Method Article
Technique for Isolation and Culture of Rat Jaw Bone Marrow Mesenchymal Stem Cells
In This Article
Summary
Mesenchymal stem cells from the jaw bone marrow have significant functions in diverse differentiation, self-renewal, and immune modulation. They have emerged as a crucial reservoir of precursor cells in gene therapy, tissue engineering, and regenerative medicine. Here, we present a unique method for isolating jaw bone marrow mesenchymal stem cells in rats.
Abstract
Bone marrow mesenchymal stem cells (BMMSCs) are a type of stem cell with multi-directional differentiation potential. Compared with BMMSCs derived from appendicular bones, BMMSCs derived from the jaw have greater proliferative and osteogenic differentiation ability, gradually becoming important seed cells for jaw defect repair. However, the mandible has a complex bony structure and less cancellous content than appendicular bones. It is difficult to acquire a large number of high-quality jaw-derived marrow mesenchymal stem cells using traditional methods. This study presents a 'niche-based approach on stemness' for isolating and culturing rat jaw bone marrow mesenchymal stem cells (JBMMSCs). Primary rat JBMMSCs were isolated and cultured using the whole bone marrow adherent method combined with the bone slice digestion method. The isolated cells were identified as JBMMSCs through cell morphology observation, detection of cell surface markers, and multi-directional differentiation induction. The cells extracted by this method exhibit a 'fibroblast-like' spindle shape. The cells are long, spindle-shaped and fibroblast-like. The flow cytometry analysis shows these cells are positive for CD29, CD44, and CD90 but negative for CD11b/c, CD34, and CD45, which is congruent with BMMSCs characteristics. The cells show strong proliferation capacity and can undergo osteogenic, adipogenic, and chondrogenic differentiation. This study provides an effective and stable method for obtaining enough high-quality JBMMSCs with strong differentiation ability in a short time, which could facilitate further studies of the exploration of biological function, regenerative medicine, and related clinical applications.
Introduction
Mesenchymal stem cells (MSCs) were first discovered in bone marrow, which showed the ability to form adhesive colonies in culture and strong osteogenic potential1. Pittinger et al.2 further found their multi-directional differentiation potential towards bone, fat, and cartilage. Although all mesenchymal stem cells from different sources have the potential for multi-directional differentiation, bone marrow mesenchymal stem cells have the strongest chondrogenic differentiation potential compared with mesenchymal stem cells derived from other tissues, making them considered the best candidate cells for bone tissue engineering3. However, many studies have proved that BMMSCs from different origins present site-specific characteristics and properties such as osteogenic differentiation capability and cellular proliferative activity4,5. This may be due to different germ layers between the jaw and appendicular bones or the iliac crest6.
Jaw bone marrow mesenchymal stem cells (JBMMSCs) arise from neural crest cells of the neuroectoderm, while femur-derived BMMSCs originate from the mesoderm7. Compared with BMMSCs derived from long bones and the iliac crest, JBMMSCs have a higher proliferation rate, ALP activity, and osteogenic potential8. Besides, the application effect of BMMSCs in tissues and organs may vary depending on different cell types and environments9. Repair of jaw defects mainly depends on the recruitment of jaw-derived mesenchymal stem cells. Therefore, studying JBMMSCs can provide an experimental basis for its clinical application in jaw bone tissue engineering10. However, basic research and clinical applications mainly focus on BMMSCs derived from appendicular and axial bones11. Research on JBMMSCs is limited, and this may be due to the low content of cancellous bone in the mandible and the fact that rats' jaws have even less cancellous bone content12. Therefore, it is difficult to separate jaw bone-derived marrow mesenchymal stem cells using the ordinary bone marrow flushing method, which is commonly used in isolating BMMSCs from appendicular bones or the iliac crest13. Based on the methods of Hong et al.14 and Cheng et al.15, we hypothesized that the combination of dense bone digestion and the bone marrow flushing method could efficiently isolate rat JBMMSCs.
This study aims to establish an efficient method for isolating rat JBMMSCs and provide sufficient seed cell sources for jaw bone tissue engineering.
Protocol
The protocol was approved by the Institutional Animal Ethics Committee of the Chinese PLA General Hospital. Thirteen-week-old male Wistar rats were used for the experiment. Details on the animals, reagents, and equipment are listed in the Table of Materials.
1. Experimental preparation
- Sterilize all surgical instruments, including ophthalmic scissors, tweezers, and bone rongeurs, at high temperature and pressure.
- Prepare culture media in advance.
- Prepare α-MEM complete medium containing 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin.
- Prepare 0.1% type II collagenase in phosphate-buffered saline (PBS).
- Prepare 50 mL of PBS with 1% penicillin-streptomycin in a disposable sterile 50 mL centrifuge tube.
2. Isolation and cultivation of rat JBMMSCs
- Isolate the rat mandible following the steps below:
NOTE: To maintain sample sterility, all metal surgical instruments must be disinfected at high temperature on an alcohol lamp flame for at least three seconds before use.- Anesthetize the rat with 2% pentobarbital sodium (2 mL/kg) by intraperitoneal injection.
- Sacrifice the anesthetized rat by cervical vertebrae dislocation (following institutionally approved protocols) after its respiratory rate decreases and the muscles relax completely.
- Immerse the rat in 75% ethanol for 30 min to disinfect it and then transfer it to the ultra-clean table.
- Place the rat on a disposable sterile cloth or in a sterile container and keep it in the supine position.
- Cut and separate the skin and muscle from the angulus oris to the joint along the ramus, and then separate the condyle from the glenoid fossa by cutting the temporomandibular joint disc and bilateral peripheral ligaments horizontally with ophthalmic scissors.
NOTE: Determine the position of the temporomandibular joint by moving the mandible and observing the position of the condyle externally. - Cut and separate the muscles and connective tissues of the lateral mandible using scissors from the vestibular groove on the buccal side of the lower incisors to the left and right sides.
- Incise the lingual muscles of the mandible from the lingual frenum to the posterior region of the mandible.
- Remove the mandible from the skull.
- Divide the mandible into halves by cutting the symphysis between the lower incisors with ophthalmic scissors.
- Remove the remaining attached muscles, fascia, and other soft tissues on the mandible carefully using ophthalmic scissors and forceps.
- Isolate JBMMSCs.
- Place the beak of the rongeur at the junction of the incisors and the mesial part of the first molar, cut off the connection between the lower incisors and the mandible with the rongeur, and extract the lower incisors completely.
- Remove all molars with a bone rongeur.
- Remove the mandibular ramus with a bone rongeur and expose the marrow cavity by separating the central part of the mandible along the distal edge of the last molar16.
- Suck α-MEM complete medium with a 1 mL disposable sterile syringe. Insert the needle into the bone marrow cavity and flush the bone marrow repeatedly into the culture dish with α-MEM complete medium until the bone turns white.
- Collect the bone marrow flushing solution into a 15 mL centrifuge tube and centrifuge it at 800 x g for 3 min at room temperature.
- Discard the supernatant with a pipette, resuspend the cells with 10 mL of α-MEM complete medium, and plate them into a new 10 cm culture dish.
- Divide the flushed mandible into 1-3 mm³ bone slices with a rongeur and digest them with 3 mL of 0.1% type II collagenase for 90 min in a 200 rpm/min shaker at 37 °C.
- Centrifuge the digested mandible at 800 x g for 3 min at room temperature and discard the supernatant.
- Culture the harvested cells and digested bone slices with α-MEM complete medium at 37 °C in a 5% CO2 humidified incubator.
- Change half of the culture medium with fresh medium after 72 h and then change it completely every 2 days.
NOTE: Do not move the culture dish for the first three days. - Passage the adherent cells at a ratio of 1:2 when they reach 80%-90% confluency. Remove bone slices during the second subculture. Use the cells of the P2 or P3 generations for experiments.
3. Flow cytometry for Identification of cell surface markers
- Resuspend JBMMSCs of the P2 generation in flow cytometry buffer. Count the cells using an automated cell counter and adjust the concentration to 3 × 106 cells/mL.
- Add 100 µL of cell suspension and 2 µL of monoclonal antibodies (CD11b/c, CD29, CD34, CD44, CD45, and CD90) to each microcentrifuge tube. Incubate for 30 min at 4 °C17,18.
- Wash the sample twice with 200 µL of flow cytometry buffer and centrifuge it for 5 min at 250 x g at room temperature. Remove the supernatant.
- Add 100 µL of flow cytometry buffer and 2 µL of PE-labeled fluorescent secondary antibody to each tube. Incubate at 4 °C for 30 min.
- Repeat step 3.3.
- Resuspend JBMMSCs in 300-500 µL of flow cytometry buffer per tube.
- Conduct the flow cytometric analysis using a flow cytometer.
NOTE: Use homologous antibodies as negative controls to eliminate background staining caused by the non-specific combination of antibodies.
4. Cell proliferation determination
- Seed JBMMSCs of the P3 generation into a 96-well plate at a density of 5 × 103 cells per well.
- Add 10 µL of CCK-8 solution to each well.
- Place the 96-well plate in the incubator for 2 h and measure the absorbance (OD value at 450 nm) using a microplate reader.
NOTE: Perform the test at the same time every day for seven consecutive days.
5. Colony formation capability
- Seed 1000 JBMMSCs from passage 3 into 10 cm culture dishes and culture at 37 °C with 5% CO2.
- Change the culture medium every 3 days.
- Perform crystal violet staining after continuous cultivation for 15 days.
- Next, fix the cells with methanol for 5 min and stain with 2% crystal violet for 5 min.
6. Multilineage differentiation of JBMMSCs
NOTE: Use JBMMSCs of P3 generation for multilineage differentiation. The Control group was cultured with α-MEM complete medium.
- Perform osteogenic differentiation.
- Inoculate 1 × 105 JBMMSCs per well in a six-well plate.
- When the cell confluence reaches 70%, change the medium to osteogenic induction medium.
NOTE: Change the osteogenic induction medium every 3 days. - Perform Alkaline Phosphatase (ALP) staining after 7 days of osteogenic induction.
- Remove the medium from the six-well plate, fix the cells with 4% paraformaldehyde at room temperature for 30 min, and then wash them with PBS three times.
- Stain the cells with ALP staining solution at room temperature for 30 min and protect them from light.
- Rinse the cells until no staining solution remains, and observe the samples under a microscope.
- Perform Alizarin red staining after 21 days of osteogenic induction.
- Stain the cells with Alizarin red staining solution for 5 min after fixing the cells with 4% paraformaldehyde for 30 min.
- Wash the cells with PBS until no Alizarin red staining solution remains.
- Observe the number of calcium nodules under a microscope.
- Perform adipogenic differentiation.
- Inoculate 1 × 105 JBMMSCs per well in a six-well plate.
- When the cell confluence reaches 70%, change the medium to adipogenic induction medium.
NOTE: Change the adipogenic induction medium every three days. - Perform Oil red O staining after 14 days of adipogenic induction.
- Remove the medium from the six-well plate, fix the cells with Oil red O fixing solution for 20-30 min, and then wash them with PBS three times.
- Remove the fixing solution and wash the cells with distilled water twice.
- Immerse the cells in 60% isopropanol for 20-30 s.
- Remove 60% isopropanol and stain the cells with Oil red O staining for 20-30 min.
- Remove the staining solution, rinse the cells with 60% isopropanol for 20-30 s, and then wash them with distilled water.
- Stain the nucleus with hematoxylin solution for 1-2 min.
- Remove the hematoxylin solution and wash the cells 2-3 times with distilled water. Add Oil red O buffer for 1 min and remove it.
- Observe the number of lipid droplets under a microscope.
- Perform chondrogenic differentiation.
- Inoculate 1 × 105 JBMMSCs per well in a six-well plate.
- When the cell confluence reaches 70%, change the medium to chondrogenic induction medium.
NOTE: Change the chondrogenic induction medium every 3 days. - Perform Alcian blue staining after 21 days of chondrogenic induction.
- Remove the medium from the six-well plate, fix the cells with 4% paraformaldehyde at room temperature for 30 min, and then wash them with PBS three times.
- Stain the cells with Alcian blue staining at 37 °C for 1 h.
- Rinse the cells until no staining solution remains, and observe them under a microscope.
7. Real-time PCR
- Extract total RNA using the RNA extraction kit and measure the RNA concentration with an enzyme marker (following the manufacturer's instructions).
- Synthesize cDNA using 500 ng of RNA19.
- Perform qRT-PCR on a Real-time system with 10 µL of qRT-PCR mix containing 1 µL of cDNA, 3.5 µLof double-distilled water, 5 µL of SYBR, and 0.5 µL of primer mix (0.1 µM).
NOTE: Primer sequences are listed in Table 1. The thermal cycling conditions are as follows: 50 °C for 2 min, 95 °C for 2 min, followed by 40 cycles of 95 °C for 15 s, 60 °C for 2 min, and then increments of 0.5 °C for 5 s from 60 °C to 95 °C for the melting curve. - Use GAPDH as an internal control16.
Results
After 72 h of cell inoculation, most cells were suspended and round in shape, with very few adhered to the wall (Figure 1B). By the fifth day, adherent cell colonies appeared, exhibiting spindles or fibroblast-like shapes (Figure 1C). By the seventh day, adherent cells reached 90% confluency, forming a "fish school" shape with a small number of intermittent suspended cells (Figure 1D). Passaged cells grew rapidly and were passaged every 3 days. The cell morphology was relatively homogeneous, predominantly spindle-shaped, and arranged in a vortex-like pattern after confluency (Figure 1E-G).
Flow cytometry analysis revealed high expression levels of mesenchymal stem cell surface markers CD90, CD29, and CD44, with positive rates of 97.2%, 97.7%, and 95.7%, respectively. Conversely, hematopoietic stem cell surface markers CD45, CD34, and CD11b/c showed low expression, with positive rates of 1.37%, 0.66%, and 1.49%, respectively (Figure 2A). After 2 weeks of culture, JBMMSCs demonstrated colonization, as evidenced by crystal violet staining (Figure 2B). They exhibited stable expansion ability in vitro, with the cell growth curve following a typical 'S-like' pattern (Figure 2C). The cell proliferation process included a latent period, logarithmic growth phase, and plateau phase. Rapid growth commenced after two days, followed by a slowdown after six days. Individual cells exhibited characteristics of self-replication and proliferation.
The cells exhibited blue-purple ALP staining particles on the 7th day and red mineralized nodules on the 21st day. Additionally, after 14 days of adipogenic induction, the cells displayed red beaded lipid droplets, while chondrogenic differentiation induction resulted in the formation of blue cartilage-like tissue (Figure 3A). Furthermore, after 7 days of osteogenic induction, the mRNA expression levels of ALP, OCN, and Runx2 in JBMMSCs were elevated15 (Figure 3B).
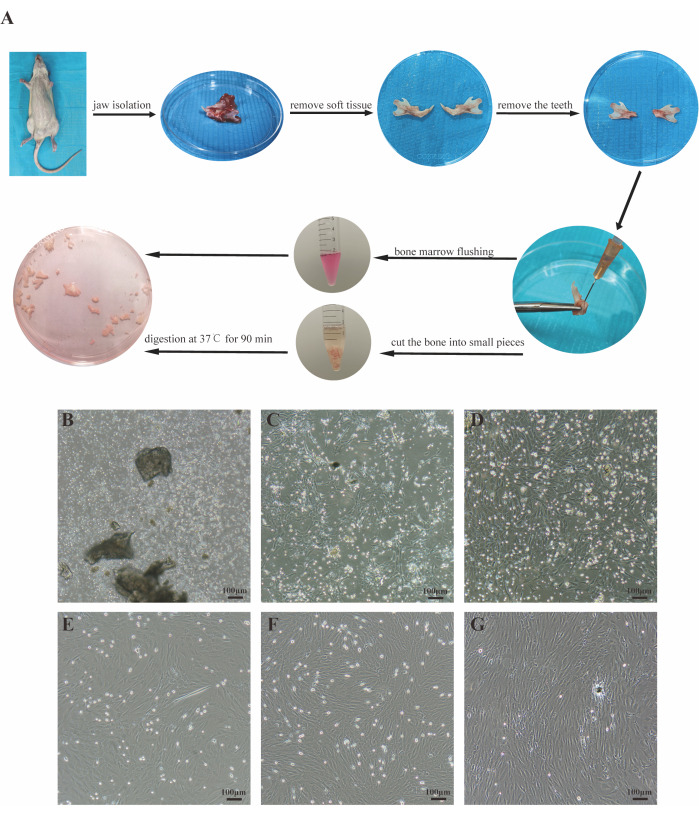
Figure 1: Isolation and culture of JBMMSCs. (A) An overview of the protocol. (B-D) Images of cells cultured for 3 days, 5 days, and 7 days in primary culture, respectively. (E-G) Images of the P1, P2, and P3 cells, respectively. Scale bars: 100 µm. Please click here to view a larger version of this figure.
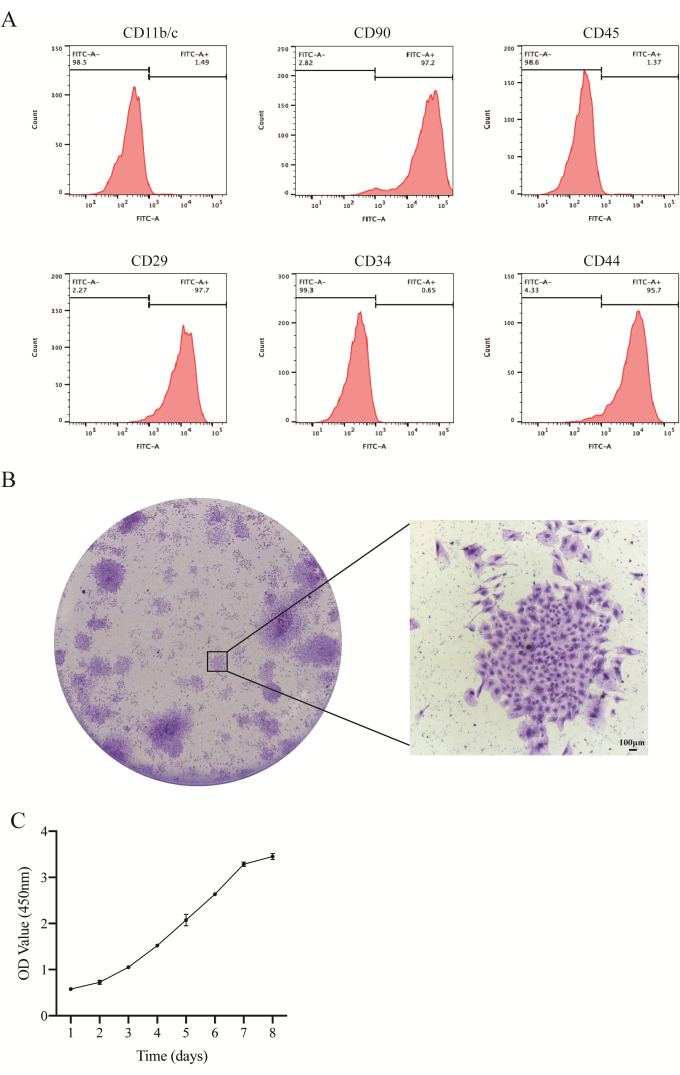
Figure 2: Identification of JBMMSCs. (A) Flow cytometry analysis results of rat JBMMSCs. Flow cytometry analysis revealed that these cells were positive for CD29, CD44, and CD90, consistent with BMMSCs' characteristics. Conversely, they were negative for CD11b/c, CD34, and CD45. (B) Representative images of JBMMSCs colonies stained with crystal violet (scale bars: 100 µm). (C) The cell growth curve showed the cell proliferation rate.The data and the error bars denote mean ± standard deviation, n = 3. Please click here to view a larger version of this figure.
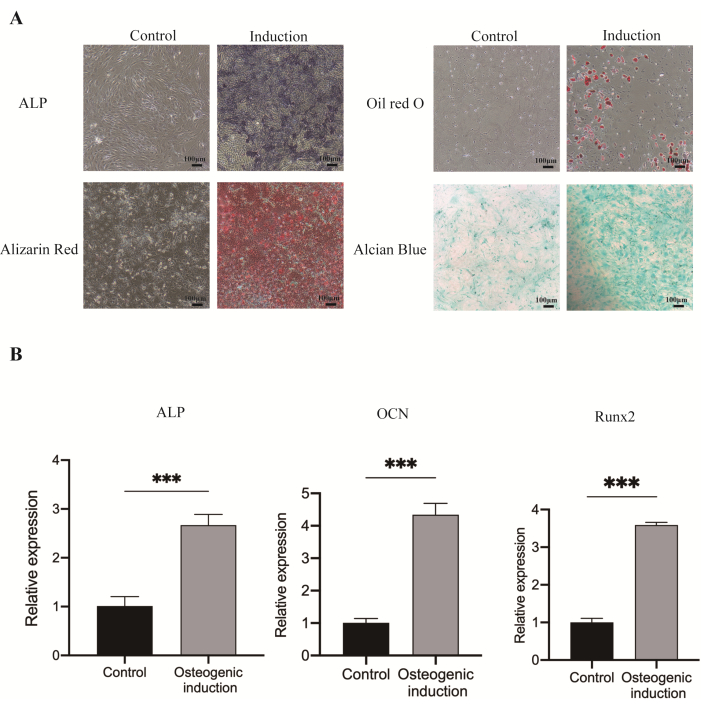
Figure 3: Multilineage (osteogenic, adipogenic, and chondrogenic) differentiation of JBMMSCs. (A) After 7 days of osteogenic induction, increased ALP activity was observed. At 21 days post-osteogenic induction, numerous mineralized nodules were stained red with Alizarin red staining. Oil red O staining revealed a significant number of lipid droplets. Alcian blue staining highlighted the cells induced into chondrocytes following chondrogenic induction. (B) mRNA expression of osteogenic related gene ALP, OCN, and Runx2 of JBMMSCs after osteogenic induction for 7 days. (***P < 0.001, vs. the control group). The data and the error bars denote mean ± standard deviation, n = 3. Scale bars: 100 µm. Please click here to view a larger version of this figure.
| Gene | primer sequences (5'-3') |
| GADPH | F: ACCCAGAAGACTGTGGATGG |
| R: CACATTGGGGGTAGGAACAC | |
| ALP | F: CACGTTGACTGTGGTTACTGCTGA |
| R: CCTTGTAACCAGGCCCGTTG | |
| OCN | F: GGTGGTGAATAGACTCCGGC |
| R: GCAACACATGCCCTAAACGG | |
| Runx2 | F: GCACCCAGCCCATAATAGA |
| R: TTGGAGCAAGGAGAACCC |
Table 1: Primers used in Real-time PCR.
Discussion
Bone marrow mesenchymal stem cells (BMMSCs) represent a subset of non-hematopoietic stem cells residing in bone marrow, characterized by their self-renewal capabilities, multi-directional differentiation potential, and supportive functions for hematopoiesis. These cells play pivotal roles in various physiological processes such as tissue regeneration, angiogenesis, and the regulation of cellular activities20. Consequently, BMMSCs are frequently utilized in tissue repair and regenerative engineering as an optimal seed cell source21.
Jaw bone marrow mesenchymal stem cells (JBMMSCs), initially isolated from human jaw bone marrow aspirates in 2005 by Matsubara et al.22, exhibit distinctive differentiation characteristics attributable to the unique origins and developmental pathways of the jaw compared to other skeletal sites4,23. Given the distinct developmental and pathological mechanisms governing craniofacial bone, prioritizing JBMMSCs in the repair of craniofacial bone defects appears imperative, owing to their homologous developmental traits23. Furthermore, JBMMSCs exhibit heightened histocompatibility with the oral microenvironment owing to their shared embryonic tissue origins24,25. Despite these advantages, a standardized and safe isolation protocol for JBMMSCs remains elusive, and research on JBMMSCs remains relatively limited.
The isolation and cultivation of BMMSCs currently lack standardization, with commonly employed methods including immunomagnetic bead sorting, flow cytometry sorting, density gradient centrifugation, and whole bone marrow adherent culture26. Among these, the immunomagnetic bead method and flow cytometry sorting method isolate cells by recognizing specific antigens on the cell surface. However, these methods involve cumbersome operations, require specialized instruments, and may affect cell activity despite yielding high-purity cells27. The density gradient centrifugation method separates cells through centrifugation and stratification, which can alter the cell microenvironment, leading to slower cell growth and increased cellular aging28. Conversely, the whole bone marrow adherent culture method separates and purifies BMMSCs by intermittently replacing the medium and passaging based on varying adherent abilities among different cell types in the bone marrow. This method inflicts minimal damage to cells and is straightforward to execute16.
Compared with the femur, the jaw is smaller in size and has a narrower bone marrow cavity, with interference from cells in the tooth or periodontal ligament. The bone marrow flushing and adhesion method requires a relatively larger amount of bone marrow and is suitable for large animals and humans29. JBMMSCs in rats extracted by applying the traditional bone marrow flushing method are rare in number, slow to proliferate, and of poor quality30,31. The content of JBMMSCs in bone marrow is very low, and they are mainly found in the compact bone and endosteum. Bu-Kyu Lee et al.32 used the mandibular aspirates to isolate JBMMSCs, while the total aspiration time for 10 mL of marrow blood for the mandible was five times longer than that for the iliac crest, and the initial yield of MSCs from the mandible was three times lower than that from the iliac crest. Flushing the bone marrow alone cannot effectively isolate the cells in the compact bone and endosteum.
In recent years, studies have found that compact bone is a new and reliable source of BMMSCs, and a relatively simple method of BMMSC isolation has been derived, the bone slice digesting culture method33. BMMSCs isolated by this method have high purity34. Guo et al.35have successfully isolated bone mesenchymal stem cells from mouse femurs using the bone slice digesting culture method. Yamazaet al.12 and Cheng et al.15 applied the bone slice digesting culture method to the isolation of mouse mandibular bone marrow mesenchymal stem cells and verified MSC properties by cell proliferation, immunophenotype, and multilineage differentiation. This experiment used a combination of flushed whole bone marrow adhesion and bone slice digesting to isolate and culture primary rat JBMMSCs. The bone marrow was thoroughly washed out from the cavity without filtration to maintain the original cellular composition and growth factors in the bone marrow. Then, the bone slices were cut into small pieces and digested using type II collagenase to facilitate cells to climb out of bone fragments. Finally, the bone slices were inoculated onto a culture dish and cultured to allow cells to crawl out of the bone slices. The combination method makes the culture system of the primary cell simultaneously contain components of hematopoietic stem cells, cancellous bone, and cortical bone, which can better simulate the microenvironment of bone marrow mesenchymal stem cells in the body and is more conducive to maintaining the original biological characteristics of cells. This method is therefore called "a niche-based approach on stemness"36. Luet al.37 also emphasized the importance of stem cell niches in the isolation of BMMSCs, but they only involved the microenvironment in the bone marrow without cortical bone. This method has been successfully applied in isolating bone marrow mesenchymal stem cells from mice jaw38.
We also successfully isolated and cultivated rat JBMMSCs with this niche-based approach. First, the isolated cells exhibited a fibroblast-like shape and adhered to plastic culture plates in vitro. Second, the cells positively expressed the CD90, CD29, and CD44 surface markers and negatively expressed CD45, CD34, and CD11b/c. Third, the cells could differentiate into osteocytes, adipocytes, and chondrocytes. The obtained cells had a good morphology, a fast proliferation rate, and differentiation ability, which met the minimum criteria for identifying human-derived mesenchymal stem cells proposed by the International Society for Cellular Therapy17.
The isolation of JBMMSCs has been developed and used to isolate cells in humans. Matsubara et al.22 were the earliest to isolate JBMMSCs from human alveolar bone marrow samples during oral surgery. Possible complications may occur during the mandible aspiration process, including damage to adjacent tissue, infection due to oral bacterial contamination, etc. Zong et al.39 flushed the bone marrow cavity of the cancellous bone specimen pieces to obtain human JBMMSCs. Park et al.40 isolated human JBMMSCs from alveolar bone chips obtained during implant drilling using a sequential digestion method. Mason et al.41 directly isolated human JBMMSCs from the jaw bone marrow cores and aspirate of patients undergoing routine dental implant placement. There was a high success rate of isolating cells from either aspirates or core and a higher success rate of the combination. However, although the above methods have successfully isolated JBMMSCs from humans, the differences in effectiveness between methods need to be compared. Currently, this presented method has only been used in mice and rats, and there is no research on applying this method to the isolation of human JBMMSCs. There are limitations to this method, especially when it is applied to humans. First, this method of combining bone marrow flushing and bone slice digestion is relatively complicated and requires more steps. Second, complex processes place higher demands on the sterility of operations, which is critical to the protocol. Third, it is impossible to flush the entire bone marrow cavity of the human jawbone, so exploring some equivalent methods, such as flushing cancellous bone fragments, is necessary.
In summary, this study successfully isolated and cultured rat JBMMSCs using a combination of bone marrow flushing and bone slice digestion based on the principle of "stem cell niche." The cell identification results confirmed that this method can isolate sufficient and high-purity JBMMSCs, providing ample cell sources for jawbone tissue engineering, particularly in bone defect repair, which is of great significance.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This study was supported by the Health Care Projects of the Military Commission Logistics Department (19BJZ22), Beijing Natural Science Foundation (7232154), and Fourth Mil Med Univ. clinical research projects (2021XB025).
Materials
| Name | Company | Catalog Number | Comments |
| Alizarin Red S Solution 0.2% | Solarbio | G1450 | |
| BCIP/NBT Alkaline Phosphatase Color Development Kit | Beyotime | C3206 | |
| Bio-Rad CFX96 Real-Time System | Bio-Rad | ||
| CCK8 Kit | Dujindo | CK04 | |
| Cell culture dish 10 cm | Corning | 353003 | |
| Centrifuge | Eppendorf | 5810R | |
| Centrifuge Tube 15 mL | Corning | 430790 | |
| Centrifuge Tube 50 mL | Corning | 430828 | |
| CO2 incubator | Thermo Fisher | 3111 | |
| Constant-temperature oscillator | Shanghai Zhicheng Analysis Instrument Manufacturing Co., Ltd. | ZWY-100H | |
| Fetal bovine serum | BI | 04-001-1ACS | |
| Flow cytometer | BD | FACS C6 | |
| Inverted phase-contrast microscope | Olympus | CKX41 | |
| Mesenchymal Stem Cell (Rat) Surface marker Detection Kit | Oricell | RAXMX-09011 | |
| Multifunctional microplate reader | BioTek | Synergy LX Multi-Mode | |
| Oil Red O Stain Kit | Solarbio | G1262 | |
| Paraformaldehyde 4% | Solarbio | P1110 | |
| PBS | MACGENE | CC008 | |
| penicillin-streptomycin 0.25% | MACGENE | CC004 | |
| PowerUp SYBR Green Master Mix | Thermo Fisher | A25742 | |
| PrimeScript RT Master Mix | Takara | RR036A | |
| Rat Bone Marrow Mesenchymal Stem Cells Adipogenic Differentiation kit | Oricell | RAXMX-90031 | |
| Rat Bone Marrow Mesenchymal Stem Cells Chondrogenic Differentiation kit | Oricell | RAXMX-90041 | |
| Rat Bone Marrow Mesenchymal Stem Cells Osteogenic Differentiation kit | Oricell | RAXMD-90021 | |
| RNA extraction kit | TIANGEN | DP419 | |
| Super-clean bench | Beijing Yataikelong Instrument Technology Co. Ltd. | KLCZ-1220A | |
| Trypsin-EDTA 0.25% | MACGENE | CC012 | |
| Type II collagenase | Solarbio | C8150 | |
| Wistar rat | Beijing Yataikelong Instrument Technology Co. Ltd. | ||
| α-MEM culture medium | Gibco | C12571500BT |
References
- Friedenstein, A. J., Petrakova, K. V., Kurolesova, A. I., Frolova, G. P. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 6 (2), 230-247 (1968).
- Pittenger, M. F., et al. Multilineage potential of adult human mesenchymal stem cells. Science. 284 (5411), 143-147 (1999).
- Shao, J., Zhang, W., Yang, T. Using mesenchymal stem cells as a therapy for bone regeneration and repairing. Biol Res. 48, 62 (2015).
- Aghaloo, T. L., et al. Osteogenic potential of mandibular vs. Long-bone marrow stromal cells. J Dent Res. 89 (11), 1293-1298 (2010).
- Mendi, A., Ulutürk, H., Ataç, M. S., Yılmaz, D. Stem cells for the oromaxillofacial area: Could they be a promising source for regeneration in dentistry. Adv Exp Med Biol. 1144, 101-121 (2019).
- Couly, G. F., Coltey, P. M., Le Douarin, N. M. The triple origin of skull in higher vertebrates: A study in quail-chick chimeras. Development. 117 (2), 409-429 (1993).
- Leucht, P., et al. Embryonic origin and hox status determine progenitor cell fate during adult bone regeneration. Development. 135 (17), 2845-2854 (2008).
- Jeyaraman, M., et al. Is mandible derived mesenchymal stromal cells superior in proliferation and regeneration to long bone-derived mesenchymal stromal cells. World J Methodol. 13 (2), 10-17 (2023).
- Mouraret, S., et al. The potential for vertical bone regeneration via maxillary periosteal elevation. J Clin Peri.odontol. 41 (12), 1170-1177 (2014).
- Li, T. Q., Meng, X. B., Shi, Q., Zhang, T. Research progress in biological characteristics and influencing factors of jaw bone marrow mesenchymal stem cell. Zhonghua Kou Qiang Yi Xue Za Zhi. 57 (1), 107-112 (2022).
- Arthur, A., Gronthos, S. Clinical application of bone marrow mesenchymal stem/stromal cells to repair skeletal tissue. Int J Mol Sci. 21 (24), 9759 (2020).
- Yamaza, T., et al. Mouse mandible contains distinctive mesenchymal stem cells. J Dent Res. 90 (3), 317-324 (2011).
- Chen, L., et al. Directional homing of glycosylation-modified bone marrow mesenchymal stem cells for bone defect repair. J Nanobiotechnology. 19 (1), 228 (2021).
- Hong, Y., et al. Isolation and cultivation of mandibular bone marrow mesenchymal stem cells in rats. J Vis Exp. (162), e61532 (2020).
- Cheng, B., et al. Isolation, culture and biological characteristics of high-purity orofacial-bone-derived mesenchymal stem cells of the rats. Zhongguo Zuzhi Gongcheng Yanjiu. 25 (1), 67-72 (2021).
- Lee, D. J., et al. Osteogenic potential of mesenchymal stem cells from rat mandible to regenerate critical sized calvarial defect. J Tissue Eng. 10, 2041731419830427 (2019).
- Dominici, M., et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 8 (4), 315-317 (2006).
- Fathi, E., Mesbah-Namin, S. A., Vietor, I., Farahzadi, R. Mesenchymal stem cells cause induction of granulocyte differentiation of rat bone marrow c-kit(+) hematopoietic stem cells through jak3/stat3, erk, and pi3k signaling pathways. Iran J Basic Med Sci. 25 (10), 1222-1227 (2022).
- Xu, W., et al. Exosomes from microglia attenuate photoreceptor injury and neovascularization in an animal model of retinopathy of prematurity. Mol Ther Nucleic Acids. 16, 778-790 (2019).
- Chu, D. T., et al. An update on the progress of isolation, culture, storage, and clinical application of human bone marrow mesenchymal stem/stromal cells. Int J Mol Sci. 21 (3), 708 (2020).
- Liu, Y., et al. Systemic infusion of mesenchymal stem cells improves cell-based bone regeneration via upregulation of regulatory T cells. Tissue Eng Part A. 21 (3-4), 498-509 (2015).
- Matsubara, T., et al. Alveolar bone marrow as a cell source for regenerative medicine: Differences between alveolar and iliac bone marrow stromal cells. J Bone Miner Res. 20 (3), 399-409 (2005).
- Zhang, G., Li, Q., Yuan, Q., Zhang, S. Spatial distributions, characteristics, and applications of craniofacial stem cells. Stem Cells Int. 2020, 8868593 (2020).
- Akintoye, S. O. The distinctive jaw and alveolar bone regeneration. Oral Dis. 24 (1-2), 49-51 (2018).
- Park, J. B., Kim, I., Lee, W., Kim, H. Evaluation of the regenerative capacity of stem cells combined with bone graft material and collagen matrix using a rabbit calvarial defect model. J Periodontal Implant Sci. 53 (6), 467-477 (2023).
- Ni, X., et al. Isolation, culture and identification of SD rat bone marrow mesenchymal stem cells in tissue engineering. Materials Exp. 12, 817-822 (2022).
- Abdallah, B. M., Khattab, H. M. Recent approaches to isolating and culturing mouse bone marrowderived mesenchymal stromal stem cells. Curr Stem Cell Res Ther. 16 (5), 599-607 (2021).
- Tan, S. L., Ahmad, T. S., Selvaratnam, L., Kamarul, T. Isolation, characterization and the multilineage differentiation potential of rabbit bone marrow-derived mesenchymal stem cells. J Anat. 222 (4), 437-450 (2013).
- Mason, S., Tarle, S. A., Osibin, W., Kinfu, Y., Kaigler, D. Standardization and safety of alveolar bone-derived stem cell isolation. J Dent Res. 93 (1), 55-61 (2014).
- Kagami, H., Agata, H., Tojo, A. Bone marrow stromal cells (bone marrow-derived multipotent mesenchymal stromal cells) for bone tissue engineering: Basic science to clinical translation. Int J Biochem Cell Biol. 43 (3), 286-289 (2011).
- Liu, J., Watanabe, K., Dabdoub, S. M., Lee, B. S., Kim, D. G. Site-specific characteristics of bone and progenitor cells in control and ovariectomized rats. Bone. 163, 116501 (2022).
- Lee, B. K., Choi, S. J., Mack, D., Oh, S. H. Isolation of mesenchymal stem cells from the mandibular marrow aspirates. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 112 (6), e86-e93 (2011).
- Zhu, H., et al. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nat Protoc. 5 (3), 550-560 (2010).
- Short, B. J., Brouard, N., Simmons, P. J. Prospective isolation of mesenchymal stem cells from mouse compact bone. Methods Mol Biol. 482, 259-268 (2009).
- Guo, Z., et al. In vitro characteristics and in vivo immunosuppressive activity of compact bone-derived murine mesenchymal progenitor cells. Stem Cells. 24 (4), 992-1000 (2006).
- Pal, B., Das, B. In vitro culture of naïve human bone marrow mesenchymal stem cells: A stemness based approach. Front Cell Dev Biol. 5, 69 (2017).
- Lu, L. L., et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica. 91 (8), 1017-1026 (2006).
- Cao, W., et al. Mir-344d-3p regulates osteogenic and adipogenic differentiation of mouse mandibular bone marrow mesenchymal stem cells. PeerJ. 11, e14838 (2023).
- Zong, C., Zhao, L., Huang, C., Chen, Y., Tian, L. Isolation and culture of bone marrow mesenchymal stem cells from the human mandible. J Vis Exp. (182), e63811 (2022).
- Park, J. C., et al. Acquisition of human alveolar bone-derived stromal cells using minimally irrigated implant osteotomy: In vitro and in vivo evaluations. J Clin Periodontol. 39 (5), 495-505 (2012).
- Mason, S., Tarle, S. A., Osibin, W., Kinfu, Y., Kaigler, D. Standardization and safety of alveolar bone-derived stem cell isolation. J Dent Res. 93 (1), 55-61 (2014).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved