Method Article
Preparing Activated Platelet-Rich Plasma for Culturing Human Adipose-Derived Stem Cells
В этой статье
Резюме
In this report, we describe a double-spin method for preparing activated platelet-rich plasma (PRP). Using autologous thrombin, human adipose-derived stem cells (hASCs) were cultured. Activated PRP was shown to promote proliferation of hASCs.
Аннотация
Activated platelet-rich plasma (PRP) prepared from whole blood via centrifugation demonstrated a proliferation-stimulating effect in several kinds of cultured cells, implying a possible use in regenerative medicine. Here, a double-spin method was used to prepare PRP from whole blood. PRP was further activated by autologous thrombin. The platelet count was measured in the activated PRP and the proliferation-stimulating effect in human adipose-derived stem cells (hASCs) was examined. The resulting platelet count was 11.5-times higher in PRP than in whole blood plasma. The proliferation of hASCs was markedly enhanced by incubation with 1% PRP. The described method can be used to reproducibly prepare PRP with a high concentration of platelets. PRP prepared by this method markedly promotes proliferation of hASCs.
Введение
Activated platelet-rich plasma (PRP) is prepared by centrifugation of whole blood and is shown to contain platelets well above baseline levels1. Autologous RPP has been widely used in surgical treatment, including wound healing2, bone injury3, and aesthetic surgeries4,5. After activation of platelets in PRP, the α-granules present in platelets release several growth factors, such as platelet-derived growth factor (PDGF), epidermal growth factor (EGF), insulin-like growth factors (IGFs), transforming growth factor beta (TGF-β), vascular endothelial growth factor (VEGF), and others1,6,7. These growth factors play an important role in cell proliferation8, migration9, and differentiation9.
To date, several studies have reported the proliferation-stimulating effect of PRP in different kinds of cells10,11,12,13,14,15,16. One of these cell types is human adipose-derived stem cells (hASCs); hASCs exist in human adipose tissue and can be easily collected in large numbers. The regenerative effect of hASCs further suggests a potential use in clinical applications8. Our previous studies reported that compared to non-activated PRP, activated PRP had a marked proliferative effect on hASCs and human dermal fibroblasts (hDFs)8. In addition, we reported that PRP promotes the proliferation of hDFs through an ERK1/2 signaling pathway17. Recently, we also reported that PRP promotes the proliferation of hASCs through the ERK1/2, JNK and Akt signaling pathways18. In hASCs, PRP plays an important role as a supplement that promotes proliferation. Knowledge about the effect of PRP on hASCs will help the development of large-scale culture methods and enable further studies on the mechanism of proliferation in hASCs.
In this report, we introduce and describe a method for preparing PRP from whole blood using centrifugation. This method uses the double spin method to easily prepare a stable sample of PRP. To assess the biological function of PRP, we measured concentrated platelet counts and the concentration of several proliferation factors. We also confirmed the growth stimulatory effect by using the prepared PRP for culturing hASCs.
протокол
The study was approved by the Ethics Review Board of Kansai Medical University in accordance with the ethical guidelines of the Helsinki Declaration of 1975. All specimens were collected and used with informed consent from the donors.
1. Preparation
- After obtaining informed consent, take blood from healthy adult donors. Here, blood was collected from four male blood donors between the ages of 28-38.
- Recommend donors to drink 500 mL of water 6 h before blood collection.
NOTE: The reference ranges for hemoglobin (Hb), red blood cell (RBC) count, and platelet (PL) concentration in healthy adults are described by Vajpayee19. In order to be suitable for blood donation, the donor’s blood analysis is required to be within these ranges.
- Recommend donors to drink 500 mL of water 6 h before blood collection.
2. Blood collection
- Have the blood donors sit during the procedure.
- Wear gloves. Fasten a tourniquet on the donor’s upper arm while the donor makes a fist.
- Once a suitable vein is found, sterilize the skin twice with 70% alcohol and insert the needle.
- Use a 21 G syringe to collect blood. Do not loosen the tourniquet during the blood draw.
- Use an 8.5 mL blood collection tube to collect the blood. Observe the state of the blood in the tube and change to a new tube as soon as the blood flow slows down.
- Collect a sufficient amount of blood to make the PRP. Collect four tubes of anticoagulated blood with a total volume of 34 mL. Invert the tubes 10 times to mix the blood with the anticoagulant.
- Use a 10 mL serum blood collection tube (see Table of Materials) without anticoagulant to collect blood for making the activator (see Step 4). Collect 10 mL of non-anticoagulant blood.
3. Double-spin method to make PRP
- Take 40 μL of anticoagulated whole blood for the platelet count. Use filtered pipette tips to protect the pipette from blood contamination.
- Centrifuge the anticoagulated whole blood for 7 min at 450 x g and room temperature. This is the first spin to obtain layered blood samples. The upper layer is the plasma fraction, the middle layer is the thin buffy boat, and the bottom-most layer consists of red blood cells.
- Use a marker to make a line at 2 mm below the buffy coat.
- Use a 20 mL syringe with a long cannula to collect the plasma just to the 2 mm mark below the buffy coat. The yellow plasma with buffy coat contains platelets, leukocytes, and some erythrocytes. Collect the plasma from two tubes into a serum blood collection tube. Combine the contents of 4 tubes into 2 tubes for a total volume of 16 mL of plasma collected.
- Centrifuge the plasma with the buffy coat for 5 min at 1,600 x g and room temperature. This is the second spin. The supernatant of the layered blood samples is platelet-poor plasma (PPP).
- Use a 20 mL syringe with a long cannula to transfer the PPP to a 50 mL serum blood collection tube containing 1 mL of liquid as a measure. Platelets that accumulated in the thrombocyte pellet in the remaining 1 mL plasma were used as the PRP. The total volume of PRP collected was 2 mL.
- Vortex the PRP in each tube and then pool into one tube (total 2 mL).
- Take 400 µL of the PRP for a platelet count.
- Aliquot the PRP into several 1.5 mL tubes, containing approximately 400 µL in each tube. There is a total of four tubes. Approximately 500 µL PRP per tube is recommended.
4. Prepare the activator
- Allow the 10 mL of blood without anticoagulant in a serum blood collection tube (step 2.3.3) to sit for 30 min at room temperature.
- Centrifuge the blood sample without anticoagulant for 8 min at 2,015 x g and room temperature in a laboratory centrifuge.
- Collect the supernatant as autologous thrombin.
- Mix 0.5 M CaCl2 and autologous thrombin in a 1:1 (v/v) ratio as an activator. For example, 500 µL of CaCl2 was mixed with 500 µL of autologous thrombin. Make a total volume of 1 mL of the activator.
5. PRP activation
- Mix PRP and the activator in a 10:1 (v/v) ratio. Mix 400 µL of PRP with 40 µL of the activator in each 1.5 mL tube, and then incubate for 10 min at room temperature. This is activated PRP in a coagulated form.
6. Storage of PRP
- Centrifuge activated PRP at 9,000 x g for 10 min in a 4 °C laboratory centrifuge.
- Collect the supernatant via pipette into a proper volume syringe.
- Filter the supernatant through a 0.22 µm membrane.
- Store the final PRP at −80 °C until use.
7. Measurement of platelet concentrations and growth factor levels
- Count the platelet number in whole plasma and PRP using an automated hematology system (see Table of Materials).
- Analyze concentrations of PDGF-BB, IGF, and EGF levels in whole plasma and activated PRP using commercially available ELISA kits (see Table of Materials).
8. Cell proliferation assay
- Isolate hASCs using a previously described method18.
- Seed hASCs at a density of 1.0 x 104 cells/well in 24-well culture plates and incubate in DMEM containing 10% FBS and antibiotics overnight at 37 °C. Use hASCs from passages 7-9 in experiments.
- Replace cell media with serum-free DMEM. After 6 h, add PRP at the designated concentrations and incubate further for 48 h at 37 °C.
- Incubate with WST-8 solution (see Table of Materials) for 1 h at 37 °C. Read absorbance at 450 nm with a multi-well plate reader.
- Construct a standard curve: Seed hASCs at densities of 0, 6,250, 12,500, 25,000, 50,000, and 100,000 cells/well in 24-well plates in DMEM with 10% FBS for 3 h. Read the absorbance at 450 nm after incubation with WST-8 solution for 1 h at 37 °C
- Draw the standard curve by plotting the number of cells versus the A450 nm.
- Estimate the number of hASCs from the absorbance based on the standard curve.
Результаты
Enriched concentrations of platelet and PDGF-BB in PRP
Concentrations of platelets and PDGF-BB in PRP increased 11.5-fold and 25.9-fold, respectively, as high as those in whole plasma. However, the concentrations of EGF in PRP were not changed and IGF was only 70% of that of whole plasma (Table 1). The experiments were replicated four times by the double-spin method.
Enhanced proliferation of hASCs by PRP stimulation
Cell proliferation was increased by treatment with 0.2% PRP (P < 0.05 vs control), and to a greater extent with 1% PRP (P < 0.05 vs control and P < 0.05 vs 0.2% PRP). Figure 1A demonstrates that proliferation of hASCs was stimulated by PRP in a dose-dependent manner. The enhanced proliferation of hASCs by PRP stimulation was confirmed by phase-contrast microscopy (Figure 1B). Data were provided as the mean value ± standard deviation (SD). The Mann–Whitney U test was used to evaluate differences among groups. P < 0.05 was considered statistically significant.
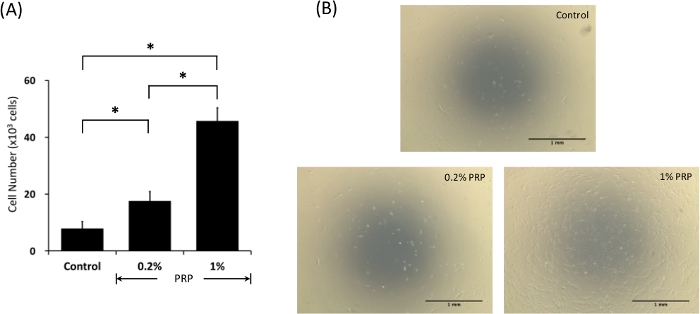
Figure 1: Enhanced proliferation of hASCs by PRP stimulation. Cells were incubated with PRP in serum-free DMEM for 48 h. Cell proliferation was determined with WST-8 by reading the absorbance at 450 nm. (A) PRP stimulated hASC proliferation in a dose-dependent manner (n = 4). *P < 0.05. (B) Phase-contrast micrographs showing an increased growth of hASCs by stimulation with PRP. Please click here to view a larger version of this figure.
| Platelets (x 1010/L) | PDGF-BB (ng/mL) | EGF (pg/mL) | IGF-I (ng/mL) | |
| Plasma | 17.92 ± 1.4 | 1.5 ± 0.1 | 537 ± 7 | 224 ± 4 |
| PRP | 205.3 ± 34.8 | 38.8 ± 0.8 | 640 ± 21 | 147 ± 3 |
| Ratio (PRP/Plasma) | 11.5 | 25.9 | 1.2 | 0.7 |
Table 1: Platelet concentrations and growth factor levels in the serum and PRP. Concentrations of platelets and PDGF-BB in PRP were 205.3 ± 34.8 x 1010/L and 38.8 ± 0.8 ng/mL, which increased 11.5-fold and 25.9-fold, respectively, as high as those in whole plasma. However, the concentrations of EGF in PRP were not changed and IGF was only 70% of that of whole plasma.
Обсуждение
After PRP activation, several growth factors, such as PDGF, EGF, IGF, TGF-β, and VEGF1,6,7 "activate" cells and tissues of wounds promoting wound healing20,21. In the case of cosmetic reconstruction surgery, activated cells have been shown to induce healing and improve aesthetics22,23. Alternatively, human adipose-derived stem cells can be used without PRP stimulation in aesthetic and reconstructive fields24,25,26. PRP can also be combined with insulin in culture, favoring chondrogenic and osteogenic differentiation of human adipose-derived stem cells in three-dimensional collagen scaffolds27.
PRP can be prepared using different methods, depending on the institution or physician. The issues observed in PRP preparation include use of PRP without activation and low platelet concentrations. The main goal of PRP preparation is collecting as many platelets as possible from blood samples of individual patients. In this way, cytokines can be extracted after activation in order to observe the effect on cells and wounded areas.
An important factor in PRP preparation is the concentration of platelets in the collected whole blood. Two centrifugation methods, the single spin and double spin methods, are used for PRP preparation. Centrifugation is specified by the number of rotations (centrifugal gravity) and centrifugal time (minutes). Generally, 900–3200 rotations/min as the number of rotations and 6–18 min as the centrifugal time have been reported. PRP preparation using the double spin yields a platelet concentration that is enhanced 4–7.9-fold4,28,29 over the baseline concentration. Thus, the double spin method yields higher platelet concentrations than the single spin method. Marx recommended the double spin method due to the increased platelet yield and efficacy as the single spin method cannot effectively separate and concentrate platelets for clinical treatments28. We also recommend the double spin method and have used the method for in vivo studies and clinical applications9,30,31,32,33.
In the double spin method, red blood cell and plasma layers are separated during the first centrifugation. Platelets exist between these two layers (in the buffy coat: containing white blood cells and platelets). We applied suction to collect the contents of this layer (until 2-3 mm below this layer) and transferred it to another test tube. This layer was subsequently separated by a second centrifugation into a yellow layer, mainly consisting of plasma, separating an upper white blood cell layer (PPP), and a lower red layer (PRP) containing concentrated platelets.
The single spin method has the advantage of easier, more rapid automation. However, the reported concentration rate at the present stage for the single spin method is 359%, which indicates that it can only concentrate platelets to approximately 3.38%34. Although the double spin method has disadvantages of requiring more time and effort due to two centrifugation steps, it can prepare platelets with a higher concentration rate than the single spin method.
Measurement of platelets and growth factors in PRP is important to assess the effects of their concentration from whole blood8,35. After preparing PRP by several methods from several blood samples from a single person, Castelioa et al.35 concluded that comparisons of platelet and growth factor concentrations were necessary to assess each PRP preparation. Kushida et al.31 collected whole blood from a single donor to prepare PRP using seven different commercial PRP separation systems and then to compare platelets and growth factor concentrations. There are two types of commercial separation systems: one uses a fully automated centrifugal separator, while the other requires centrifugation of manually collected platelet fractions. The representative centrifugal separator used in the double spin method is described by Kushida et al.31. The advantage of this system is greater uniformity due to reduced potential for technical errors and ease of PRP preparation. The main disadvantages are the high costs for the kit and the centrifugal separator.
The PRP preparation method introduced in this report is a double spin method that yields a high platelet concentration. An additional advantage of this method is that less expensive equipment is required including readily available syringes, blood-collecting vessels, and a common centrifugal separator.
Upon adding autologous thrombin and calcium chloride to activate PRP, α granules in platelets release high concentrations of PDGF, TGF-β, EGF, and VEGF. Kakudo et al.8,9 reported that they prepared PRP by the same double spin methods as described here, and then added autologous thrombin and calcium chloride to activate PRP. Kakudo et al.8 also reported that the amount of PDGF and TGF-β1 released when PRP was activated in the same manner was markedly higher than those in whole blood or pre-activated PRP.
When adding PRP into cultured cells, including adipose stem cells, the following three methods were important: 1) Use the double spin method to prepare PRP with high platelet concentrations; 2) Activate PRP to obtain PRP with high growth factor concentrations; 3) Before adding PRP, thoroughly remove debris by strong centrifugation and/or filtration. This paper presents a useful PRP preparation method that adheres to the three conditions above and can quickly and economically obtain activated PRP that will amplify adipose stem cells.
Раскрытие информации
The authors declare that they have no competing financial interests.
Благодарности
Not applicable.
Материалы
| Name | Company | Catalog Number | Comments |
| 20 mL Syringe, Terumo syringe lock type | Terumo, Tokyo, Japan. | SS-20LZP | |
| 50 mL Tube, Polypropylene Conical Tube | Corning, NY, USA. | 352070 | |
| Automated Hematology System | Sysmex Corp., Tokyo, Japan | XE-2100 | |
| Blood Collection Needle, SafeTouch PSV set with luer adapter, 21 G x 3/4” | Nipro, Osaka, Japan. | 32-384 | |
| Blood Collection Tube, ACD Solution A Blood Collection Tube, 8.5 mL | BD Vacutainer, NJ, USA. | 364606 | |
| Blood Collection Tube, Serum Blood Collection Tube/monovette, 10 mL | BD Vacutainer, NJ, USA. | 366430 | |
| Calcium Chloride, 1 mEq/ mL, | Otsuka Pharmaceutical Factory, Tokushima, Japan. | 3215400A1061 | |
| Cannula, BS non-bevel needle, 18 G (1.2 mm) x 75 mm | BS Medical, Tokyo, Japan. | BS-81007 | Not for sale |
| Cell Counting Kit-8 | Dojindo Molecular Technologies, Kumamoto, Japan | CK04 | |
| Centrifuge | Kokusan, Tokyo, Japan. | H-19F | |
| Centrifuge | Eppendorf, Hamburg, Germany. | 5415R | |
| EnSpire 2300 Multilabel Reader | PerkinElmer, Inc., Waltham, MA, USA | ||
| Filter Unit | Merck Millipore, Co. Cork, Ireland. | SLGP033RS | |
| Human EGF Quantikine ELISA Kit | R&D Systems, Minneapolis, MN, USA | DEG00 | |
| Human IGF-I Quantikine ELISA Kit | R&D Systems, Minneapolis, MN, USA | DG100 | |
| Human PDGF-BB Quantikine ELISA Kit | R&D Systems, Minneapolis, MN, USA | DBB00 | |
| Pipette Tip, ART 1000 Reach Barrier Tip | Thermo Scientific, MA, USA. | 2079-05-HR | |
| Pipette, Nichipet EXII 100-1000 μL | Nichiryo, Saitama, Japan. | 00-NPX2-1000 | |
| Sterling Nitrile Power-Free Exam Gloves | Kimberly-Clark | 50707 | |
| Yamazen Alcohol for Disinfection | Yamazen Pharmaceutical, Osaka, Japan. | A7L07 |
Ссылки
- Eppley, B. L., Woodell, J. E., Higgins, J. Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plastic and Reconstructive Surgery. 114 (6), 1502-1508 (2004).
- Salcido, R. S. Autologous platelet-rich plasma in chronic wounds. Advances in Skin & Wound. 26 (6), 248 (2013).
- Marx, R. E., et al. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 85 (6), 638-646 (1998).
- Bhanot, S., Alex, J. C. Current applications of platelet gels in facial plastic surgery. Facial Plastic Surgery. 18 (1), 27-33 (2002).
- Man, D., Plosker, H., Winland-Brown, J. E. The use of autologous platelet-rich plasma (platelet gel) and autologous platelet-poor plasma (fibrin glue) in cosmetic surgery. Plastic and Reconstructive Surgery. 107 (1), 238 (2001).
- Eppley, B. L., Pietrzak, W. S., Blanton, M. Platelet-rich plasma: a review of biology and applications in plastic surgery. Plastic and Reconstructive Surgery. 118 (6), 147-159 (2006).
- Lubkowska, A., Dolegowska, B., Banfi, G. Growth factor content in PRP and their applicability in medicine. Journal of biological regulators and homeostatic agents. 26, 3-22 (2012).
- Kakudo, N., et al. Proliferation-promoting effect of platelet-rich plasma on human adipose-derived stem cells and human dermal fibroblasts. Plastic and Reconstructive Surgery. 122 (5), 1352-1360 (2008).
- Kakudo, N., Morimoto, N., Kushida, S., Ogawa, T., Kusumoto, K. Platelet-rich plasma releasate promotes angiogenesis in vitro and in vivo. Medical Molecular Morphology. 47 (2), 83-89 (2014).
- Liu, Y., Kalen, A., Risto, O., Wahlstrom, O. Fibroblast proliferation due to exposure to a platelet concentrate in vitro is pH dependent. Wound Repair and Regeneration. 10 (5), 336-340 (2002).
- Lucarelli, E., et al. Platelet-derived growth factors enhance proliferation of human stromal stem cells. Biomaterials. 24 (18), 3095-3100 (2003).
- Kilian, O., et al. Effects of platelet growth factors on human mesenchymal stem cells and human endothelial cells in vitro. European Journal of Medical Research. 9 (7), 337-344 (2004).
- Kanno, T., Takahashi, T., Tsujisawa, T., Ariyoshi, W., Nishihara, T. Platelet-rich plasma enhances human osteoblast-like cell proliferation and differentiation. Journal of Oral and Maxillofacial Surgery. 63 (3), 362-369 (2005).
- Gruber, R., et al. Platelet-released supernatants increase migration and proliferation, and decrease osteogenic differentiation of bone marrow-derived mesenchymal progenitor cells under in vitro conditions. Platelets. 15 (1), 29-35 (2004).
- Koellensperger, E., von Heimburg, D., Markowicz, M., Pallua, N. Human serum from platelet-poor plasma for the culture of primary human preadipocytes. Stem Cells. 24 (5), 1218-1225 (2006).
- Kocaoemer, A., Kern, S., Kluter, H., Bieback, K. Human AB serum and thrombin-activated platelet-rich plasma are suitable alternatives to fetal calf serum for the expansion of mesenchymal stem cells from adipose tissue. Stem Cells. 25 (5), 1270-1278 (2007).
- Hara, T., et al. Platelet-rich plasma stimulates human dermal fibroblast proliferation via a Ras-dependent extracellular signal-regulated kinase 1/2 pathway. Journal of Artificial Organs. 19 (4), 372-377 (2016).
- Lai, F., et al. Platelet-rich plasma enhances the proliferation of human adipose stem cells through multiple signaling pathways. Stem Cell Research and Therapy. 9 (1), 107 (2018).
- Vajpayee, N., Graham, S. S., Bem, S. Basic examination of blood and bone marrow. Henry's Clinical Diagnosis and Management by Laboratory Methods. 22, 509-535 (2011).
- Cervelli, V., et al. Use of platelet-rich plasma and hyaluronic acid in the loss of substance with bone exposure. Advances in Skin & Wound. 24 (4), 176-181 (2011).
- Nicoli, F., et al. Severe hidradenitis suppurativa treatment using platelet-rich plasma gel and Hyalomatrix. International Wound Journal. 12 (3), 338-343 (2015).
- Cervelli, V., et al. platelet rich lipotransfert: our experience and current state of art in the combined use of fat and PRP. BioMed Research International. 2013, 434191 (2013).
- Gentile, P., et al. Platelet-Rich Plasma and Micrografts Enriched with Autologous Human Follicle Mesenchymal Stem Cells Improve Hair Re-Growth in Androgenetic Alopecia. Biomolecular Pathway Analysis and Clinical Evaluation. Biomedicines. 7 (2), (2019).
- Gentile, P., Scioli, M. G., Orlandi, A., Cervelli, V. Breast Reconstruction with Enhanced Stromal Vascular Fraction Fat Grafting: What Is the Best Method. Plastic and Reconstructive Surgery. Global Open. 3 (6), 406 (2015).
- Gentile, P., Casella, D., Palma, E., Calabrese, C. Engineered Fat Graft Enhanced with Adipose-Derived Stromal Vascular Fraction Cells for Regenerative Medicine: Clinical, Histological and Instrumental Evaluation in Breast Reconstruction. Journal of Clinical Medicine. 8 (4), (2019).
- Gentile, P., Piccinno, M. S., Calabrese, C. Characteristics and Potentiality of Human Adipose-Derived Stem Cells (hASCs) Obtained from Enzymatic Digestion of Fat Graft. Cells. 8 (3), (2019).
- Scioli, M. G., Bielli, A., Gentile, P., Cervelli, V., Orlandi, A. Combined treatment with platelet-rich plasma and insulin favours chondrogenic and osteogenic differentiation of human adipose-derived stem cells in three-dimensional collagen scaffolds. Journal of Tissue Engineering and Regenerative Medicine. 11 (8), 2398-2410 (2017).
- Marx, R. E., Garg, A. K. . Dental and craniofacial applications of platelet-rich plasma. , (2005).
- Kakudo, N., Kushida, S., Kusumoto, K. Platelet-rich plasma: the importance of platelet separation and concentration. Plastic and Reconstructive Surgery. 123 (3), 1135-1136 (2009).
- Kakudo, N., Kushida, S., Minakata, T., Suzuki, K., Kusumoto, K. Platelet-rich plasma promotes epithelialization and angiogenesis in a splitthickness skin graft donor site. Medical Molecular Morphology. 44 (4), 233-236 (2011).
- Kushida, S., et al. Platelet and growth factor concentrations in activated platelet-rich plasma: a comparison of seven commercial separation systems. Journal of Artificial Organs. 17 (2), 186-192 (2014).
- Morimoto, N., et al. Exploratory clinical trial of combination wound therapy with a gelatin sheet and platelet-rich plasma in patients with chronic skin ulcers: study protocol. British Medical Journal Open. 5 (5), 007733 (2015).
- Kushida, S., Kakudo, N., Morimoto, N., Mori, Y., Kusumoto, K. Utilization of Platelet-Rich Plasma for a Fistula With Subcutaneous Cavity Following Septic Bursitis: A Case Report. Eplasty. 15, 31 (2015).
- Eby, B. W. Platelet-rich plasma: harvesting with a single-spin centrifuge. Journal of Oral Implantology. 28 (6), 297-301 (2002).
- Castillo, T. N., Pouliot, M. A., Kim, H. J., Dragoo, J. L. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. The American Journal of Sports Medicine. 39 (2), 266-271 (2011).
Перепечатки и разрешения
Запросить разрешение на использование текста или рисунков этого JoVE статьи
Запросить разрешениеThis article has been published
Video Coming Soon
Авторские права © 2025 MyJoVE Corporation. Все права защищены