchart shown in Figure 1.
- Cell Culture
- Culture the cells at 37 °C and 5% CO2 in Dulbecco's Modified Eagle's Medium (DMEM), supplemented with 10% v/v fetal bovine serum (FBS), 1,000 U/mL penicillin, 100 µg/mL streptomycin, and Amphotericin B.
- Wash the cells with 1 mL of 1x phosphate-buffered saline (PBS), harvested using 0.25% v/v trypsin/EDTA.
- Collect the cells in a 15 mL centrifuge tube.
- Centrifuge the cells at room temperature at 100 x g for 5 min.
- Aspirate the supernatant carefully with a pipette and keep the cell pellet.
- Resuspend the cell pellet in DMEM culture medium with 10% v/v FBS and seed into optical bottom 96-well plates (0.05 x 106 cells/well).
NOTE: Cells are incubated at 37 °C and 5% CO2 for 24 h before proceeding with subsequent treatments.
- Autophagy induction by serum starvation
- Remove the cell culture medium and incubate the cells in serum-deprived DMEM medium to remove serum and induce autophagy for 6 h.
- Use bafilomycin A1 200 nM to inhibit LD lipophagy. Diacylglycerol acyltransferase-1 (DGAT1) inhibitor T863 (50 µM) inhibits LD biogenesis. Use the Carnitine Palmitoyltransferase I (CPT1) inhibitor Etomoxir (100 µM) to inhibit FFAs conversion to acyl-carnitine. Additionally, lipolysis can be inhibited by using ATGLstatin (10 µM).
- Cell fixation
- Wash the cells three times with ice-cold PBS-CM (200 µL/well).
- Fix the cells with 4% paraformaldehyde diluted in PBS supplemented with 0.1 mM CaCl2 and 1 mM MgCl2 (PBS-CM) at room temperature for 15 min16.
- Wash the cells three times with PBS-CM (200 µL/well).
- Permeabilize with 0.2% Triton X-100 in PBS-CM at room temperature for 15 min.
- Wash the cells three times with PBS-CM.
- Organelle labeling
- Incubate the cells with BODIPY 493/503 diluted at 0.5 µM and DAPI (125 mg/mL) in PBS-CM for 30 min at 37 °C.
- Wash three times with PBS-CM.
- Keep the fixed and stained cells in 200 µL of PBS per well until image acquisition.
NOTE: The 96-well plate can be stored at 4 °C for several weeks, protected from light and dehydration. Mounting with commonly used antifade mounting media is not required.
- Automated confocal microscopy (fixed cells)
- Perform nuclei staining for automated image segmentation16.
NOTE: Image acquisition uses a spinning-disk microscope with a 40x (N.A 1.1) water immersion objective. DAPI acquisition is performed using 305 nm LED illumination (Ex: 355-385 nm; Em: 430-500 nm), and BODIPY 493/503 acquisition is performed using 488 nm LED illumination (Ex: 460-490 nm; Em: 550-550 nm).
- Perform nuclei staining for automated image segmentation16.
- Image analysis
NOTE: Image analysis16 is performed using compatible software with a dedicated LD analysis module. The software used in this study (see Table of Materials) has a ready-made solution (RMS Lipid Droplet Analysis) algorithm combination for image segmentation and quantification.- Identify the nuclei by selecting the DAPI channel and adjust the threshold based on signal and background.
- Identify the cytoplasm. Select the appropriate method based on the LDs fluorescence channel.
- Identify spots by adjusting parameters: Radius (1-1.5 µm), Contrast (0.2-0.25), Uncorrected Spot to Region Intensity (0.4-0.6), Distance (0.3-0.4 µm), Spot Peak Radius (0.2-0.25 µm).
- Calculate morphology properties: Select population LDs, Region Spot, and standard method, then select Area.
- Calculate intensity properties: Select LDs channel, population LDs, Region Spot, and standard method.
- Calculate properties: Select the Population of all cells, select the method by related population LDs, and then select the number of LDs, area, and intensity (mean and sum; the sum is integrated fluorescence density/intensity). This will yield LD measurements related to the cell.
- Define results by selecting Standard Output, Object Count, number of LDs per cell expressed as mean ± SD, Average LDs per area, Total LDs per area, Fractions of LD per area, and Total LDs intensity.
Results
Confocal live cell imaging
LDs are dynamic and transiently interact with p62/SQSTM1-positive autophagosomes. When lipophagy is induced, these interactions decrease the number of LDs and their total fluorescent intensity. This protocol used phospho-mutant versions of the autophagy receptor p62/SQSTM1 to examine these effects16.
The number and fluorescence intensity of LDs are regulated by lipophagy, dependent on the expression variants of p62/SQSTM1. Expression of p62/SQSTM1-S182A increases the number of lipid droplets and their fluorescence intensity, while p62/SQSTM1-S182E decreases the number of lipid droplets and their total fluorescence intensity. This mechanism occurs through inhibiting lipophagy by p62/SQSTM1-S182A or its activation by p62/SQSTM1-S182E, which leads to the inhibition or activation of LDs degradation. Quantitative changes include increased LDs by about 20%-25% after 48 h of expression and increased total fluorescence intensity by 30%-35%. Conversely, LDs decrease by 50%-60% after 48 h, with fluorescence intensity reduced by 60% (Figure 2).
PKA activation also reduces LDs' number and total fluorescence intensity compared to the p62/SQSTM1-S182 wild-type control, as enhancing lipophagy increases LDs degradation. Quantitative changes show a reduction in total fluorescence intensity by 20%-25% after 30 min of PKA activation induced by lipophagy. Expression of p62/SQSTM1-S182A leads to a reduction in LDs by blocking lipophagy, evidenced by a reduction in total fluorescence intensity of LDs by less than 10% after 30 min of PKA activation (Figure 3).
When lipophagy is induced by p62/SQSTM1-S182E expression, transient contacts between LDs and autophagosomes increase compared to control cells, and LDs speed increases. In contrast, cells expressing p62/SQSTM1-S182A show decreased contacts between LDs and autophagosomes and decreased LDs speed. LD/autophagosome contacts decrease by 40%-50% when lipophagy is blocked, with no changes in LD speed observed with p62/SQSTM1-S182A expression. LDs/autophagosome contacts increase by 35%-50% when lipophagy is induced, and LDs speed increases by 100%-120% with p62/SQSTM1-S182E expression (Figure 4).
Complete automated confocal image acquisition and image analysis in fixed cells
LDs parameters are modulated by lipophagy-mediated LDs metabolism. Lipophagy induction by serum deprivation will reduce the number of LDs, LDs per area, total LDs per area, fractions per area, and total LDs intensity. Bafilomycin will block changes induced by lipophagy. Etomoxir will increase the number of LDs, the number of LDs per area, the total LDs per area, the fractions of LDs per area, and the total intensity of LDs. T863 will reduce LDs number, LDs per area, total LDs per area, fractions of LDs per area, and total LDs intensity.
Serum deprivation increases lipophagy, requiring lysosome activity to increase FFAs availability. CPT1 transfers FFAs to mitochondria. T863 inhibits DGAT1 to block LD formation. LDs number, LDs per area, total LDs per area, fractions of LDs per area, and total LDs intensity variations will depend on the cell type, ranging from 20% to 60% (Figure 1).
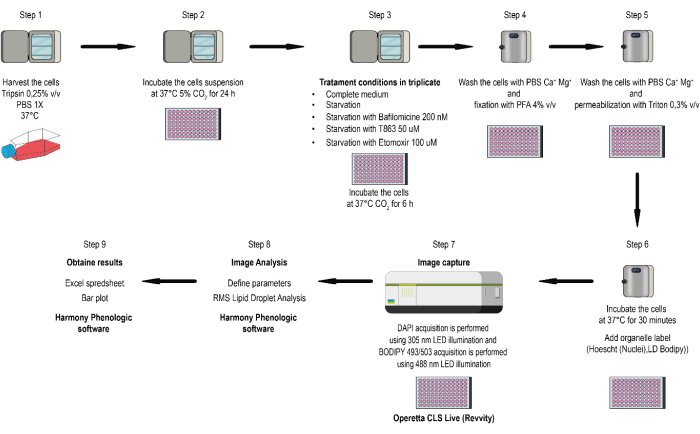
Figure 1: Flow chart of the study. Cells are cultured at 37 °C and 5% CO2 in DMEM, 10% v/v FBS, 1,000 U/mL penicillin, 100 µg/mL streptomycin, and Amphotericin B. Harvested using 0.25% v/v trypsin/EDTA and centrifuged at 100 x g for 5 min. The supernatant is then aspirated. Cells (0.05 x 106 cells/well) are suspended in DMEM culture medium with 10% v/v FBS and seeded into optical bottom 96-well plates. Image acquisition uses a live spinning-disk microscope equipped with a 40x (N.A 1.1) water immersion objective. DAPI acquisition is performed using 305 nm LED illumination (Ex: 355-385 nm; Em: 430-500 nm), and BODIPY 493/503 acquisition is performed using 488 nm LED illumination (Ex: 460-490 nm; Em: 550-550 nm). Image analysis uses phenologic software with RMS Lipid Droplet Analysis algorithm combination for image segmentation and quantification. Please click here to view a larger version of this figure.

Figure 2: Lipophagy activation/inhibition switches the number of LDs and their total fluorescence. Cells were transfected to express wild-type or lipophagy deficient p62/SQSTM1-mcherry variant (S182A), and LDs were stained (as indicated). Live-cell images were acquired at 5-min intervals during PKA activation by 8-Br-cAMP (100 mM), and the total fluorescence intensity was measured. PKA activation reduced LD intensity. The expression of the autophagy-defective p62/SQSTM1 version reduces PKA response. The effect of PKA is reversible by using the PKA inhibitor PKI (50 mM, myristoylated-PKI peptide). Data are means ± SEM. The figure is modified from Tapia et al.16. Please click here to view a larger version of this figure.
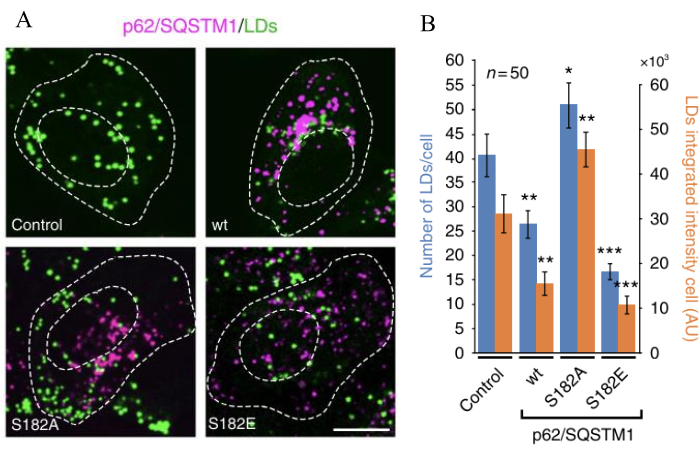
Figure 3: Lipophagy activation or inhibition switches the number of LDs and their total fluorescence. (A) Cells were transfected to express either of the indicated p62/SQSTM1-mcherry variants (magenta), and LDs were stained and quantified (green). Scale bar: 10 µm. (B) The number and fluorescent intensity of LDs were measured in control (non-transfected cells) and in p62/SQSTM1-cherry expressing p62/SQSTM1-cherry-wt (wild type) or the indicated S182A and S182E phospho-variant versions. Data are means ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001 (Student's t tests). All t-tests were conducted comparing to control cells. The figure is modified from Tapia et al.16. Please click here to view a larger version of this figure.
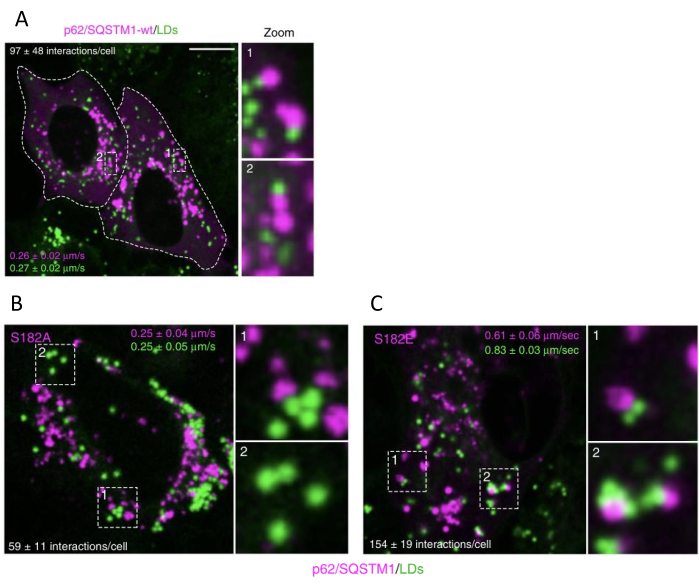
Figure 4: Lipophagy activation or inhibition changes the number of LDs speed and autophagosome interactions. (A) p62/SQSTM1-cherry-wt (wild type), or the indicated S182A (B) and S182E (C) phospho-variant versions, were transfected and followed by LD staining. Live-cell images were acquired, and the number of LDs and p62/SQSTM1-cherry-wt puncta interactions and the movement speed were measured. Scale bar: 10 µm. Data are means ± SEM. The figure is modified from Tapia et al.16. Please click here to view a larger version of this figure.
Supplementary File 1: Interactions spreadsheet. Cells were transiently transfected to express p62/SQSTM1-mcherry-wt (wild type) and then stained for lipid droplets (LDs). Live-cell images were obtained, and the number of interactions between LDs and p62/SQSTM1-mcherry-wt puncta was quantified. Please click here to download this File.
Supplementary File 2: Displacement and speed spreadsheet. Cells were transiently transfected to express p62/SQSTM1-mcherry-wt (wild type) and subsequently stained for lipid droplets (LDs). Live-cell images were then captured to quantify the number of interactions between LDs and p62/SQSTM1-mcherry-wt puncta, as well as to measure movement speed and displacement. Please click here to download this File.
Discussion
Quantitative imaging techniques, such as confocal microscopy and image analysis protocols, have provided valuable insights into the dynamics of LDs during lipophagy16,42,43. These technologies enable real-time visualization and quantification of LDs, allowing for the analysis of their number, size, and interactions with other organelles16. However, one of the most critical steps in this protocol is the correct LDs labeling, and to achieve this objective is required the use of fluid-phase LDs markers with detection ranges that allow quantifying minor differences in LDs with high precision and specificity. A more quantitative method for organelle-organelle contacts has been developed based on SPLICS reporter44.
BODIPY 493/503 marker has been extensively used but has experimental limitations, such as restricted detection of low FAs concentrations and changes in fluorescence emission that can lead to misinterpretations of results41. Other fluorescent dyes, like Nile red, are difficult to use for double labeling because of their broad fluorescence emission range from 550-750 nm45. On the other hand, BODIPY 558/568 C12 (Red C12) can be a good alternative for detecting low FA content in LDs in both live and fixed cells46. However, depending on the cell type, it must be used at the appropriate concentration, as it can also label FAs contained in other organelles, such as the endoplasmic reticulum and mitochondria.
Here, automated image acquisition and analysis protocols are detailed that streamline the study of lipophagy in live and fixed cells. This approach considered the pitfalls of previous molecular methods and accounts for its limitations, usually associated with selecting the LDs markers47,48. Through this protocol, in addition to using appropriate image acquisition equipment and software, it is possible to perform precise and real-time quantification of LDs and their interactions with lysosomes. This approach provides reliable, unbiased, and robust data crucial for understanding the role of lipophagy on LDs metabolism in cancer cell models.
In conclusion, advanced imaging technologies combined with automated image acquisition and quantitative analysis methods simplify and expedite data collection while providing robust results. The insights gained from this data enhance our understanding of the interplay between cell organelle biology and lipid metabolism. Future research should further elucidate the molecular mechanisms underlying lipophagy regulation in cancer cells and explore its therapeutic potential in cancer treatment.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
The Operetta robotized confocal microscope was financed by Fondo de Equipamiento Mediano (FONDEQUIP) N° EQM220072 grant. C.L. was supported by Vicerrectoria de Investigación y Doctorados (VRID), Universidad San Sebastian PhD scholarship. C.S. was supported by the Agencia Nacional de Investigación y Desarrollo (ANID) scholarship. D.T. and J.C. were supported by the Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT) N°1221374 grant.
Materials
| Name | Company | Catalog Number | Comments |
| 35 mm glass-bottom dishes | MatTek | P35G-1.5-14-C | |
| Bafilomycin A1 | Tocris | 1334 | 200 nM |
| BODIPY 493/503 | Invitrogen | D3922 | 0.5 mM |
| CaCl2 | Merck | 102378 | 0.1 mM |
| ComDet V Plugin | ImageJ | ImageJ FIJI | |
| DAPI | Invitrogen | D1306 | 125 mg/mL |
| Dulbecco’s Modified Eagle’s Medium (DMEM) | Gibco | 12800017 | |
| ES-qualified HEPES buffer | Cytiva HyClone AdvanceSTEM | SH3085101 | 10 mM |
| Etomoxir | SigmaAldrich | E1905 | 100 mM |
| Fetal Bovine Serum | Cytiva HyClone AdvanceSTEM | SH3039603 | 10% v/v |
| Forma Series II Water-Jacketed CO2 Incubator | Thermo Scientific | 3111 | 37 °C, 5% CO2 |
| Harmony Phenologic software | Revvity | image analysis software | |
| HeLa cells | ATCC | CCL-2 | Maintain cells at a low passage number, ideally between 8 and 10, to ensure optimal cellular characteristics. |
| HEPES | Merck | 110110 | 10 mM |
| High-speed clinical centrifuge | DLAB | DM0412 | |
| Immersion Oil | Leica | 11513859 | |
| MgCl2 | Merck | 814733 | 1 mM |
| Operetta CLS Live spinning-disk microscope | Revvity | HH16000020 | |
| Optical bottom 96-well plates | Thermo Scientific | 165305 | |
| Paraformaldehyde | Electron Microscopy Sciences | 157-8 | 4%v/v |
| penicillin/streptomycin/Amphotericin B | Biological Industries | 030331b | (1000 µ/mL, 100 mg/mL, 100 mg/mL) |
| Phosphate-buffered saline (PBS) | Sartorius | 020235A | 1x |
| Red-phenol free DMEM | Gibco | 31053028 | |
| T863 | Merck | SML0539 | 50 mM |
| TCS SP8 Leica confocal microscope | Leica Microsystems | ||
| TransIT-LT1 Transfection Reagent | Mirus | MIR 2304 | |
| Triton X-100 | Merck | T9284 | 0.20% |
| Trypsin/EDTA | Gibco | 252000056 | 0.25% v/v |
| UNO-TEMP controller | Okolab | OK-H401-T-CONTROLLER | 37 °C |
References
- Yamamoto, H., Matsui, T. Molecular mechanisms of macroautophagy, microautophagy, and chaperone-mediated autophagy. J Nippon Med Sch. 91 (1), 2-9 (2024).
- Mejlvang, J., et al. Starvation induces rapid degradation of selective autophagy receptors by endosomal microautophagy. J Cell Biol. 217 (10), 3640-3655 (2018).
- Kaushik, S., Cuervo, A. M. The coming of age of chaperone-mediated autophagy. Nat Rev Mol Cell Biol. 19 (6), 365-381 (2018).
- Galluzzi, L., Pietrocola, F., Levine, B., Kroemer, G. Metabolic control of autophagy. Cell. 159 (6), 1263-1276 (2014).
- He, C., Klionsky, D. J. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 43, 67-93 (2009).
- Suzuki, K., Ohsumi, Y. Current knowledge of the pre-autophagosomal structure (pas). FEBS Lett. 584 (7), 1280-1286 (2010).
- Neufeld, T. P. Contribution of ATG1-dependent autophagy to tor-mediated cell growth and survival. Autophagy. 3 (5), 477-479 (2007).
- Mizushima, N., Yoshimori, T., Ohsumi, Y. The role of ATG proteins in autophagosome formation. Annu Rev Cell Dev Biol. 27, 107-132 (2011).
- Mari, M., et al. An ATG9-containing compartment that functions in the early steps of autophagosome biogenesis. J Cell Biol. 190 (6), 1005-1022 (2010).
- Itakura, E., Mizushima, N. Characterization of autophagosome formation site by a hierarchical analysis of mammalian ATG proteins. Autophagy. 6 (6), 764-776 (2010).
- Kabeya, Y., et al. LC3, GABARAP AND GATE16 localize to autophagosomal membrane depending on form-ii formation. J Cell Sci. 117 (Pt 13), 2805-2812 (2004).
- Ahmad, R., et al. P62/SQSTM1 binds with claudin-2 to target for selective autophagy in stressed intestinal epithelium. Commun Biol. 6 (1), 740 (2023).
- Bento, C. F., et al. Mammalian autophagy: How does it work. Annu Rev Biochem. 85, 685-713 (2016).
- Jahreiss, L., Menzies, F. M., Rubinsztein, D. C. The itinerary of autophagosomes: From peripheral formation to kiss-and-run fusion with lysosomes. Traffic. 9 (4), 574-587 (2008).
- Pu, J., Guardia, C. M., Keren-Kaplan, T., Bonifacino, J. S. Mechanisms and functions of lysosome positioning. J Cell Sci. 129 (23), 4329-4339 (2016).
- Tapia, D., et al. KDEL receptor regulates secretion by lysosome relocation- and autophagy-dependent modulation of lipid-droplet turnover. Nat Commun. 10 (1), 735 (2019).
- Ichimiya, T., et al. Autophagy and autophagy-related diseases: A review. Int J Mol Sci. 21 (23), 8974 (2020).
- Ciechanover, A. Proteolysis: From the lysosome to ubiquitin and the proteasome. Nat Rev Mol Cell Biol. 6 (1), 79-87 (2005).
- Khaminets, A., et al. Regulation of endoplasmic reticulum turnover by selective autophagy. Nature. 522 (7556), 354-358 (2015).
- Ashrafi, G., Schwarz, T. L. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 20 (1), 31-42 (2013).
- Koerver, L., et al. The ubiquitin-conjugating enzyme ube2ql1 coordinates lysophagy in response to endolysosomal damage. EMBO Rep. 20 (10), e48014 (2019).
- Deosaran, E., et al. NBR1 acts as an autophagy receptor for peroxisomes. J Cell Sci. 126 (Pt 4), 939-952 (2013).
- Olzmann, J. A., Carvalho, P. Dynamics and functions of lipid droplets. Nat Rev Mol Cell Biol. 20 (3), 137-155 (2019).
- Xu, C., Fan, J. Links between autophagy and lipid droplet dynamics. J Exp Bot. 73 (9), 2848-2858 (2022).
- White, E. The role for autophagy in cancer. J Clin Invest. 125 (1), 42-46 (2015).
- Amaravadi, R. K., Kimmelman, A. C., Debnath, J. Targeting autophagy in cancer: Recent advances and future directions. Cancer Discov. 9 (9), 1167-1181 (2019).
- Levy, J., et al. Intestinal inhibition of ATG7 prevents tumour initiation through a microbiome-influenced immune response and suppresses tumour growth. Nat Cell Biol. 17 (8), 1062-1073 (2015).
- Trentesaux, C., et al. Essential role for autophagy protein ATG7 in the maintenance of intestinal stem cell integrity. Proc Natl Acad Sci U S A. 117 (20), 11136-11146 (2020).
- Zheng, J. Energy metabolism of cancer: Glycolysis versus oxidative phosphorylation (review). Oncol Lett. 4 (6), 1151-1157 (2012).
- Martin-Perez, M., Urdiroz-Urricelqui, U., Bigas, C., Benitah, S. A. The role of lipids in cancer progression and metastasis. Cell Metab. 34 (11), 1675-1699 (2022).
- Shiratori, R., et al. Glycolytic suppression dramatically changes the intracellular metabolic profile of multiple cancer cell lines in a mitochondrial metabolism-dependent manner. Sci Rep. 9 (1), 18699 (2019).
- Danielli, M., Perne, L., Jarc Jovicic, E., Petan, T. Lipid droplets and polyunsaturated fatty acid trafficking: Balancing life and death. Front Cell Dev Biol. 11, 1104725 (2023).
- Butler, L. M., et al. Lipids and cancer: Emerging roles in pathogenesis, diagnosis and therapeutic intervention. Adv Drug Deliv Rev. 159, 245-293 (2020).
- Nagarajan, S. R., Butler, L. M., Hoy, A. J. The diversity and breadth of cancer cell fatty acid metabolism. Cancer Metab. 9 (1), 2 (2021).
- Vasseur, S., Guillaumond, F. Lipids in cancer: A global view of the contribution of lipid pathways to metastatic formation and treatment resistance. Oncogenesis. 11 (1), 46 (2022).
- Yang, K., et al. The role of lipid metabolic reprogramming in tumor microenvironment. Theranostics. 13 (6), 1774-1808 (2023).
- Tan, Y., et al. Metabolic reprogramming from glycolysis to fatty acid uptake and beta-oxidation in platinum-resistant cancer cells. Nat Commun. 13 (1), 4554 (2022).
- Safi, R., Menendez, P., Pol, A. Lipid droplets provide metabolic flexibility for cancer progression. FEBS Lett. 598 (10), 1301-1327 (2024).
- Iwahashi, N., et al. Lipid droplet accumulation independently predicts poor clinical prognosis in high-grade serous ovarian carcinoma. Cancers (Basel). 13 (20), 5251 (2021).
- Luo, W., et al. Adding fuel to the fire: The lipid droplet and its associated proteins in cancer progression. Int J Biol Sci. 18 (16), 6020-6034 (2022).
- Ohsaki, Y., Shinohara, Y., Suzuki, M., Fujimoto, T. A pitfall in using bodipy dyes to label lipid droplets for fluorescence microscopy. Histochem Cell Biol. 133 (4), 477-480 (2010).
- Rambold, A. S., Cohen, S., Lippincott-Schwartz, J. Fatty acid trafficking in starved cells: Regulation by lipid droplet lipolysis, autophagy, and mitochondrial fusion dynamics. Dev Cell. 32 (6), 678-692 (2015).
- Nguyen, T. B., Olzmann, J. A. Lipid droplets and lipotoxicity during autophagy. Autophagy. 13 (11), 2002-2003 (2017).
- Giamogante, F., et al. A SPLICS reporter reveals-synuclein regulation of lysosome-mitochondria contacts which affects TFEB nuclear translocation. Nat Commun. 15 (1), 1516 (2024).
- Greenspan, P., Mayer, E. P., Fowler, S. D. Nile red: A selective fluorescent stain for intracellular lipid droplets. J Cell Biol. 100 (3), 965-973 (1985).
- Nguyen, T. B., et al. DGAT1-dependent lipid droplet biogenesis protects mitochondrial function during starvation-induced autophagy. Dev Cell. 42 (1), 9-21.e5 (2017).
- Murugan, S., Amaravadi, R. K. Methods for studying autophagy within the tumor microenvironment. Adv Exp Med Biol. 899, 145-166 (2016).
- Mallela, S. K., et al. Detection and quantification of lipid droplets in differentiated human podocytes. Methods Mol Biol. 1996, 199-206 (1996).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved