Method Article
Sampling of N-methyl-N-nitrosourea-induced Rat's Retina Damage and Multi-index Evaluation of Pathological Changes
W tym Artykule
Podsumowanie
This protocol describes a procedure for rat retinal sampling and utilizing hematoxylin-eosin staining, TUNEL assay, and Western blot to detect pathological changes and apoptosis in the retina after intraperitoneal injection of N-Methyl-N-Nitrosourea.
Streszczenie
Retinopathy can be observed in most ocular diseases and the late complications of diabetes mellitus. The specific pigment epithelial cells and optic cells' damage and degeneration are the main features of retinopathy. Many traditional medicines have shown substantial clinical efficacy in treating retinopathy. How to obtain a retina quickly and completely is a key step in traditional medicine research for the treatment of retinopathy. In this study, we aim to provide a standardized and exercisable procedure for sampling of N-methyl-N-nitrosourea (MNU)-induced rat's retina damage and multi-index evaluation of pathological changes. Rats were injected intraperitoneally with 60 mg/kg MNU once to induce retina damage, and retina samples were obtained after 7 days. Additionally, we performed hematoxylin-eosin staining to assess retinal pathological changes. Determination of the apoptosis rate and apoptosis protein by TUNEL and Western blot. These standardized protocols for retinal sampling and evaluation of pathological changes are helpful in promoting the exploration of the mechanism of retinopathy and the discovery of novel and effective traditional herbs.
Wprowadzenie
Retinitis pigmentosa (RP) is a hereditary and blinding retinal disease1. The incidence of RP is between 1/3500 and 1/5000, affecting the visual function of about 2.5 million people in the world. It is one of the most well-known diseases leading to visual impairment in human beings, imposing a significant burden on the whole society2. The disease is characterized by the gradual loss of retinal pigment epithelial cell function and the progressive apoptosis of photoreceptors. In the early stage, patients experience night blindness, which manifests as peripheral visual field defects and eventually leads to the loss of central vision3. Therefore, inhibition of retinal photoreceptor apoptosis is the pointcut for the prevention and treatment of RP.
Retinal cell apoptosis is a common feature of human RP and model animals4. Intraperitoneal injection of 60 mg/kg N-Methyl-N-Nitrosourea (MNU) in rats for 7 days can induce apoptosis and loss of retinal photoreceptor cells, and it is a commonly used animal model of RP5,6. Analyzing the changes in pathological structure, specific cell apoptosis, and apoptosis-related protein expression of retinal tissue in model animals can provide effective experimental data and theoretical support in studying the pathogenesis of human RP and screening drugs7,8. Therefore, the quality of retinal specimens determines the reliability of experimental data. However, due to the particularity of ocular tissue, there are very few reports on how to get the rat retina9.
This paper provides a simple, fast, standardized, and operable procedure for retinal sampling in rats to overcome the above shortcomings. Hematoxylin-eosin staining (HE), terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate-digoxigenin nick-end labeling (TUNEL) staining, and Western blot are used to analyze the pathological changes in retinal tissue damage and apoptosis in the rats. All the methods are derived from our research group's experience with the specific operational processes.
Protokół
All protocols and surgical procedures were approved by the Ethics Committee of Ningxia Medical University (Ethics number: NO. 2020-Q066). SPF Sprauge-Dawley (SD) male rats, aged 7-8 weeks and weighing 200-220 g, were purchased from Ningxia Medical University with the animal license number SCXK (Ning) 2020-0001. All the animals were raised in the Laboratory Animal Center of Ningxia Medical University. The temperature and humidity were suitable, the day-night light cycle was maintained, and the food and water were free and sufficient.
1. Preoperative preparation
- Preparation of materials
- Prepare rat plates, inhalation anesthesia devices, embedded boxes, frozen storage tubes, disposable surgical gloves and masks, wide-mouth bottles, 4% paraformaldehyde, ice packs, ophthalmic scissors, ophthalmic tweezers, surgical blades and knife handles, 1.5 mL microcentrifuge tubes and sterilize them in advance (see Table of Materials).
- Animal preparation
- Randomly divide 20 male SD rats into model group (n=10) and control group (n=10). Inject model rats intraperitoneally with 60 mg/kg MNU and inject control rats intraperitoneally with the same volume of saline.
- After 7 days of injection, fast the rats and allow them to drink freely the night before sampling.
- The next day, place the rat supine on the operating table and use a mask to administer anesthesia using 4% isoflurane and 2 L/min oxygen. Measure the depth of anesthesia by pinching the center of the foot to observe the rats' reactions.
2. Retinal sampling for protein factor detection
- Fix the eye socket of the rat with the left hand and remove the eye with moderate force with the right hand using an ophthalmic bending forceps. Place the eyeball in a sagittal position in a cold glass dish.
- With the left hand holding ophthalmic straight tweezers to fix the eyeball and the right hand holding the surgical blade, make a 2 mm longitudinal incision near the lens site.
- Cut off the cornea with the ophthalmic scissors along the incision, and gently peel off the lens, vitreous, and expose eye cup.
- Place the bottom of the eye cup at the tip of the 1.5 mL microcentrifuge tube, turn the eye cup over the tube, and expose the retina (the retina is faint yellow).
- Remove the entire retina along the scleral wall of the eyeball with ophthalmic bending tweezers, then place it in a frozen tube and store it in a liquid nitrogen tank for future use (Figure 1).
3. Retinal sampling for pathological examination and specific cell staining
- With the left hand, use the eye tweezers to lift the corner of the rat's eye. With the right hand, use a surgical blade to cut a 4 mm opening along the corner of the rat's eye.
- Use the eye tweezers to clamp the edge of the eyeball at the opening in the left hand. Use ophthalmic scissors to cut off the surrounding tissues along the edge of the eyeball while paying attention to cleaning the surrounding tissues of the eyeball.
- Separate the tissues around the optic nerve at the posterior pole of the eyeball with eye tweezers, cut the optic nerve, and pay attention to preserving it. Then, remove the eyeball.
- Wash the removed eyeball in saline to remove any remaining blood, roll it on filter paper to absorb excess water, and place it on cold tin foil.
- Fix the eye with ophthalmic tweezers. Make a 4 mm longitudinal incision along the junction of the cornea and retina with a surgical blade.
- Remove the cornea with ophthalmic scissors, make a circular cut along the incision, and gently remove the lens and vitreous body.
- Place the remaining eye cup with an optic nerve in an embedding box and fix it in 4% paraformaldehyde.
- Embed paraformaldehyde-fixed samples in paraffin and cut into 4 µm slices for HE staining and TUNEL staining7.
4. HE staining of rat retina
- Dewaxing and hydration: Place the slices in xylene for 10 min, repeat the process for another 10 min, followed by absolute ethanol for 5 min, repeat ethanol treatment for another 5 min, followed by treatment in 95% alcohol for 5 min, then in 85% alcohol for 5 min, and finally in 75% alcohol for 5 min.
- Hematoxylin staining: Rinse the slice with deionized water for 5 min. Use 100 µL of hematoxylin staining solution and stain for 5 min, rinse with deionized water for 5-10 s. Add 100 µL of 1% hydrochloric acid ethanol for 30 s, rinse with deionized water for 20 min, and add 100 µL of 0.2% ammonia water for 1 min. Rinse with deionized water for 5 min.
- Eosin staining: Use 100 µL of eosin staining solution to stain for 5 min and rinse with deionized water for 30 s.
- Dehydration and seal: Immerse the slices in 70% alcohol for 5 min, then 85% alcohol for 5 min, followed by 90% alcohol for 5 min, and absolute ethanol for 5 min. Repeat the absolute ethanol treatment for another 5 min, then immerse in xylene for 5 min, and repeat the process for another 5 min. Finally, seal by adding approximately 100 µL of neutral resin to the surface of the slices and then cover it with a cover glass.
- Imaging the slides: Position the stained slices on the microscope, adjust the microscope's focus until the image is clearly visible, set the magnification at 400x, and capture the image.
- Measure the total retinal thickness and outer retinal thickness: Measure the total retinal thickness and outer retinal thickness (thickness of the outer nuclear layer and photoreceptor layer), and calculate the outer retinal thickness percentage
Outer retinal thickness (%) = (outer retinal thickness/ total retinal thickness) x 100.
5. Determination of apoptosis rate of retinal photoreceptor cells with TUNEL method
- Dewaxing and hydration: Place the slices in xylene for 10 min, repeat the process for another 10 min, then place in absolute ethanol for 5 min, and repeat this step for another 5 min. Perform dehydration by placing in 95% alcohol for 5 min, 90% alcohol for 5 min, 80% alcohol for 5 min, 70% alcohol for 5 min, and rinse with deionized water for 5 min.
- Rinse with 0.85% sodium chloride solution for 5 min and PBS for 5 min. Fix with paraformaldehyde using 4% paraformaldehyde for 15 min. Rinse with PBS for 5 min, and repeat the process for another 5 min.
- Incubation and equilibration7: Incubate with 20 µg/mL proteinase K solution for digestion for 15 min, equilibrate in the equilibrium buffer for 10 min, and incubate with recombinant terminal deoxynucleotidyltransferase (rTdT) buffer at 37 °C for 60 min.
- Stopping reaction: Add 100 µL the standard citrate solution (2x SSC solution) for 15 min. Rinse with PBS for 5 min, and repeat the process for another 5 min.
- Nuclear dye: Add 100 µL 4 ′, 6-diamidine-2-phenindl (DAPI) reagent for 5 min. Wash with deionized water for 5 min, and repeat the process for another 5 min.
- Seal: Wipe excess xylene around the slices, and seal by adding approximately 100 µL of neutral resin to the surface of the slices, and then cover it with a cover glass.
- Microscopy: Place the stained slices on the microscope, adjust the microscope's focus for clear visibility, set the magnification to 400x, and capture the image. Observe the green fluorescence at 520 ± 20 nm using a standard fluorescence filter. Observe the blue DAPI at 460 nm.
- Calculate the apoptosis rate: measure the green fluorescence values and blue fluorescence values of the outer retinal nucleus layer. Calculate the apoptosis rate as
Apoptosis cell rate = green fluorescence value/blue fluorescence value x 100.
6. Western blot analysis 10
- Lysis buffer treatment: Mince and homogenize one frozen retina with 50 µL of cold lysis buffer (1 mL of lysis buffer containing 5 µL of phosphatase inhibitor, 1 µL of protease inhibitor, and 5 µL of 100 mM PMSF).
- Subject to a 28 kHz sonication for 5 s on an ice bath; repeat 3x. Centrifuge at 4 °C for 5 min at 10,000 x g to remove cellular debris. Keep the supernatant.
- em>6.3Quantification of protein: Determine the protein concentration using a BCA protein assay reagent kit.
- em>6.4Electrophoresis: Load 50 µg protein sample and separate on 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE).
- Transfer to membrane: Electrophoretically transfer to PVDF membranes using an electrophoretic transfer system.
- Subdivide membranes: Subdivide the membranes, and analyze each protein of interest, β-actin and GAPDH from a single transfer.
- Blocking: Block the membranes for 1 h in TBST containing 5% non-fat milk at room temperature.
- Incubate primary antibody: Incubate with primary antibody of cleaved caspase-9 (1:200 dilution), cleaved caspase-3 (1:200 dilution), cleaved caspase-7 (1:200 dilution), and β-actin (1:1000 dilution) overnight at 4 °C. Wash the membranes with TBST for 5 min, repeat 3x.
- Incubate second antibody: Incubate with horseradish peroxidase-conjugated secondary antibodies (1:2000) for 2 h at room temperature. Wash the membranes with TBST for 5 min, repeat 3x.
- Exposure: Visualize the labeled proteins using Western blot substrate, and finally, expose the membranes to visualize the protein bands in an automatic exposure mode.
- Calculate the protein content7 by measuring the protein band density.
7. Statistical analysis
- Analyze the data using the SPSS 19.0 software and present it as the mean ± standard deviation. Perform statistical analyses of observed values using one-way analysis of variance. Take p< 0.05 as statistically significant.
Wyniki
After HE staining, all retinal layers of rats in the control group had clear tissue structure, orderly cell arrangement, and uniform staining, while the retinal structure of rats in the model group was significantly damaged, the outer retinal layer was thinner, the outer nuclear layer was almost completely integrated with the inner core layer, the arrangement of cells in the layer was extremely disordered, the number of cell layers was significantly reduced, and the thickness of the photosensitive cell layer and outer nuclear layer was significantly thinner. The thickness of the outer retinal layer in the control group and model group was 48% and 23%, respectively (p < 0.01; Figure 2).
The TUNEL method showed that no apoptotic cells were detected in the control group, but there were some apoptotic cells in the outer nuclear layer of rats in the model group (p < 0.01), and the TUNEL-positive ratio was 9.6% (Figure 3).
The caspase family of proteins is one of the major families that execute apoptosis. Among them, cleaved caspase 3, cleaved caspase 7, and cleaved caspase 9 are classical indicators used to evaluate cell apoptosis7. The results of Western blot showed that compared with the control group, the expression of cleaved caspase 3, cleaved caspase 7, and cleaved caspase 9 in the retina of the model group was significantly increased (p<0.01; Figure 4). This indicated that MNU can clearly cause cell apoptosis in the retina.

Figure 1: The experimental workflow. After intraperitoneal injection of MNU for 7 days (7d), the retina of anesthetized rats was obtained for HE and TUNEL staining. Abbreviations: MNU = N-methyl-N-nitrosourea; HE = hematoxylin-eosin; TUNEL = terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate-digoxigenin nick-end labeling. Please click here to view a larger version of this figure.

Figure 2: Representative histopathological HE staining images on day 7 after MNU injection. (A) HE staining on 7 days (7d) after MNU administration. (B) Outer retinal ratio. Data are presented as mean ± SD (n=6). Statistical analyses of observed values were performed using one-way analysis of variance. ** p < 0.01 vs control. Abbreviations: GCL = ganglion cell layer; IPL = inner plexiform layer; INL = the kernel layer; OPL = outer plexiform layer; ONL = outer nuclear layer; PRL = photoreceptor layer; MNU = N-methyl-N-nitrosourea; HE = hematoxylin-eosin. n = 6. Please click here to view a larger version of this figure.
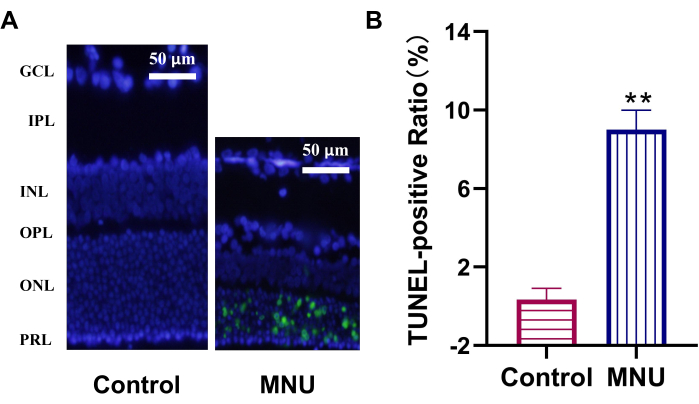
Figure 3: TUNEL assay shows photoreceptors apoptosis induced by MNU in rats. (A) At 7 days (7d) after MNU injection, TUNEL-positive cells (green) were presented in the ONL in MNU-treated rats. (B) TUNEL-positive ratio. Data are presented as mean ± SD (n=6). Statistical analyses of observed values were performed using one-way analysis of variance. ** p < 0.01 vs control. Abbreviations: GCL = ganglion cell layer; IPL = inner plexiform layer; INL = the kernel layer; OPL = outer plexiform layer; ONL = outer nuclear layer; PRL = photoreceptor layer; MNU = N-methyl-N-nitrosourea; TUNEL = terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate-digoxigenin nick-end labeling. n = 6. Please click here to view a larger version of this figure.

Figure 4: Expression of cleaved caspase 3, cleaved caspase 7, and cleaved caspase 9 in rat retinas on day 7 after MNU injection. (A) Representative data for cleaved caspase expression. (B) Ratio of cleaved caspase 3/β-actin. (C) Ratio of cleaved caspase 7/β-actin. (D) Ratio of cleaved caspase 9/β-actin. Data are presented as mean ± SD (n=6). Statistical analyses of observed values were performed using one-way analysis of variance. ** p < 0.01 vs control. Please click here to view a larger version of this figure.
Dyskusje
RP is a common retinal disease in clinics. The onset, severity, and progression of RP are related to genes and genetic modes and are affected by the environment8,11. RP includes familial RP and occasional RP, of which familial RP accounts for about 60% of the patients. Through tracing the family genetic history, 83 genes related to RP have been identified so far, while occasional RP accounts for about 40%, which means this kind of patient lacks a family history and has an irregular genetic pattern2. Although the apoptosis of retinal photoreceptor cells, causing the loss of visual function in RP patients is a general recognition, the underlying mechanism of photoreceptor death in RP remains unclear12,13,14. Due to the limited access to human retinal tissue, which is mainly obtained during vitrectomy, the extraction of retinal tissue from model animals is still a common method in the basic research of RP14,15.
The retina is a transparent membrane located in the inner layer of the bulb of the eye, with the vitreous cavity inside and the choroid membrane close to the outside. The retina starts from the serrated edge in the front and ends at the optic disc in the back. It is the initial part of vision formation16. Retinal samples of model animals are mainly used for retinal PCR analysis, Western blot detection, pathological tissue structure observation, immunohistochemical staining, immunofluorescence examination, microvascular density evaluation, cell isolation, and culture, etc.17,18. The requirements of retinal sampling for different test indexes are different. Retinal sampling for protein factor detection in model animals and retinal sampling for paraffin-embedded sections are common operational techniques for screening drugs for RP8. However, due to the very fine and complex structure of the eyeball of the model animal, the process of removing interfering tissues in the eye and obtaining retinal specimens is complicated. During the sampling process, there may be retinal tissue loss, contamination, protein degradation, and retinal detachment. These factors will have an impact on the quality and quantity of retinal protein extraction, as well as the effect of section staining and the detection results of experimental indicators. Therefore, an appropriate retinal sampling procedure is needed.
It is critical to simplify the complex and grasp the key link of harvesting in order to improve the technique of retinal harvesting in model animals. Rapid exposure of the retina and complete removal of the retina as far as possible are the key steps of retinal sampling for protein factor detection in model rats. In this study, the eyeballs were extracted directly after anesthesia to save the sampling time. A trick to expose the retina is to turn the eye cup over a 1.5 mL tube, which was cost-effective and practical and could completely peel the retina from the scleral wall. This operation procedure is simple, fast, cost-saving, and conducive to the experimental personnel operation practice, so it can be completed in 5 min. In the process of retinal sampling for pathological examination and specific cell staining, maintaining the freshness and structural integrity of the retina is a key step to obtaining high-quality staining. Perfusion with formaldehyde from the left atrium and then obtain the retina is one of the methods researchers often use, but it is time-consuming, and formaldehyde smells bad and brings discomfort to the operator19. In this study, the tissue around the eyeball is separated into a ring after anesthesia to maintain its integrity. After the optic nerve has been cut and retained, separate the tissue around it at the posterior pole of the eyeball and remove the eyeball. Different parts of the eyeball were accurately located with the optic nerve. Finally, with the support of the eye contents, the incision was made, the cornea was cut off in a ring, the lens and vitreous were peeled off, and the eye cup connecting the optic nerve was obtained (the retina is located on the scleral wall inside the eyecup). In this process, the operation should be gentle to maintain the integrity of the retina. The operation procedure does not only greatly improve the speed of collecting materials, but it can also maintain the complete normal structure and shape of the eyeball and can be accurately located in the different parts of the eyeball after taking out the eyeball. After a skillful operation, the operation can be completed in 8 min. By using the above retinal sampling methods, our research group obtained relatively ideal protein factor detection results, HE staining results, and TUNEL staining results.
Although the current retinal sampling method has some advantages, it still needs to be improved. In the process of retinal sampling for protein factor detection, after exposing the retina, there is also the risk of incomplete retinal dissection or loss when taking the retina by bending tweezers due to the limited support point of bending tweezers. Therefore, it is necessary to improve the tools for retinal dissection. On the other hand, in the process of retinal extraction for pathological examination and specific cell staining, after isolating the eyeball, during the process of lens and vitreous stripping, the eyeball wall will collapse due to the loss of support from the eye, which is easy to cause retinal stripping. Thus, the operation needs to be fast, gentle, and of appropriate strength, and the operator needs to practice frequently. Therefore, our retinal sampling procedures still need to be improved in order to promote the research on the protective effect and mechanism of traditional medicine against RP to a greater extent.
Ujawnienia
The authors have nothing to disclose.
Podziękowania
This work was supported by the Scientific Research Project of the Higher Education Department of Ningxia (NYG2022029).
Materiały
| Name | Company | Catalog Number | Comments |
| Amersham Imager | GE | 680 | |
| Ammonium persulfate | Boster Biological Technology Co.,Ltd | A8090 | |
| Analytical Balance | Mettler Toledo | ME104E | |
| BCA protein quantization kit | Ken Gen Biotech. Co. Ltd | KGP902 | |
| cleaved caspase-3 antibody | Cell Signaling Technology | #9664 | |
| cleaved caspase-7 antibody | Cell Signaling Technology | #8438 | |
| cleaved caspase-9 antibody | Cell Signaling Technology | #9507 | |
| DeadEnd Fluorometric TUNEL System | Promega | G3250 | |
| Glycine | Boster Biological Technology Co.,Ltd | AR1200 | |
| Goat Anti-Rabbit IgG H&L (HRP) | Bioss Antibodies | bs-80295G-HRP | |
| Goat serum | Biosharp | BL210A | |
| High speed crusher | Thermo Fisher Scientific | AG22331 | |
| Immobilon-P SQ Transfer membranes | Merck Millipore. Ltd | ISEQ00010 | |
| Isoflurane | RWD Life Science | R510-22-10 | |
| Methanol | Chengdu Kelong Chemical Co., Ltd | 20230108 | |
| Microplate Reader | Thermo Fisher Scientific | 1510 | |
| Microscope | Olympus | IX73 | |
| Microscope slide | Citotest Labware Manufacturing Co., Ltd | 7105P-G | |
| Mini-PROTEAN Tetra | BIO-RAD | 1658001 | |
| N-Methyl-N-Nitrosourea | sigma-Aldrich | N4766–100G | |
| Oven | Shanghai Yuejin Medical Equipment Co., Ltd | DHG-8145 | |
| Page Pre-solution (30% ) | Doublehelix Biology Science and Technology Co.,Ltd | L3202A | |
| PageRuler Prestained Protein Ladder | Thermo Fisher Scientific | 26617 | |
| PBS buffer | Biosharp | G4202 | |
| SDS-PAGE Protein loading buffer (5×) | Beyotime Biotechnology | P0015 | |
| Skim milk powder | BioFroxx | 1172GR500 | |
| Sprague Dawley rats | Ningxia Medical University | SCXK (Ning) 2020-0001 | |
| TEMED | Boster Biological Technology Co.,Ltd | AR1165 | |
| Total protein extraction kit | Ken Gen Biotech. Co. Ltd | KGP2100 | |
| Trans-Blot Module | BIO-RAD | 1703935 | |
| Tris base | Boster Biological Technology Co.,Ltd | AR1162 | |
| Tweezer | Changde BKMAM Biotechnology Co., Ltd | 130302027 | |
| β-actin | Cell Signaling Technology | #4970 |
Odniesienia
- Cehajic-Kapetanovic, J., et al. Association of a novel intronic variant in RPGR with hypomorphic phenotype of X-linked retinitis pigmentosa. JAMA Ophthalmol. 138 (11), 1151-1158 (2020).
- Pan, M. Y., et al. Mice deficient in UXT exhibit retinitis pigmentosa-like features via aberrant autophagy activation. Autophagy. 17 (8), 1873-1888 (2021).
- Xiong, Y. C., et al. 17β-Oestradiol attenuates the photoreceptor apoptosis in mice with retinitis pigmentosa by regulating N-myc downstream regulated gene 2 expression. Neuroscience. 452, 280-294 (2021).
- Zhang, S., et al. Müller cell regulated microglial activation and migration in rats with -Methyl--Nitrosourea-Induced retinal degeneration. Front Neurosci. 14, 606486 (2020).
- Karine, B., et al. Transferrin non-viral gene therapy for treatment of retinal degeneration. Pharmaceutics. 12 (9), 836 (2020).
- Yan, W. M., et al. Protection of retinal function and morphology in MNU-induced retinitis pigmentosa rats by ALDH2: an in-vivo study. BMC Ophthalmol. 20 (1), 55 (2020).
- Zhu, Y. F., et al. Lycium barbarum polysaccharides attenuates N-methy-N-nitrosourea-induced photoreceptor cell apoptosis in rats through regulation of poly (ADP-ribose) polymerase and caspase expression. J Ethnopharmacol. 191, 125-134 (2016).
- He, S. Q., et al. Effects and mechanisms of water-soluble Semen cassiae polysaccharide on retinitis pigmentosa in rats. Food Sci Technol. 40 (1), 84-88 (2020).
- Chen, Q., Cheng, Z. H., Hu, B. J. Current situation of vitreous and retinal related tissue specimens collection and application. Chinese J Ocular Fundus Dis. 36 (5), 396-399 (2020).
- Zhang, Y., et al. Salidroside modulates repolarization through stimulating Kv2.1 in rats. Eur J Pharmacol. 977, 176741 (2024).
- Deng, F. Y., Han, M. Y., Deng, T. T., Jin, M. Research progress of gene therapy for retinitis pigmentosa. Int Eye Sci. 21 (7), 1205-1208 (2021).
- Lin, B., Youdim, M. B. H. The protective, rescue and therapeutic potential of multi-target iron-chelators for retinitis pigmentosa. Free Radic Biol Med. 174, 1-11 (2021).
- Carullo, G., et al. Retinitis pigmentosa and retinal degenerations: deciphering pathways and targets for drug discovery and development. ACS Chem Neurosci. 11 (15), 2173-2191 (2020).
- Zhang, Z. J., et al. Quantification of microvascular change of retinal degeneration in Royal College of Surgeons rats using high-resolution spectral domain optical coherence tomography angiography. J Biomed Opt. 28 (10), 106001 (2023).
- Loiseau, A., Raîche-Marcoux, G., Maranda, C., Bertrand, N., Boisselier, E. Animal models in eye research: focus on corneal pathologies. Int J Mol Sci. 24 (23), 16661 (2023).
- Gregory-Evans, K. A review of diseases of the retina for neurologists. Handb Clin Neurol. 178, 1-11 (2021).
- Li, X. M., et al. Targeting long noncoding RNA-AQP4-AS1 for the treatment of retinal neurovascular dysfunction in diabetes mellitus. EBioMedicine. 77, 103857 (2022).
- Lee, D., et al. Retinal degeneration induced in a mouse model of ischemia-reperfusion injury and its management by pemafibrate treatment. FASEB J. 36 (9), e22497 (2022).
- Zhang, Q. L., Zhao, N., Li, Z. J. Effects of salidroside on retinopathy in diabetic rats based on COX-2/PGE2/VEGF pathway. J China Medical Uni. 52 (8), 731-935 (2023).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone