Method Article
Implanting Glass Spinal Cord Windows in Adult Mice with Experimental Autoimmune Encephalomyelitis
В этой статье
Резюме
We describe a method for implanting and maintaining glass windows over the exposed spinal cords of adult mice for studying experimental autoimmune encephalomyelitis. These windows provide chronic optical access to the spinal cord for monitoring cell dynamics at the subcellular level in live animals using two-photon microscopy.
Аннотация
Experimental autoimmune encephalomyelitis (EAE) in adult rodents is the standard experimental model for studying autonomic demyelinating diseases such as multiple sclerosis. Here we present a low-cost and reproducible glass window implantation protocol that is suitable for intravital microscopy and studying the dynamics of spinal cord cytoarchitecture with subcellular resolution in live adult mice with EAE. Briefly, we surgically expose the vertebrae T12-L2 and construct a chamber around the exposed vertebrae using a combination of cyanoacrylate and dental cement. A laminectomy is performed from T13 to L1, and a thin layer of transparent silicone elastomer is applied to the dorsal surface of the exposed spinal cord. A modified glass cover slip is implanted over the exposed spinal cord taking care that the glass does not directly contact the spinal cord. To reduce the infiltration of inflammatory cells between the window and spinal cord, anti-inflammatory treatment is administered every 2 days (as recommended by ethics committee) for the first 10 days after implantation. EAE is induced only 2-3 weeks after the cessation of anti-inflammatory treatment.
Using this approach we successfully induced EAE in 87% of animals with implanted windows and, using Thy1-CFP-23 mice (blue axons in dorsal spinal cord), quantified axonal loss throughout EAE progression. Taken together, this protocol may be useful for studying the recruitment of various cell populations as well as their interaction dynamics, with subcellular resolution and for extended periods of time. This intravital imaging modality represents a valuable tool for developing therapeutic strategies to treat autoimmune demyelinating diseases such as EAE.
Введение
Multiple sclerosis is a CNS auto-immune disease that causes progressive myelin and axon damage. The standard research model for CNS autoimmune demyelinating diseases is EAE in adult rodents. However, the dynamic cellular events underlying EAE progression remain largely unexplored. One approach is to use intravital two-photon microscopy to visualize cellular populations with fluorescent markers for extended periods of time in the spinal cords of adult mice with EAE.
Intravital two-photon microscopy is a powerful way to study cellular physiology, cellular interactions, and the dynamic progression of disease in living animals with subcellular resolution1. However, a major hurdle to the approach is that it requires optical access to the region of interest. For example, cranial glass windows are a well-established methodology for repeatedly imaging the same microstructures in the brain over several weeks2,3. Adaptation of this methodology to spinal cord imaging is, however, not straightforward and it requires several technical modifications to immobilize the dorsal spinal tissue within the articulated vertebrae.
Previously, optical access to the spinal cord required surgically reexposing the spinal cord at the beginning of each imaging session and suturing the skin closed at the end of each session4-12. These repeated surgeries are traumatic for the animal, limit the number and length of imaging sessions, and increases the probability of surgery related artifacts such as inflammation and damage to the spinal cord through mechanical perturbation. To overcome these shortcomings, we recently developed a protocol to implant glass windows over the exposed spinal cords of adult fluorescent transgenic mice with traumatic spinal cord injuries for long-term in vivo two-photon microscopy experiments without repeated surgeries13. Here we present a detailed adaptation of the implantation protocol in the context of its application to study the dynamic cellular events underlying the progression of EAE in adult mice.
протокол
1. Window and Support Structure Preparation
- Window preparation - prepare several windows of various sizes (width = 1.8-2.2 mm; length = 4.5-5.2 mm) for implantation.
- Making the modified drill burr to cut the cover glass - using a generous amount of dental cement attach a drill burr to a shaft (in our case old Bulldog clamps). Be sure that the burr is ~45° relative to the shaft for easier handling.
Note: diamond scribes also work well for cutting cover glass. - Making slide-A - using a standard microscope slide, tape a short piece of ruler paper (~2 cm) to the bottom of the slide so that you can see the ruler through the glass. Next, place a piece of tape along the edge of the 0 mm mark of the ruler to act as a fixture for the cover glass in the next step.
- Using the modified drill burr, slide-A, and an unmodified microscope slide (slide-B), position a piece of cover glass (no. 1.5 thickness) to the 0 mm mark on slide-A. Use slide-B to hold the cover glass in place and as a jig for the intended cut line. Cut the cover glass into a long rectangle of approximately 5 x 22 mm. Rotate the cut glass 90° and cut the rectangle into smaller rectangle of ~2 x 5 mm.
- Making the modified drill burr to cut the cover glass - using a generous amount of dental cement attach a drill burr to a shaft (in our case old Bulldog clamps). Be sure that the burr is ~45° relative to the shaft for easier handling.
- Staple modifications - two modified staples will be implanted to support and fix the window chamber to the vertebrae (see section 2.7-8).
- Using a standard small staple (crown = 8 mm; leg = 4 mm), bend the middle of the crown ~90° so that the tips of the legs are nearly touching.
- Bend the tips of the legs outwards ~45° at the half-way point, so that the distal half of the legs are parallel.
- Bend the distal half of the legs ~30° upward relative to the plane of the staple. Place the modified staples into ethanol prior to surgery for sterilization.
Note: Staples can also be sterilized using ethylene oxide or autoclaved.
- Paperclip modifications - prepare one modified paperclip to be implanted as part of the chamber (see sections 2.9-10) and to act as a holding clip for surgery and imaging.
- Using a standard 25 mm paperclip, remove the small bend leaving an elongated U-shape.
- Using pliers or needle-drivers, reduce the diameter of the bend to ~2 mm while keeping the tips parallel.
- Bend the tips outward ~90° at about 8-10 mm from the bend. Make any adjustments necessary so that it lies flat.
2. Window Implantation
Note: All surgical and animal care procedures were conducted in accordance with the European Communities Council Directive and were approved by the National Animal Studies Committee of France (authorization no. 13,300).
- Anesthetize the mouse (Ket/Xyl; induction dose: 120 mg/kg, 12 mg/kg; supplemental dose, 40 mg/kg, 4 mg/kg), apply eye ointment to the eyes.
- Shave its back approximately ~2 cm wide by ~5 cm long, centered on the thoracic hump, and clean the exposed skin using iodine soap followed by iodine solution. Clean gloves by spraying with 90% ethanol.
- Using tissue scissors cut the dorsal skin longitudinally over vertebrae T11 to L1 (~2 cm). Using a scalpel, make bilateral longitudinal incisions between the spinous and transverse processes from T11 to L1. Note: If gloves or instruments touch the fur or skin of the animal, reclean the gloves or instrument using a cold sterilizer such as 90% ethanol or Germ-X.
- Suspend the mouse in the spinal fork stereotaxic - Begin by clamping the top right spinal fork into position. Grasp T11 using Adson forceps and position the T11 transverse process over the fixed spinal fork. While holding the vertebra in place, fasten the contralateral spinal fork under the contralateral transverse process.
- Using the same procedure as step 2.4, position L1 within the spinal fork.
- Using a scalpel (no. 23), gently scrape away the muscles between the spinous and transverse processes from T11 to L1 so that the laminae are clearly visible. For a better view of the pedicles of T11, cut the tendon projecting rostrally from the tip of the transverse process.
- Rinse the modified staples with PBS, and insert the tips of the staples lateral to the vertebral pedicles of T11 and L1 adjusting the distance between the tips so that they are lightly touching the pedicles.
Note: Use only PBS containing dexamethasone (0.1%), penicillin (1,000 U/ml), and streptomycin (1 mg/ml), to reduce postsurgical inflammation and to improve window clarity. - Glue the staples in place with a small drop of cyanoacrylate at each insertion point and remove the mouse from the spinal forks.
- Apply a thin layer of cyanoacrylate to the cut edges of the skin and exposed muscles around the cleared vertebrae being careful to not glue the vertebrae. Bend the tips of the modified paperclip so that they enter under the protruding sections of the implanted staples and rest parallel to the long axis of the isolated vertebral column and leave in this position.
- Starting at the skin-muscle interface, apply several layers of dental cement to cover the entire opening except the exposed vertebrae, being sure to implant the paper clip securely within the dental cement.
- Once the dental cement is cured (5-15 min), attach the mouse to the 'clip holder' by the exposed portion of the implanted paper clip. Using forceps, remove the muscle and other soft tissue between the spinous processes and laminae of the exposed vertebrae.
Note: Any device that can securely grasp the exposed portion of the paperclip without causing stress to the clip/chamber, and can be maneuvered to reorient the mouse throughout surgery can be used as a 'clip holder'. - Using the bone removal drill, thin the laminae of T12 and T13 bilaterally. To remove the dorsal bone, grasp the spinous process with blunt forceps and lift taking care to apply no downward force towards the spinal cord. If the bone does not easily come away, continue to thin the laminae until the spinous process can be easily removed.
- Using the bone removal drill, continue to shape the lateral edges of the vertebrae to form a support structure for the window. Gently remove all of the small bone fragments touching the dura mater using blunt forceps. Be sure to maintain the humidity of the dura mater using PBS.
- Choose one of the prepared windows that is slightly wider than the laminectomy opening and test how well it rests on the exposed bone. Ideally, the window will rest bilaterally on both vertebrae so that the middle of the window touches the dura mater (Figure 1a). The window should not, however, compress the spinal cord even when pushing the window towards the spinal cord. If the window is unbalanced or rests above the dura, repeat step 2.13.
- Once satisfied with the laminectomy, place a small piece of autoclaved tissue-paper soaked in PBS onto the spinal cord to prevent drying of the dura and set the mouse aside.
- Cleaning the window - place the window in a solution of window cleaner for several seconds. Using a piece of microscope lens tissue, wipe the window dry. With the surgical microscope verify that the window is clean, and repeat the cleaning process if not. Place the window in a clean area with easy access for rapid retrieval and placement of the window in step 2.19.
- Retrieve the mouse and remove the tissue paper. Gently remove any debris from the dura using blunt forceps and allow the dura to dry until slightly tacky.
- Quickly fill the silicone (Kwik-sil) mixer tube and extrude silicone onto a clean slide until there are no bubbles emerging from the tip. Immediately administer a small bead of silicone along the midline of the exposed spinal cord.
- Retrieve the cleaned windows using blunt forceps and immediately place over the silicone. Using the blunt forceps, apply a small amount of pressure to the window to spread the silicone to the edges of the window and hold in place for ~1 min.
Note: Prior to mixing, silicone must be stored below 27 °C otherwise the cure-time can increase significantly and it will not adhere to the window or dura mater leading to reduced optical clarity. - Slowly release pressure from the window. If the window does not move, proceed immediately to step 2.21. If, however, the window rises when the pressure is released (i.e. the vertebrae move), proceed to step 2.21 while maintaining pressure on the window to hold it in place.
- Apply cyanoacrylate rostral and caudal of the window. If the cyanoacrylate does not cover the rostral and caudal edges of the window, use a toothpick to guide some of the liquid cyanoacrylate over these edges. Next, guide the glue along the lateral edges of the window (Figure 1a) to cover all of the surrounding muscles. Note: The cyanoacrylate should cover the surface of the outer edges of the window. Too much cyanoacrylate can compromise optical clarity, not enough cyanoacrylate will result in air bubbles between the silicone and window.
- To protect the cyanoacrylate and increase structural integrity of the chamber, apply dental cement to all regions covered with cyanoacrylate (Figure 1a). As with the cyanoacrylate, start at the rostral and caudal edges and use a small wood stick to guide the dental cement along the edges of the window.
3. Postoperative Care, EAE Induction, and Window Maintenance
- Inject Dexamethasone (0.2 mg/kg) and Rimadyl (5 mg/kg) s.q. over the pelvic regions. Transfer the mouse to a warm cage (~26 °C) with nesting material. Mice should be fully mobile, exploring their cage, and grooming after the effects of the anesthetic have fully subsided (usually 2-3 hr). House animals individually and make sure they have easy access to food and water for the first 1-2 days. Once the mouse begins to recover from anesthetic, inject buprenorphine (0.05 mg/kg) s.q. Following full recovery from anesthetic, the mouse should show no signs of pain. Note: Once fully recovered from anesthetic, any signs of pain or abnormal motor activity indicate that the window was not implanted correctly.
- Inject Dexamethasone(0.2 mg/kg) and Rimadyl (5 mg/kg) every 2 d for the first 10 d after surgery (Figure 1a). To inject mice with windows, lightly sedate the animal with isoflurane in an induction chamber (~1.75% isoflurane in air for 1-2 min). Remove the mouse from the chamber and immediately inject the drugs. The mouse should be fully awake in <1 min.
Note: Do not scruff mice with implanted windows since this can cause separation of the skin from the implant. It is possible to inject mice i.p. without sedation by allowing them to grab a cage lid with their forepaws and gently raising their hindlimbs. However, in this position the mouse will sometimes pull away causing stress to the implanted window. This can lead to bleeding between the window and spinal cord, thus compromising optical clarity. - EAE can be induced at 2-3 weeks after the cessation of anti-inflammatory treatment (~3-4 weeks after surgery). Lightly sedate the mouse as described in step 3.2. Remove the mouse from the induction chamber and maintain the sedation with an isoflurane mask placed over the nose.
- Inject MOG35-55 peptide (75 µg in Freund's adjuvant containing 800 µg of mycobacterium tuberculosis) into 3 different s.q. locations (bilaterally over the femur, and at the base of the tail), and pertussis toxin (400 ng) i.p.14,15
Note: Mice with EAE require supplemental care compared to mice with windows only. Weigh EAE mice every day and be sure that they have easy access to food and water at all times. Supplement with a 3% agarose jelly containing to maintain animal hydration and weight. Glucose (1-3%) can also be added to the jelly for additional calories. - Two days after EAE induction, repeat the sedation protocol outlined in step 3.2 and inject a second dose of pertussis toxin (400 ng) i.p.
- To image the spinal cord, first sedate the animal as described in step 3.2, and lightly anesthetize with ketamine/xylazine (100 mg/kg; 10 mg/kg) i.p. This will provide ~ 1 hr of imaging.
- For imaging sessions lasting <2 hr, light anesthesia can be maintained with supplementary injections of ketamine/xylazine (50 mg/kg; 5 mg/kg) every 45-60 min. Alternatively, for longer imaging sessions, mice can be continuously sedated with isoflurane (0.75-1.25% isoflurane in humidified air).
Note: windows can be cleaned under a surgical microscope by gently wiping away debris with the corner of a tissue or a cotton-tipped swab. Do not use alcohol to clean the window because it can degrade some types of dental cement and it can cool the spinal cord. - Once lightly anesthetized, the mouse is attached firmly to a clip holder by the exposed portion of the implanted paperclip (Figure 1b).
- The spinal cord is aligned perpendicular to the objective lens by adjusting the positioning of the clip within the holder, adjusting the position of the holder, and adjusting the position of the stage. The dorsal surface of the spinal cord is rapidly imaged, and the dorsal vasculature is used to determine the orientation of the spinal cord.
Результаты
Using chronically implanted glass windows in combination with intravital two-photon microscopy and EAE models may be a useful tool to understand the cellular mechanisms underlying autoimmune demyelinating disease pathology. Using our implantation method we found that most windows were clear at the time of EAE induction, and remained clear throughout EAE progression (Figure 1c). Using this approach, we found that windows remained clear for the entire postimplantation period (average ~68 d±7 s.e.m.; n = 21 mice), and two-photon imaging could be done repeatedly before or after EAE induction up to 12x over 36 days in our hands.
As a first step towards testing the feasibility of this approach, we compared EAE clinical progression in animals with and without implanted windows (Figure 1d). We found that EAE mice had a slight delay in the onset of clinical signs (Figure 1e), but following onset there was no difference in the overall progression or peak clinical score (Figure 1f).
EAE is a progressive neurodegenerative disease characterized by the loss of myelin and axons. We therefore examined the time-line of axon degeneration in the spinal cord over the course of EAE progression using intravital two-photon microscopy via the implanted windows. Similar to previous findings10, we observed examples of uninjured axons (Figure 2a), as well as axons in different stages of degeneration, such as axons with focal swellings (Figure 2b) and fragmented axons (Figures 2c and 2d). Figure 2e shows the average number of uninjured axons at multiple time-points relative to the onset of clinical signs (n = 5 mice). For this, we counted the number of axons within the same spinal region from each imaging session using vascular markers to relocate the region of interest. We found that the number of axons drops dramatically following the onset of clinical signs. Moreover, we quantified the number of axons with focal swellings (Figure 2f) and fragmented axons (Figure 2g) within the regions of interest. The number of damaged axons increases with the onset of EAE clinical signs, and axon deterioration progresses from swellings to fragmentation at later time-points.
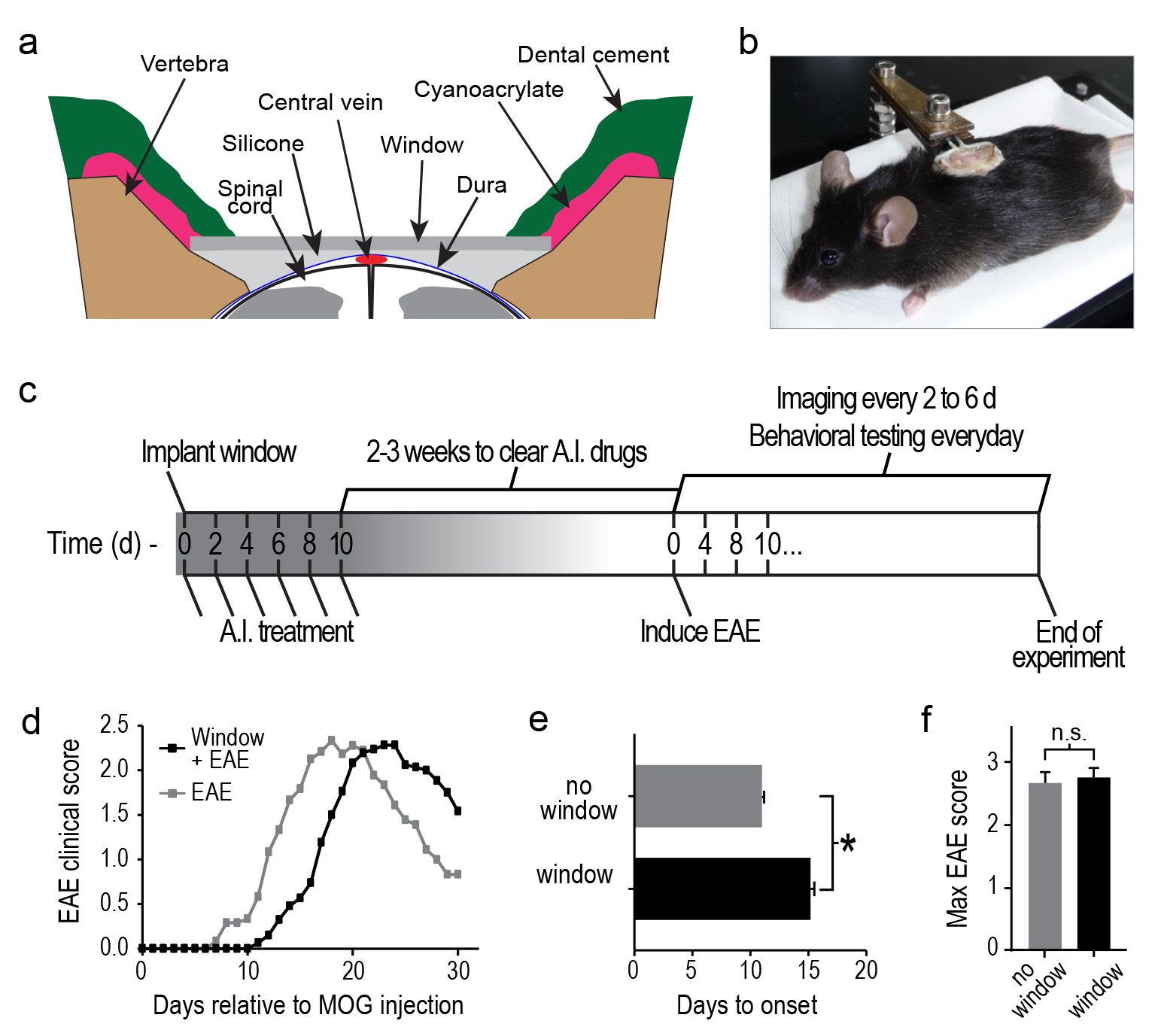
Figure 1. EAE is induced in mice with implanted windows. A, diagram showing the layers of implanted dental cement, cyanoacrylate, and silicone elastomer relative to spinal cord, and implanted window. Dorsal, up; Ventral, down. B, Image of an anesthetized mouse with an implanted window and is supported by the implanted paperclip. A and B were adapted with permission from Figure 1, Fenrich et al.13 C, Time-line showing the anti-inflammatory (A.I.) treatment paradigm, posttreatment waiting period, and EAE induction schedule relative to the day of window implantation. D, Graphs showing the progression of clinical signs in mice with (black) and without (grey) implanted windows. E, Average time to onset of EAE clinical signs in mice with (black) and without (grey) implanted windows. F, Average peak EAE clinical score in mice with (black) and without (grey) implanted windows. Error bars, s.e.m. *p < 0.05, Mann-Whitney U test. Click here to view larger image.

Figure 2. Windows are useful for tracking axon deterioration at a subcellular level throughout EAE progression. a-d, Average intensity projection of two-photon image stacks of the same spinal region from 16d to 30d post-MOG injection. Arrows point to a degenerating axon that was uninjured at 16 d (a), had focal swellings at 20 d (b), and was fragmented at 22 d (c) and 30 d (d) post-MOG injection. Inset shows a higher power image of the same degenerating axon (highlighted in orange) compared to uninjured axons. Stars show shadows caused by a small piece of debris located between the dura mater and silicone. Notice that the debris remains constant and that these regions have a diminished intensity throughout the duration of the experiment. Scale bar, 50 μm; inset, 10 μm. e-g, Line graphs showing the average number of uninjured axons (e); percentage, normalized to first imaging session before EAE induction), axons with swellings (f), and fragmented axons (g) within the same spinal regions over time(n = 5 mice). Data in e-f are grouped according to the onset of EAE clinical symptoms. Error bars, s.e.m. Click here to view larger image.
Обсуждение
We described a method for implanting and maintaining glass windows over the exposed spinal cords of adult mice to study the dynamic progression of EAE over time. A similar glass window implantation approach has been used to study spinal cord injury models13 and may also be useful for studying other spinal cord pathologies such as glioblastomas, meningitis, or the cellular physiology of the normal spinal cord. For our method, vertebral stabilization was achieved by building an exoskeleton around four tightly fixed vertebrae while the glass window was anchored to this exoskeleton. In these experiments windows were implanted at vertebral levels T13-L1. For some preliminary experiments we implanted windows at more rostral and caudal levels, but a detailed examination of the reliability of these windows remains to be done. Spinal tissue movements inside the vertebral lumen were reduced by gluing the dura to the glass with a thin layer of transparent silicone. This cement, glue, and silicone approach thus offers unique tailoring possibilities to ensure a tight seal at all points of the chamber and for every animal. Avoiding air bubbles and adherence losses, we minimized not only movements but also fibrotic tissue formation that would otherwise impede fluorescence imaging. As a result, we successfully imaged through implanted windows for an average of nearly 10 weeks, compared to a 50% success rate at just five weeks reported with other techniques16.
A severe inflammatory response between the silicone and dura mater, and/or within the spinal cord is the main source of reduced window clarity and experimental failure. Inflammation between the silicone and dura mater is rare and usually occurs when the dura is not cleaned properly or if the dura is not sufficiently tacky prior to application of the silicone bead (steps 2-17, 18). Inflammation within the spinal cord is more common, and is usually caused by applying too much force to the spinal cord during bone removal (step 2-12) or while cleaning the dura mater (steps 2-13 and 2-17), by allowing too much time between silicone application and window implantation (steps 2-18-19, and example shown in video), and/or by compromising the integrity of the dura. When done properly, there should be virtually no inflammation at the implantation site. To minimize surgery induced spinal inflammation and to reduce the risk of axon damage, the dura mater must remain intact throughout surgery. To verify the integrity of the dura mater, it should appear slightly inflated with a thin layer of cerebrospinal fluid (CSF) between the spinal cord and dura mater. If there is a hole in the dura mater, the dura mater will be touching the spinal cord. Moreover, windows must be clear of any debris, especially cellular debris (e.g. red blood cells that adhered while testing the window size) or other contaminants (e.g. oils from skin), prior to implantation. Dirty windows do not adhere well to silicone and are far more susceptible to the formation of bubbles and recruitment of inflammatory cells between the silicone and window.
Steps 2.18 and 2.19 are the most important steps of the entire procedure, and are very time sensitive. It should take less than 30 sec from filling the silicone mixer to the final placement of the window. This short time-frame is to avoid damaging the spinal cord since the silicone will typically become viscous in less than 30 sec. If too much time elapses between the mixing of the silicone and window placement, pressure from the window will compress the spinal cord because the silicone will not migrate to the edges. In addition, when gluing the windows in place it is important to note that cyanoacrylate does not adhere to silicone. If there is excessive amounts of silicone along the lateral edges of the window, or if there is silicone on the external surface of the window, the silicone must be removed before gluing the lateral edges of the window. Failure to do so will compromise the bond between the window and the silicone, resulting in air bubbles and reduced optical clarity. We do not recommend removal and replacement of poorly implanted windows. In our experience successful window replacement is rare, and often leads to rapid loss of optical clarity and damage to spinal cord axons.
Because well implanted windows remain clear for several months it is possible to prepare animals several weeks prior EAE induction. Large groups of animals can thus be implanted over an extended period of time while disease onset can still be synchronized in the whole population thanks to delayed induction. Delaying induction also has the benefit of reducing the influence of postsurgical inflammation that could otherwise modulate the MOG induced autoimmune reaction17. In our case, postoperative anti-inflammatory treatment was applied to accelerate mouse recovery and to minimize eventual fibrotic tissue formation between the dura mater and the silicone layer. Animals were then left in their cage without treatments for at least two weeks to ensure complete wash out of the drugs prior EAE induction.
EAE is an animal model of Multiple Sclerosis that recapitulates at least the immunological features of the human disease18. EAE was induced by auto-immunization against MOG35-55 peptide. As immune response is highly dependent on physiological status including temperature19,20, stress and hormonal conditions21, the fact that animals with windows have a delayed onset of the disease is not surprising. Importantly, we show here that the spinal chamber does not affect progression kinetics once the disease is initiated.
Focal demyelination of axons by MOG targeted T-cells is an acknowledged hallmark of MS but demyelination does not necessarily lead to axonal degeneration18. Because axonal losses have been shown to be a major correlate of permanent neurological deficit in MS patients22, correlating axonal changes with clinical scores over the course of EAE progression should provide valuable information for the planning of treatment protocols. The window is well tolerated and motor scores can be accurately determined in our model. Moreover our minimally invasive imaging methods eases the interpretation of minor morphological changes such as swelling or fragments that could otherwise be understood as surgery related events.
Раскрытие информации
The authors declare that they have no competing financial interests.
Благодарности
We thank C. Ricard, M-C. Amoureux, A. Jaouen, and S. Meijia-Gervacio for helpful discussions; M. Hocine and C. Meunier, and the staff of the animal and PicSIL imaging facilities of the IBDML for technical support. This work was supported by an institutional grant from Centre National de la Recherche Scientifique, and by grants from the Association de Recherche sur la Sclérose en Plaque (ARSEP) (to G.R. and K.K.F), Agence Nationale de la Recherche (ANR JCJC), Fédération de Recherche sur le Cerveau (FRC) Institut de Recherche sur la Moelle Epinière (IRME) (to FD). K.K.F. was supported by an ARSEP fellowship. Imaging was performed on the PIcSIL imaging facility of the IBDML.
Материалы
| Name | Company | Catalog Number | Comments |
| Bone removal drill | World Precision Instruments | IDEAL MICRO-DRILL | Also sold by Harvard Apparatus. Any high quality surgical bone drill will suffice. |
| Drill burr (#1/4 or 1/2 Carbide Round Burr) | World Precision Instruments | 501860 (#1/4) 501860 (#1/2) | Also sold by Harvard Apparatus |
| Staples | Lyreco | 5.002.567 | Crown = 8 mm Leg = 4 mm |
| Paperclip | Any standard 25 mm paperclip | ||
| Needle drivers | Fine Science Tools | 12003-15 | |
| Tissue scissors | Fine Science Tools | 14028-10 | |
| Adson forceps | Fine Science Tools | 11027-12 | 1 x 2 teeth |
| Blunt forceps | Fine Science Tools | 11293-00 | |
| Spinal forks | Harvard Apparatus | 724813 | |
| Dental cement | GACD | 12-565 & 12-568 | |
| Silicone elastomer | World Precision Instruments | KWIK-SIL | Also avaiable through Coherent Scientific and Fisher Scientific |
| Cyanoacrylate | Eleco-EFD | Cyanolit 201 | |
| Microscope | Zeiss | 7MP | Our microscope is in an upright configuration and is equipped with 5 NDD detectors. |
| Objective lens | Zeiss | W Plan-Apochromat 20x/1.0 DIC M27 75mm | Working distance = 1.8 mm |
Ссылки
- Kim, J. V., Dustin, M. L. Innate response to focal necrotic injury inside the blood-brain barrier. J. Immunol. 177 (8), 5269-5277 (2006).
- Xu, H. -. T., Pan, F., Yang, G., Gan, W. -. B. Choice of cranial window type for in vivo imaging affects dendritic spine turnover in the cortex. Nat. Neurosci. 10 (5), 549-551 (2007).
- Holtmaat, A., Bonhoeffer, T., et al. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat. Protoc. 4 (8), 1128-1144 (2009).
- Dray, C., Rougon, G., Debarbieux, F. Quantitative analysis by in vivo imaging of the dynamics of vascular and axonal networks in injured mouse spinal cord. Proc. Natl. Acad. Sci. U.S.A. 106 (23), 9459-9464 (2009).
- Kerschensteiner, M., Schwab, M. E., Lichtman, J. W., Misgeld, T. . In vivo imaging of axonal degeneration and regeneration in the injured spinal. 11 (5), 572-577 (2005).
- Ylera, B., Ertürk, A., et al. Chronically CNS-injured adult sensory neurons gain regenerative competence upon a lesion of their peripheral axon. Curr. Biol. 19 (11), 930-936 (2009).
- Dibaj, P., Nadrigny, F., et al. NO mediates microglial response to acute spinal cord injury under ATP control in vivo. Glia. 58 (9), 1133-1144 (2010).
- Dibaj, P., Steffens, H., et al. In Vivo imaging reveals distinct inflammatory activity of CNS microglia versus PNS macrophages in a mouse model for ALS. PloS One. 6 (3), (2011).
- Johannssen, H. C., Helmchen, F. In vivo Ca2+ imaging of dorsal horn neuronal populations in mouse spinal cord. J. Physiol. 588 (18), 3397-3402 (2010).
- Nikić, I., Merkler, D., et al. A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis). Nat. Med. 17 (4), 495-499 (2011).
- Di Maio, A., Skuba, A., et al. In vivo imaging of dorsal root regeneration: rapid immobilization and presynaptic differentiation at the CNS/PNS border. J. Neurosci. 31 (12), 4569-4582 (2011).
- Laffray, S., Pagès, S., Dufour, H., De Koninck, P., De Koninck, Y., Côté, D. Adaptive movement compensation for in vivo imaging of fast cellular dynamics within a moving tissue. PloS One. 6 (5), (2011).
- Fenrich, K. K., Weber, P., Hocine, M., Zalc, M., Rougon, G., Debarbieux, F. Long-term in vivo imaging of normal and pathological mouse spinal cord with subcellular resolution using implanted glass windows. J. Physiol. 590 (16), 3665-3675 (2012).
- Kuerten, S., Angelov, D. N. Comparing the CNS morphology and immunobiology of different EAE models in C57BL/6 mice - a step towards understanding the complexity of multiple sclerosis). Ann. Anat. 190 (1), 1-15 (2008).
- Mendel, I., Kerlero de Rosbo, N., Ben-Nun, A. A myelin oligodendrocyte glycoprotein peptide induces typical chronic experimental autoimmune encephalomyelitis in H-2b mice: fine specificity and T cell receptor V beta expression of encephalitogenic T cells. Eur. J. Immunol. 25 (7), 1951-1959 (1995).
- Farrar, M. J., Bernstein, I. M., Schlafer, D. H., Cleland, T. A., Fetcho, J. R., Schaffer, C. B. Chronic in vivo imaging in the mouse spinal cord using an implanted. Nat. Methods. , (2012).
- Moreno, B., Jukes, J. -. P., et al. Systemic inflammation induces axon injury during brain inflammation. Ann. Neurol. 70 (6), 932-942 (2011).
- Lassmann, H., van Horssen, J. The molecular basis of neurodegeneration in multiple sclerosis. FEBS Lett. 585 (23), 3715-3723 (2011).
- Yenari, M. A., Han, H. S. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat. Rev. Neurosci. 13 (4), 267-278 (2012).
- Kalmbach, A. S., Waters, J. Brain surface temperature under a craniotomy. J. Neurophysiol. 108 (11), 3138-3146 (2012).
- Pérez-Nievas, B. G., García-Bueno, B., Madrigal, J. L. M., Leza, J. C. Chronic immobilisation stress ameliorates clinical score and neuroinflammation in a MOG-induced EAE in Dark Agouti rats: mechanisms implicated. J. Neuroinflammation. 7, 60 (2010).
- Bjartmar, C., Kidd, G., Mörk, S., Rudick, R., Trapp, B. D. Neurological disability correlates with spinal cord axonal loss and reduced N-acetyl aspartate in chronic multiple sclerosis patients. Ann. Neurol. 48 (6), 893-901 (2000).
Перепечатки и разрешения
Запросить разрешение на использование текста или рисунков этого JoVE статьи
Запросить разрешениеThis article has been published
Video Coming Soon
Авторские права © 2025 MyJoVE Corporation. Все права защищены