Method Article
Multi-modal Pulmonary Imaging: Using Complementary Information from CT and Hyperpolarized 129Xe MRI to Evaluate Lung Structure-Function
W tym Artykule
Podsumowanie
CT and 129Xe MRI provide complementary lung structure-function information that can be exploited for regional analysis using image registration. Here, we provide a protocol that builds from the existing literature for 129Xe MR to CT image registration using open-source platforms.
Streszczenie
Hyperpolarized 129Xe gas MRI is an emerging technique to evaluate and measure regional lung function including pulmonary gas distribution and gas exchange. Chest computed tomography (CT) still remains the clinical gold standard for imaging of the lungs, though, in part due to the rapid CT protocols that acquire high-resolution images in seconds and the widespread availability of CT scanners. Quantitative approaches have enabled the extraction of structural lung parenchymal, airway and vascular measurements from chest CT that have been evaluated in many clinical research studies. Together, CT and 129Xe MRI provide complementary information that can be used to evaluate regional lung structure and function, resulting in new insights into lung health and disease. 129Xe MR-CT image registration can be performed to measure regional lung structure-function to better understand lung disease pathophysiology, and to perform image-guided pulmonary interventions. Here, a method for 129Xe MRI-CT registration is outlined to support implementation in research or clinical settings. Registration methods and applications that have been employed to date in the literature are also summarized, and suggestions are provided for future directions that may further overcome technical challenges related to 129Xe MR-CT image registration and facilitate broader implementation of regional lung structure-function evaluation.
Wprowadzenie
Hyperpolarized gas magnetic resonance imaging (MRI) first emerged as a novel functional pulmonary imaging modality to evaluate pulmonary ventilation distribution nearly three decades ago1. Since then, research studies using hyperpolarized gas MRI have revealed numerous insights into the nature of lung function in patients with chronic lung diseases such as asthma, chronic obstructive pulmonary disease (COPD), and cystic fibrosis2,3,4,5,6. Both hyperpolarized 3He and 129Xe gas have been used historically; however, 129Xe is now the primary inhaled agent because of the limited availability of 3He gas. 129Xe also freely diffuses across the alveolar membrane and is absorbed by red blood cells in the pulmonary capillaries; in this so-called 'dissolved phase,' 129Xe resonates at unique frequencies that allow for measurement of regional gas exchange in a single breath-hold scan4,7,8. For quantification, volume-matched anatomical 1H MR images are typically contemporaneously acquired for co-registration with 129Xe to delineate boundaries of the thoracic cavity. Conventional 1H MRI, though, does not provide further lung structural information. The impetus for clinical translation of hyperpolarized 129Xe MRI grew in recent years with UK NHS approval in 2015 and US FDA approval in late 20225,9, yet advanced structural characterization is still mostly missing from the pulmonary MRI arsenal.
Chest computed tomography (CT) remains the mainstay of clinical imaging assessment of the lungs, providing three-dimensional high-resolution images of lung structure using conventional imaging protocols. Quantitative approaches have enabled rapid and repeatable measurement of parenchymal integrity, such as emphysema and interstitial lung abnormalities, large airway morphology and pulmonary vasculature, and regional anatomic characterization by identification and segmentation of lung lobes10,11. In the research space, quantitative CT has been widely used to better understand structural alterations and their relationships with patient outcomes in asthma and COPD in large observational studies such as the Severe Asthma Research Program (SARP)12, Genetic Epidemiology of COPD (COPDGene)13, Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS)14, Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE)15, and Canadian Cohort of Obstructive Lung Disease (CanCOLD)16. Alternative CT methods such as expiratory imaging17,18 or computational models19 may derive functional information, but these methods are indirect, and conventional CT does not otherwise provide much for functional characterization of the lungs.
Taken together, CT and 129Xe MRI provide complementary lung structure-function information that can be exploited for regional analysis using image registration. Lung lobes identified on CT have allowed for lobar characterization of MRI ventilation patterns in asthma20,21,22, COPD23,24, bronchiectasis25, and lung cancer26,27. MRI ventilation abnormalities in asthma have also been directly spatially matched to abnormally remodeled large airways28,29,30,31 and air trapping indicative of small airways dysfunction20,32 measured on CT, and to probe regional treatment responses following whole-lung bronchial thermoplasty33. In COPD, MRI ventilation abnormalities have been linked to small airway dysfunction in milder diseases and emphysema in more severe diseases34,35,36. Beyond ventilation imaging in obstructive lung disease, heterogeneous spatial relationships between CT interstitial lung abnormalities and 129Xe MRI gas exchange patterns have also been demonstrated in idiopathic pulmonary fibrosis37. Such studies have provided a deeper understanding of regional lung structure-function in a range of lung diseases that can be used to inform future image-guided interventions.
Direct registration of anatomic CT and functional hyperpolarized gas MRI is challenging, however, due to fundamentally different imaging contrast between the two methods, the absence of hyperpolarized gas signal in regions of ventilation abnormalities, and potentially different lung volumes. Figure 1 shows four examples of 129Xe and paired anatomical 1H MRI and CT in a healthy volunteer (Figure 1A) and three participants with chronic obstructive pulmonary disease (COPD; Figure 1B-D), highlighting heterogeneous 129Xe ventilation patterns and varying missing lung boundaries in the COPD cases. The key to overcoming these challenges has been using the anatomical 1H MRI acquired contemporaneously with hyperpolarized gas MRI as an intermediate step to register hyperpolarized gas MRI to CT indirectly34,38. Early work employed side-by-side visual comparison and manual segmentation of CT structures, such as lung lobes, onto MRI space20. Advancements in computational resources and open-source image processing tools have enabled three-dimensional registration of CT and hyperpolarized gas MRI, for example, using modality independent neighborhood descriptor (MIND)23,30,34,39,40,41 or Advanced Normalization Toolkit (ANTs) registration21,22,27,31,32,37,38,42,43, both of which were top performers in a pulmonary image registration challenge44. One novel method coupled the two registrations rather than treating them independently45, which has been implemented in a full pulmonary image analysis pipeline designed for phenotyping lung disease46. Overall, hyperpolarized gas MRI to CT registration accuracy was improved using the intermediate 1H step38 and using deformable approaches over affine-only approaches38,45.
The goal here is to build from the existing literature and provide a protocol for 129Xe MR to CT image registration using open-source platforms47,48,49. The protocol is implemented using ANTsPy, and, in line with previous work38, registers a single-label lung mask from 1H MRI to the single-label lung mask from CT; the resulting transformation is subsequently applied to the 129Xe image to map it to the CT image space. The protocol outlined is intended to be appropriate for research or clinical settings, where applicable, and hyperpolarized 129Xe MRI is available.
For context, image acquisition and analysis for the examples provided herein were performed as follows. Chest CT was acquired at full inspiration (total lung capacity, TLC) according to an established low-dose research protocol50 with parameters: 64 x 0.625 collimation, 120 peak kilovoltage, tube current 100 mA, 0.5 s revolution time, spiral pitch 1.0, 1.25 mm slice thickness, 0.80 mm slice spacing, standard reconstruction kernel, display field-of-view limited to the most lateral extents of the lungs (to maximize spatial resolution). CT segmentation and analysis were performed using commercial software (see Table of Materials).
129Xe and volume-matched 1H MRI were performed according to published guidelines9. For full MRI acquisition details and protocol, readers are directed to another article in this collection51. MRI segmentation and registration were performed using a semi-automated custom pipeline using k-means clustering for 129Xe segmentation, seeded-region growing for 1H segmentation, and landmark-based affine registration to map the 1H image to the 129Xe image52. Affine registration is typically sufficient for 1H-129Xe MR registration to account for most lung inflation or patient position differences between acquisitions; deformable registration is typically not necessary. The 1H-129Xe registration step can be eliminated with simultaneously acquired 129Xe and 1H MRI in the same breath-hold53,54.
Protokół
The imaging cases shown here were approved by the University of British Columbia Providence Health Care Research Ethics Board (REB# H21-01237, H21-02149, H22-01264). Participants provided written informed consent prior to completing imaging. The overall pipeline from image acquisition to registration is outlined in Figure 2, and the protocol details here focus on the MR-CT image registration only. Image acquisition and segmentation are dependent on available or preferred imaging hardware, imaging protocols, and image analysis software tools and, therefore, are left to readers' preference. The protocol is designed to be agnostic to those prior steps using single-label masks of the lungs following image segmentation.
1. Software setup
- Download and install ANTsPy (see Table of Materials), the Python wrapper for the Advanced Normalization Tools image processing library47,48,49. Tutorials are available at the link under the 'tutorials' tab. ANTsPy is also available for Docker installation if desired.
NOTE: ANTsPy requires a Linux-based operating system or environment. For the examples here, ANTsPy was installed and used in a virtual high-performance computing environment on a local workstation. The protocol has tended to work better using a virtual computing environment in the authors' experience. - Download and install segmentation and/or visualization software of choice.
NOTE: For the present study, ITK-SNAP was used for visualization (see Table of Materials). - Download and save the reg.py script (Supplementary File 1).
2. Image pre-processing
- Click to open images and masks in the desired image visualization software to verify image and mask orientation matches for all CT, 1H, and 129Xe files. Depending on the segmentation method and/or software used, the orientation of some images or masks may need to be adjusted. As needed, we recommend adjusting 1H and 129Xe images and mask orientation to match that of the native CT image.
- Save the image DICOMs and single-label masks (adjusted as in step 2.1 as needed) as Neuroimaging Informatics Technology Initiative files (NIfTI, *.nii; six files total) using the preferred software tool, in the same folder as reg.py that is accessible to the location where ANTsPy is installed and will be run. Follow the naming conventions as mentioned below.
- 1H MRI: Proton.nii; 1H MRI mask: Proton_mask.nii.
NOTE: Use the 1H image and mask after registration to 129Xe. - 129Xe MRI: Ventilation.nii; 129Xe MRI mask: Ventilation_mask.nii
- CT: CT.nii; CT mask: CT_mask.nii.
NOTE: The file names are hard-coded into the registration script and, therefore, must follow the mentioned format, or be revised in the script to match the desired naming convention. These steps can be completed together using any of the recommended software tools listed in step 1.2. With respect to those software tools, some will write the required header information automatically when saving .nii files, whereas others require additional steps to copy and write the header information.
- 1H MRI: Proton.nii; 1H MRI mask: Proton_mask.nii.
3. CT-XeMRI registration
- Open the reg.py file in the desired Python computing environment setup in step 1.1.
NOTE: Script reg.py is based on the built-in ANTs registration tools; additional documentation is available55. - If using a virtual environment, set the number of central processing units (CPU), number threads, and RAM as desired or available in the computing environment. A virtual computing environment with 16 CPU, 1 thread per CPU, and 186 GB of available RAM was used for the examples herein.
- Set desired transformation and interpolation. The SyNAggro transformation with linear interpolation for images and generic label interpolation for single-label masks, which are default in the provided reg.py script, were used here.
NOTE: SyNAggro is a symmetric normalization transformation, which includes affine + deformable transformation plus more aggressive registration using fine-scale matching and more deformation (compared with plain SyN). Alternative transformation and interpolation algorithms are listed in the ANTs registration documentation link in step 3.155. - Set the fixed and the moving image. Here, the CT (single-label mask) was set as the fixed image, and the 1H MRI (single-label mask) was set as the moving image.
- Run reg.py in the Python computing environment. Full registration may take 5-10 min (using our parameters) or longer, depending on computing resources used or available. When complete, warped files will be automatically saved in the same directory as the original image files with the file names as follows: Proton_warped.nii.gz; Ventilation_warped.nii.gz; Ventilation_label_warped.nii.gz.
NOTE: NIfTI *.nii.gz files are just zipped versions of *.nii files and can be unzipped or opened as desired. Script reg.py can be modified as desired, for example, using different transformation or interpolation methods or mapping to or creating file directories.
4. Evaluation of registration results
- Open the CT.nii image as the base image in the desired visualization software.
- Open Ventilation_warped.nii.gz or Ventilation_label_warped.nii.gz as another image and overlay on the CT image with the desired color map.
- Review the overlap of the 129Xe image or mask with the CT image in all image planes (coronal, axial, sagittal), evaluating the visual alignment of landmarks such as the carina and lung boundaries (where available in the 129Xe image).
- Check the results. If satisfied with the results, registration is complete.
NOTE: The registered 129Xe image/mask can be multiplied by the CT mask to remove the trachea and main airways (if not removed prior to MRI segmentation) and remove any signal that falls outside of the CT lung boundaries after registration. Further quantification for regional structure-function measurements can be performed as desired. - If not satisfied with the results, evaluate and optimize alternative types of transformation and associated parameters as needed.
Wyniki
This study prospectively acquired paired CT and 129Xe MRI in a research setting for regional lung structure-function characterization and image-guided bronchoscopy across a range of lung diseases and conditions. Figure 3 shows registered 129Xe MRI ventilation and CT in coronal and sagittal planes for four representative participants with a range of MRI ventilation patterns (for the same participants of Figure 1). The registered 129Xe MR label mask shows 129Xe signal intensity clusters from ventilation defect or signal void to hyperintense, and was multiplied by the CT label mask to remove the trachea and main airways. Visual inspection shows good alignment of all lung boundaries for the healthy participant (Figure 3A) with homogenous ventilation, with the exception of the costophrenic angle in the right lung. This discrepancy could be due to resolution differences between the two modalities or geometric distortion in the 129Xe/1H MRI; however, it is a small fraction of the overall lung volume. In the three participants with COPD, there was also good alignment of lung boundaries, where available. The COPD examples herein range from diffuse ventilation abnormalities (Figure 3B), upper lobe ventilation abnormalities with absent apical lung boundaries (Figure 3C), and lower lobe ventilation abnormalities with absent diaphragmatic lung boundaries (Figure 3D).
The authors typically opt for visual inspection of the registered images and do not prospectively quantitatively evaluate registration performance because of the nature of the multi-modal contrast differences between CT and 129Xe MRI. Common quantitative metrics of registration performance are the Dice coefficient or target registration error (TRE). Dice coefficients could be evaluated between the registered 1H (moving) and CT (fixed) images; however, this would be indirect since 1H images are used as an intermediate bridge to register 129Xe images, and the measurements of interest come from 129Xe MRI. TRE may be quantified by placing fiducial landmarks on the fixed and moving images; however placing fiducials is a time-consuming manual process, and the nature of 129Xe MRI ventilation abnormalities means there may be limited anatomical landmarks available. Although the tracheal carina and costophrenic angles typically serve as easy landmarks, the participants in Figure 3C,D highlight severe examples where there are limited obvious landmarks available. Using a similar registration framework in ANTs, Tahir et al. achieved TRE of 8.8 mm to 19.7 mm38, which is small relative to the size of the lungs (typical field-of-view 350-400 mm) and therefore acceptable; the authors anticipate TRE might be similar using the current framework.
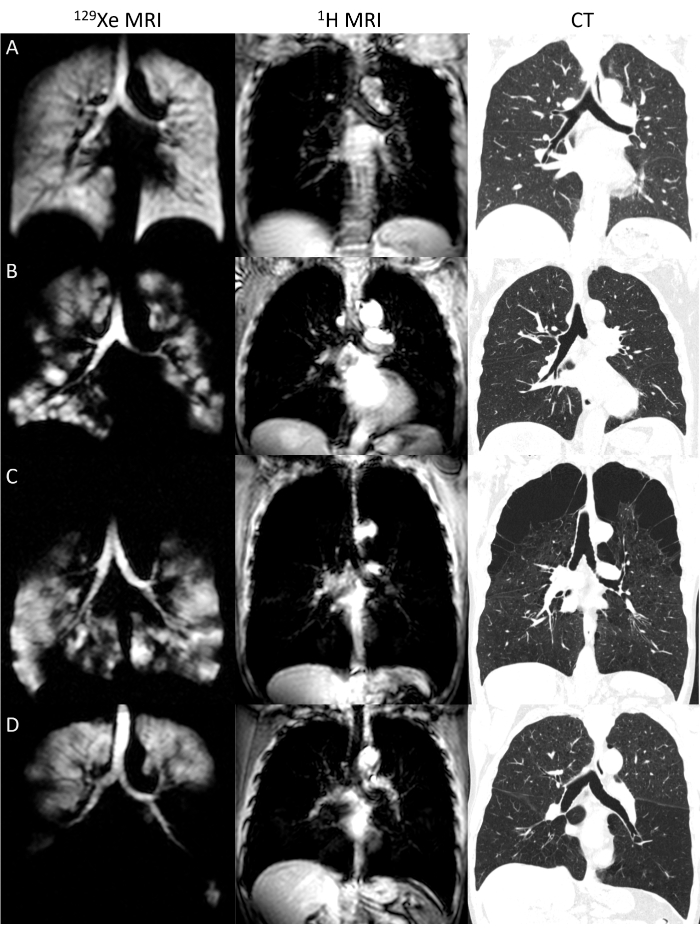
Figure 1: Pulmonary 129Xe and 1H and CT. Examples of 129Xe and volume-matched anatomical 1H MRI with paired CT for a healthy participant (A) and three participants with chronic obstructive pulmonary disease (COPD; B, C, D) showing a range of 129Xe ventilation patterns. Please click here to view a larger version of this figure.

Figure 2: Overall image analysis and registration pipeline. Input CT, 129Xe MR, and 1H MR images are first segmented to generate single-label masks. The 1H MR image/mask is first transformed to the 129Xe image/mask. All CT, 129Xe, and 1H images and masks are converted to NIfTI files, which are then used for 129Xe-CT registration. The 1H mask is transformed to the CT image space, and the transformation is subsequently applied to the 129Xe image/mask. Please click here to view a larger version of this figure.

Figure 3: 129Xe MRI-CT registration results. Registration results for the four representative participants shown in Figure 1, where 129Xe is shown in cyan overlaid on the CT in greyscale. There was good alignment of all lung boundaries for the healthy participant (A) with homogenous ventilation, with the exception of the costophrenic angle in the right lung. There was also good alignment of lung boundaries in the three participants with COPD, where available, ranging from diffuse ventilation abnormalities (B), upper lobe ventilation abnormalities with absent apical lung boundaries (C), and lower lobe ventilation abnormalities with absent diaphragmatic lung boundaries (D). Please click here to view a larger version of this figure.

Figure 4: Lobar structure-function measurements in a participant with COPD. Lobar CT emphysema measured by the low-attenuating areas less than -950 Hounsfield Units (LAA950, yellow) and 129Xe MRI ventilation defect percent (VDP, cyan) generated after 129Xe MR-CT registration, as an example regional evaluation for an image-guided treatment plan. The schematic of lobar outlines is shown in sagittal planes. RUL = right upper lobe; RML = right middle lobe; RLL = right lower lobe; LUL = left upper lobe; LLL = left lower lobe. Please click here to view a larger version of this figure.
Supplementary File 1: The reg.py script. Please click here to download this File.
Dyskusje
CT and 129Xe MRI provide complementary information to evaluate regional lung structure and function that is best facilitated using image registration. Multi-modal image registration can be non-trivial to implement, and so the protocol provided here is intended to provide the tools for readers to register 129Xe MRI to CT. The provided protocol uses ANTsPy for easier implementation for users with a broad range of image processing experience using Python rather than C++, as in conventional ANTs. Overall, ANTs provide an open-source image registration framework that reduces the need for tuning for different metrics and/or image pairs and supports reproducible research practices49. A set of three successive algorithms are typically used in ANTs to achieve optimal registration: (1) rigid registration using only rotation and translation, (2) affine registration using rotation and translation plus scaling and shearing, and (3) deformable, non-linear registration. At a deeper level, the three steps of the default protocol provided here for CT-MR registration are: (1) Initial similarity (rigid) transformation to capture gross similarities between the CT and MR images, preparing the images for subsequent, more refined transformations. This step uses Mattes mutual information similarity metric49 with 32 histogram bins, regular sampling with 0.2% pixels sampled, gradient descent optimization with step size 0.25, multi-resolution Gaussian pyramid with four levels of down-sampling factors 6 x 4 x 2 x 1 (iterations 2100 1200 1200 10) and corresponding smoothing Gaussian sigmas 3 mm x 2 mm x 1 mm x 0 mm. (2) Affine transformation using the output from the similarity stage as the initial transformation. This step uses Mattes mutual information similarity metric with 16 histogram bins, regular sampling with 0.2% pixels sampled, gradient descent optimization with step size 0.25, multi-resolution Gaussian pyramid with four levels of down-sampling factors 4 x 2 x 2 x 1 (iterations 2100 1200 1200 100) and corresponding smoothing Gaussian sigmas 3 mm x 2 mm x 1 mm x 0 mm. (3) SyNAggro transformation as the final step to further refine the transformation using non-linear, deformable registration. This step uses Mattes mutual information similarity metric with 16 histogram bins, full sampling, gradient descent optimization with step size 0.2, multi-resolution Gaussian pyramid with three levels of down-sampling factors 4 x 2 x 1 (iterations 40 20 0), and corresponding smoothing Gaussian sigmas 2 mm x 1 mm x 0 mm, Gaussian regularization kernel width of 3 voxels for smoothing of the update transform field. These are the default settings for the SyNAggro transformation algorithm.
As previously described and used for image registration between CT and hyperpolarized gas MRI38, a variation of the symmetric normalization (SyN) transformation was used here because it was shown to be a top-performing algorithm in a pulmonary image registration challenge44. The mutual information similarity metric was used because it tends to perform best for multi-modality imaging56. To further overcome the multi-modal image contrast differences, the protocol uses the volume-matched 1H MRI to indirectly register 129Xe MRI to CT as first described by Tahir et al.38, and actually registers the single-label 1H mask to the CT mask instead of the images the resulting transformation is subsequently applied to the 129Xe image and mask. Multi-label masks, for example, lobar or segmental CT masks21,22,23,45, or MRI intensity bins57, may also be used. Registration is performed by mapping 1H MRI to the CT space to maintain CT resolution for quantitation of CT structural features, although the registration direction can be reversed as desired. As input to the registration pipeline, the protocol handles images and single-label masks in NIfTI format, because, in this way, all cross-sectional slices in a 3D image are contained in a single file. We have evaluated this protocol on paired CT-129Xe MRI data from two centers independently (University of British Columbia and University of Kansas Medical Center) with good performance, and therefore anticipate the protocol will apply well in other datasets. Nevertheless, the transformation parameters may be optimized to improve performance in local datasets as needed.
The protocol is intentionally designed to be mostly agnostic to image acquisition and segmentation since these steps are dependent on available or preferred imaging hardware, imaging protocols, and image analysis software tools. The CT protocol should ideally be thin-slice, non-contrast enhanced, with standard-equivalent reconstruction kernel to enable measurement of validated quantitative parenchymal, airway and/or vascular metrics10,11,50. CT may be acquired at full inspiration, which is best validated for quantitative measurements10, or volume-matched to MRI to better facilitate CT-MRI registration and pair structure-function measurements at the same lung inflation volume24,30. Expiratory CT could also be performed and registered to inspiratory CT for quantification of air trapping17,18,34. For segmentation and quantitative analysis, various CT software tools are available commercially58 or open-source59. On the other hand, 129Xe MRI acquisition protocols have been published9, which currently recommend separate breath-hold acquisitions for 129Xe and 1H MRI. Novel protocols have been developed that acquire 129Xe and 1H MRI in the same breath-hold53,54, and therefore, can obviate the 1H-129Xe registration pre-processing step. Moreover, this protocol focuses on 129Xe MR ventilation imaging, but is similarly applicable for 129Xe gas exchange imaging. As an emerging method, 129Xe/1H MRI segmentation and quantification are not yet standardized; many methods have been reported in the literature that could be used here and have been nicely summarized in a recent review60. Regardless of how CT and 1H-129Xe MR images are acquired and their single-label masks obtained, this registration protocol is intended to be broadly applicable.
We acknowledge the limitations of the current protocol, primarily that it is somewhat manual, especially for pre-processing to prepare for registration and for evaluation of registration performance. Automated methods have been proposed previously45, and improvements in the existing protocol towards automation will be important for seamless clinical translation. The current registration is also CPU-based; while CPU processing is likely more widely available and the registration runs within approximately 10 min, implementation with graphics processing units (GPU) and/or deep learning could further shorten the run time and potentially improve registration accuracy. Finally, recommendations or methodology for CT and 1H/129Xe MR image segmentation are not provided. There are a wide range of methods available for both, so this is left to readers' choice; however, there is immense opportunity for a pipeline that encompasses automatic segmentation and registration to further accelerate clinical translation.
This registration protocol may be applied for research or clinical settings where hyperpolarized 129Xe MRI is available. In the research landscape, complementary CT and 129Xe MRI have largely supported the discovery of novel insights into regional lung structure-function, for example, in asthma20,21,22,28,29,30,31,32,COPD24,25,34,35, and IPF37,61. The bridge to clinical translation, though, comes in image-guided pulmonary interventions. Image-guided bronchial thermoplasty using CT and 129Xe MRI in patients with severe asthma afforded fewer bronchoscopic procedures, fewer peri-procedure adverse events, and non-inferior patient-reported outcomes compared to conventional whole-lung therapy62,63. In COPD, quantitative CT structure and 129Xe MRI function may suggest different bronchoscopic lung volume reduction targets based on the greatest lobar burden of CT emphysema and MRI ventilation abnormalities, highlighting the importance of considering structure and function together23. Moreover, functional lung avoidance radiotherapy schemes have been proposed40,64 to spare regions with preserved ventilatory and gas exchange function on MRI from undue radiation exposure. Additional image-guided opportunities in pulmonary interventions include surgical lung cancer resection65, airway stent and valve placement in COPD, and other novel bronchoscopic therapies for COPD or chronic bronchitis such as thermal vapor ablation, cryotherapy or rheoplasty66,67. Figure 4 illustrates lobar CT emphysema and MRI ventilation abnormalities for a patient with COPD that could be considered in treatment planning. The lungs remain one of the human body's last frontiers for image-guided interventions; together, CT and 129Xe MRI provide complementary information that has improved our understanding of lung structure-function, which can now be implemented for image-guided pulmonary interventions. The CT-129Xe MRI registration protocol provided here can enable further discovery of lung structure-function as well as image-guided interventions towards improved care, treatment, and outcomes for patients with respiratory disease.
Ujawnienia
RLE receives personal consulting fees from VIDA Diagnostics Inc. outside the submitted work. JAL has received an institutional grant from GE Healthcare and honoraria for lectures from Philips and GE Healthcare outside the submitted work.
Podziękowania
This research was supported in part through computational resources and services provided by Advanced Research Computing at the University of British Columbia, and by a University of British Columbia Department of Radiology AI Grant. RLE was supported by a Michael Smith Health Research BC Trainee Award.
Materiały
| Name | Company | Catalog Number | Comments |
| 3D Slicer | Brigham and Women's Hospital (BWH) | https://www.slicer.org/ | Image analysis/visualization software; open source |
| ANTsPy | NA | https://github.com/ANTsX/ANTsPy | Coding infrastructure; open source |
| ITK-SNAP | NA | http://www.itksnap.org/pmwiki/pmwiki.php | Image analysis/visualization software; open source |
| MAGNETOM Vida 3.0T MRI | Siemens Healthineers | NA | Can be any 1.5 T or 3.0 T scanner with broadband imaging capability |
| MATLAB | Mathworks | https://www.mathworks.com/products/matlab.html | General software, good for image analysis; available by subscription |
| reg.py | NA | NA | Registration script (Supplementary File 1) |
| Revolution HD CT scanner | GE Healthcare | NA | Can be any CT scanner with ≥64 detectors |
| VIDA Insights | VIDA Diagnostics Inc. | NA | CT analysis software; can be any to generate masks |
Odniesienia
- Albert, M. S., et al. Biological magnetic resonance imaging using laser-polarized 129Xe. Nature. 370 (6486), 199-201 (1994).
- Sheikh, K., Coxson, H. O., Parraga, G. This is what COPD looks like. Respirology. 21 (2), 224-236 (2016).
- Ebner, L., et al. The role of hyperpolarized (129)xenon in MR imaging of pulmonary function. Eur J Radiol. 86, 343-352 (2017).
- Eddy, R. L., Parraga, G. Pulmonary xenon-129 MRI: New opportunities to unravel enigmas in respiratory medicine. Eur Respir J. 55 (2), 1901987 (2020).
- Stewart, N. J., et al. Lung MRI with hyperpolarised gases: Current & future clinical perspectives. Br J Radiol. 95 (1132), 20210207 (2022).
- Kooner, H. K., et al. Pulmonary functional MRI: Detecting the structure-function pathologies that drive asthma symptoms and quality of life. Respirology. 27 (2), 114-133 (2022).
- Mugler, J. P., Altes, T. A. Hyperpolarized 129Xe MRI of the human lung. J Magn Reson Imaging. 37 (2), 313-331 (2013).
- Kaushik, S. S., et al. Single-breath clinical imaging of hyperpolarized (129)Xe in the airspaces, barrier, and red blood cells using an interleaved 3D radial 1-point Dixon acquisition. Magn Reson Med. 75 (129), 1434-1443 (2016).
- Niedbalski, P. J., et al. Protocols for multi-site trials using hyperpolarized (129) Xe MRI for imaging of ventilation, alveolar-airspace size, and gas exchange: A position paper from the (129) Xe MRI clinical trials consortium. Magn Reson Med. 86 (6), 2966-2986 (2021).
- Lynch, D. A., Al-Qaisi, M. A. Quantitative computed tomography in chronic obstructive pulmonary disease. J Thorac Imaging. 28 (5), 284-290 (2013).
- Motahari, A., et al. Repeatability of pulmonary quantitative computed tomography measurements in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 208 (6), 657-665 (2023).
- Jarjour, N. N., et al. Severe asthma: Lessons learned from the national heart, lung, and blood institute severe asthma research program. Am J Respir Crit Care Med. 185 (4), 356-362 (2012).
- Regan, E. A., et al. Genetic epidemiology of COPD (copdgene) study design. COPD. 7 (1), 32-43 (2010).
- Couper, D., et al. Design of the subpopulations and intermediate outcomes in COPD study (SPIROMICS). Thorax. 69 (5), 491-494 (2014).
- Vestbo, J., et al. Evaluation of COPD longitudinally to identify predictive surrogate end-points (eclipse). Eur Respir J. 31 (4), 869-873 (2008).
- Bourbeau, J., et al. Canadian cohort obstructive lung disease (cancold): Fulfilling the need for longitudinal observational studies in COPD. Copd. 11 (2), 125-132 (2014).
- Galbán, C. J., et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med. 18 (11), 1711-1715 (2012).
- Kirby, M., et al. A novel method of estimating small airway disease using inspiratory-to-expiratory computed tomography. Respiration. 94 (4), 336-345 (2017).
- Kim, M., Doganay, O., Hwang, H. J., Seo, J. B., Gleeson, F. V. Lobar ventilation in patients with COPD assessed with the full-scale airway network flow model and xenon-enhanced dual-energy CT. Radiology. 298 (1), 201-209 (2021).
- Fain, S. B., et al. Evaluation of structure-function relationships in asthma using multidetector CT and hyperpolarized He-3 MRI. Acad Radiol. 15 (6), 753-762 (2008).
- Zha, W., et al. Regional heterogeneity of lobar ventilation in asthma using hyperpolarized helium-3 MRI. Acad Radiol. 25 (2), 169-178 (2018).
- Tahir, B. A., et al. Comparison of CT-based lobar ventilation with 3He MR imaging ventilation measurements. Radiology. 278 (2), 585-592 (2016).
- Adams, C. J., Capaldi, D. P. I., Di Cesare, R., McCormack, D. G., Parraga, G. On the potential role of MRI biomarkers of COPD to guide bronchoscopic lung volume reduction. Acad Radiol. 25 (2), 159-168 (2018).
- Pike, D., et al. Regional heterogeneity of chronic obstructive pulmonary disease phenotypes: Pulmonary (3)He magnetic resonance imaging and computed tomography. COPD. 13 (3), 601-609 (2016).
- Svenningsen, S., Guo, F., McCormack, D. G., Parraga, G. Noncystic fibrosis bronchiectasis: Regional abnormalities and response to airway clearance therapy using pulmonary functional magnetic resonance imaging. Acad Radiol. 24 (1), 4-12 (2017).
- Sheikh, K., et al. Magnetic resonance imaging biomarkers of chronic obstructive pulmonary disease prior to radiation therapy for non-small cell lung cancer. Eur J Radiol Open. 2, 81-89 (2015).
- Tahir, B. A., et al. Spatial comparison of CT-based surrogates of lung ventilation with hyperpolarized helium-3 and xenon-129 gas MRI in patients undergoing radiation therapy. Int J Radiat Oncol Biol Phys. 102 (4), 1276-1286 (2018).
- Svenningsen, S., et al. What are ventilation defects in asthma. Thorax. 69 (1), 63-71 (2014).
- Svenningsen, S., Nair, P., Guo, F., McCormack, D. G., Parraga, G. Is ventilation heterogeneity related to asthma control. Eur Respir J. 48 (2), 370-379 (2016).
- Eddy, R. L., et al. Is computed tomography airway count related to asthma severity and airway structure and function. Am J Respir Crit Care Med. 201 (8), 923-933 (2020).
- Mummy, D. G., et al. Mucus plugs in asthma at CT associated with regional ventilation defects at (3)He MRI. Radiology. 303 (3), 184-190 (2022).
- Carey, K. J., et al. Comparison of hyperpolarized (3)He-MRI, CT based parametric response mapping, and mucus scores in asthmatics. Front Physiol. 14, 1178339 (2023).
- Thomen, R. P., et al. Regional ventilation changes in severe asthma after bronchial thermoplasty with (3)He MR imaging and CT. Radiology. 274 (3), 250-259 (2015).
- Capaldi, D. P., et al. Pulmonary imaging biomarkers of gas trapping and emphysema in COPD: (3)He MR imaging and CT parametric response maps. Radiology. 279 (3), 597-608 (2016).
- MacNeil, J. L., et al. Pulmonary imaging phenotypes of chronic obstructive pulmonary disease using multiparametric response maps. Radiology. 295 (1), 227-236 (2020).
- Kirby, M., et al. Pulmonary ventilation visualized using hyperpolarized helium-3 and xenon-129 magnetic resonance imaging: Differences in COPD and relationship to emphysema. J Appl Physiol. 114 (1985), 707-715 (2013).
- Hahn, A. D., et al. Hyperpolarized (129)Xe MR spectroscopy in the lung shows 1-year reduced function in idiopathic pulmonary fibrosis. Radiology. 305 (129), 688-696 (2022).
- Tahir, B. A., et al. A method for quantitative analysis of regional lung ventilation using deformable image registration of CT and hybrid hyperpolarized gas/1h MRI. Phys Med Biol. 59 (23), 7267-7277 (2014).
- Heinrich, M. P., et al. MIND: Modality independent neighbourhood descriptor for multi-modal deformable registration. Med Image Anal. 16 (7), 1423-1435 (2012).
- Yaremko, B. P., et al. Functional lung avoidance for individualized radiation therapy: Results of a double-masked, randomized controlled trial. Int J Radiat Oncol Biol Phys. 113 (5), 1072-1084 (2022).
- Westcott, A., et al. Chronic obstructive pulmonary disease: Thoracic CT texture analysis and machine learning to predict pulmonary ventilation. Radiology. 293 (3), 676-684 (2019).
- Tahir, B. A., et al. Comparison of CT ventilation imaging and hyperpolarised gas MRI: Effects of breathing manoeuvre. Phys Med Biol. 64 (5), 055013 (2019).
- Astley, J. R., et al. A hybrid model- and deep learning-based framework for functional lung image synthesis from multi-inflation CT and hyperpolarized gas MRI. Med Phys. 50 (9), 5657-5670 (2023).
- Murphy, K., et al. Evaluation of registration methods on thoracic CT: The empire10 challenge. IEEE Trans Med Imaging. 30 (11), 1901-1920 (2011).
- Guo, F., et al. Thoracic CT-MRI coregistration for regional pulmonary structure-function measurements of obstructive lung disease. Med Phys. 44 (5), 1718-1733 (2017).
- Guo, F., et al. Development of a pulmonary imaging biomarker pipeline for phenotyping of chronic lung disease. J Med Imaging (Bellingham). 5 (2), 026002 (2018).
- Avants, B. B., et al. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 54 (3), 2033-2044 (2011).
- Tustison, N. J., Avants, B. B. Explicit B-spline regularization in diffeomorphic image registration. Front Neuroinform. 7, 39 (2013).
- Avants, B. B., et al. The Insight ToolKit image registration framework. Front Neuroinform. 8, 44 (2014).
- Sieren, J. P., et al. SPIROMICS protocol for multicenter quantitative computed tomography to phenotype the lungs. Am J Respir Crit Care Med. 194 (7), 794-806 (2016).
- Garrison, W. J., et al. Acquiring hyperpolarized 129Xe magnetic resonance images of lung ventilation. J Vis Exp. (201), e65982 (2023).
- Kirby, M., et al. Hyperpolarized 3He magnetic resonance functional imaging semiautomated segmentation. Acad Radiol. 19 (2), 141-152 (2012).
- Niedbalski, P. J., et al. A single-breath-hold protocol for hyperpolarized (129) Xe ventilation and gas exchange imaging. NMR Biomed. 36 (8), e4923 (2023).
- Collier, G. J., et al. Single breath-held acquisition of coregistered 3D (129) Xe lung ventilation and anatomical proton images of the human lung with compressed sensing. Magn Reson Med. 82 (1), 342-347 (2019).
- . Registration Available from: https://antspy.readthedocs.io/en/latest/registration.html (2017)
- Maes, F., Vandermeulen, D., Suetens, P. Medical image registration using mutual information. Proceedings of the IEEE. 91 (10), 1699-1722 (2003).
- He, M., et al. Extending semiautomatic ventilation defect analysis for hyperpolarized (129)Xe ventilation MRI. Acad Radiol. 21 (12), 1530-1541 (2014).
- Wang, J. M., Ram, S., Labaki, W. W., Han, M. K., Galbán, C. J. CT-based commercial software applications: Improving patient care through accurate COPD subtyping. Int J Chron Obstruct Pulmon Dis. 17, 919-930 (2022).
- Estepar, R. S. J., et al. Chest imaging platform: An open-source library and workstation for quantitative chest imaging. Am J Respir Crit Care Med. 191, 4975 (2015).
- Sharma, M., et al. Quantification of pulmonary functional MRI: State-of-the-art and emerging image processing methods and measurements. Phys Med Biol. 67 (22), (2022).
- Wang, J. M., et al. Using hyperpolarized (129)Xe MRI to quantify regional gas transfer in idiopathic pulmonary fibrosis. Thorax. 73 (129), 21-28 (2018).
- Hall, C. S., et al. Single-session bronchial thermoplasty guided by (129)Xe magnetic resonance imaging. A pilot randomized controlled clinical trial. Am J Respir Crit Care Med. 202 (129), 524-534 (2020).
- Svenningsen, S., et al. Bronchial thermoplasty guided by hyperpolarised gas magnetic resonance imaging in adults with severe asthma: A 1-year pilot randomised trial. ERJ Open Res. 7 (3), 00268 (2021).
- Rankine, L. J., et al. Hyperpolarized (129)Xe magnetic resonance imaging for functional avoidance treatment planning in thoracic radiation therapy: A comparison of ventilation- and gas exchange-guided treatment plans. Int J Radiat Oncol Biol Phys. 111 (129), 1044-1057 (2021).
- Radadia, N., et al. Comparison of ventilation defects quantified by technegas spect and hyperpolarized (129)Xe MRI. Front Physiol. 14, 1133334 (2023).
- Criner, G. J. Surgical and interventional approaches in COPD. Respir Care. 68 (7), 939-960 (2023).
- Hartman, J. E., Garner, J. L., Shah, P. L., Slebos, D. J. New bronchoscopic treatment modalities for patients with chronic bronchitis. Eur Respir Rev. 30 (159), 200281 (2021).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone