Polymerization
Przegląd
Source: Vy M. Dong and Jan Riedel, Department of Chemistry, University of California, Irvine, CA
Polymers are made from macromolecules, which are composed of repeating units (the so called monomeric units). In our modern world, polymers play an important role. One of the first important polymers was nylon, which is a polyamide. It found widespread application in tooth brushes and stockings.
Zasady
There are two main types of polymerization. One is the chain growth reaction, which can be differentiated into radical, cationic, anionic, and coordinative polymerization. Step-growth polymerization is the other main method to make polymers. Bi-functional or multifunctional monomers react to ultimately form polymers. Step-growth polymerization can be further differentiated into condensation polymerization and addition polymerization.
In an addition polymerization, monomers will add to one another to form the polymer. Whereas in a condensation polymerization, two monomers will add in a condensation reaction under the release of water or another small molecule like hydrogen chloride.
In the following synthesis of a polyamide, a dicarboxylic acid chloride condenses with a diamine to form a polyamide, under the release of hydrogen chloride. The notation 6,10 in the name of polyamide-6,10 reflects the number of carbons in the diamine monomer (six in this case) and the number of carbons in the dicarboxylic acid chloride monomer (ten in this case).
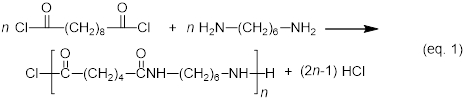
One characteristic of a step-growth polymerization is the dependence of the chain length and the conversion of the polymerization. At the beginning of the reaction most monomers will condense to mainly form dimers and trimers. With further progress, the dimers and trimers will combine to oligomers and only after high conversion rates, when most monomers have reacted, the oligomers will condense to form polymers with a high number of monomeric units. This phenomenon is illustrated in Figure 1.
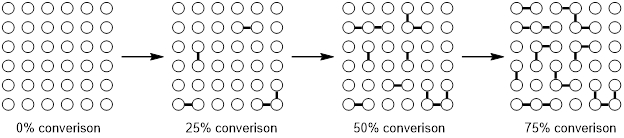
Figure 1. Condensation Polymerization.
In this experiment, the polyamide forms through a so-called surface polymerization. In an inhomogeneous solution, consisting of an aqueous and organic solution, the polymerization will take place at the interface of the two layers. The diamine monomer is dissolved in water, while the dicarboxylic acid is dissolved in an organic solvent.
Procedura
1. Preparation
- To a 250 mL beaker, add 3 mL (14 mmol) of sebacoyl chloride in 100 mL n-hexane.
- To a 150 mL beaker, add 4.4 g (38 mmol) of 1,6-diaminohexane in 50 mL distilled water.
- Add approximately 5 drops of a phenolphthalein solution to the 1,6-diaminohexane solution.
2. Surface Polymerization
- Overlay carefully the aqueous solution with the sebacoyl chloride solution.
- Note that a thin layer will form at the interface of the aqueous solution and the organic solution.
- Note that the added phenolphthalein will make the interface visible.
3. Collecting the Polymer
- Pull the formed polyamide film from the interface with tweezers and wrap it around a glass rod.
- Wind the polyamide on the glass rod.
- Wash the polymer with acetone and then with copious amounts of water.
- Dry the polymer at 50 °C under reduced pressure.
Wyniki
A hollow, long strand of polyamide is obtained.
Wniosek i Podsumowanie
This experiment showcases in a vivid way the synthesis of a polymer in a simple fashion. The condensation polymerization of 1,6-diaminohexane and sebacoyl chloride gives a polyamide-6,10, which polymerizes at the interface of two liquid layers. This surface polymerization will form a hollow strand of polyamide, after pulling the polymer from the interface and wrapping it around a glass rod to wind it.
Polymers and polyamide find a variety of different uses in our daily life. Initially used for toothbrushes and stockings. Today, polyamide is used in the production of textiles, like rain coats, outdoor clothes, lab coats, and flight jackets. Due to its strength and toughness, polyamides are also used in parachutes, climbing ropes, and sails. These applications make polyamides one of the most important polymers in use today.
Tagi
Przejdź do...
Filmy z tej kolekcji:

Now Playing
Polymerization
Organic Chemistry II
93.9K Wyświetleń

Cleaning Glassware
Organic Chemistry II
123.5K Wyświetleń

Nucleophilic Substitution
Organic Chemistry II
99.5K Wyświetleń

Reducing Agents
Organic Chemistry II
43.1K Wyświetleń

Grignard Reaction
Organic Chemistry II
149.0K Wyświetleń

n-Butyllithium Titration
Organic Chemistry II
47.8K Wyświetleń

Dean-Stark Trap
Organic Chemistry II
100.2K Wyświetleń

Ozonolysis of Alkenes
Organic Chemistry II
67.0K Wyświetleń

Organocatalysis
Organic Chemistry II
16.6K Wyświetleń

Palladium-Catalyzed Cross Coupling
Organic Chemistry II
34.4K Wyświetleń

Solid Phase Synthesis
Organic Chemistry II
41.0K Wyświetleń

Hydrogenation
Organic Chemistry II
49.6K Wyświetleń

Melting Point
Organic Chemistry II
149.9K Wyświetleń

Infrared Spectroscopy
Organic Chemistry II
214.7K Wyświetleń

Polarimeter
Organic Chemistry II
99.9K Wyświetleń
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone