Method Article
Quantification of Diabetes-induced Adherent Leukocytes in Retinal Vasculature
In This Article
Summary
We demonstrate a method to label the walls of the retinal vasculature and adherent leukocytes. These adherent leukocytes can then be counted under a fluorescence microscope as a parameter of inflammation or the response of that inflammation to therapies.
Abstract
Leukostasis refers to the attachment of leukocytes to the luminal wall of the vasculature. This interaction of leukocytes with the wall of blood vessels is characteristic of inflammation and has been causally linked to capillary occlusion in a variety of tissues and diseases, including diabetic retinopathy.
Leukostasis has been reported for years as a life-threatening complication of hyperleukocytosis and can only be diagnosed clinically. Given the importance of the phenomenon, intensive research has been done to understand the potential mechanism(s) that lead to its manifestation; however, there is no gold-standard technique in laboratory settings to visualize and quantify the severity of the event.
In the method summarized below, the vasculature is initially perfused with a buffer to remove blood, and then, concanavalin A is perfused into the vasculature where it binds to all exposed cell walls and causes especially bright staining of leukocytes. If the perfusion to remove all unbound blood cells was successful, the remaining fluorescently labeled leukocytes are bound to the vasculature, and they can be manually quantified using any available fluorescence microscope.
Introduction
Leukocytes (white blood cells, WBCs) play an important role in the optimal function of the vasculature such as maintenance of the blood fluidity and regulation of thrombus resolution1. They also play a key role in some pathological conditions, such as adhering to the luminal wall of the vasculature for prolonged periods of time leading to vessel obstruction, at least temporarily, a phenomenon known as leukostasis2,3.
Diabetic retinopathy is one of the most common complications of long-term diabetes and one of the leading causes of visual impairment and blindness in the US and worldwide for individuals 20-75 years of age4. Slow and progressive degeneration of the retinal vasculature is a clinically meaningful component of the early stages of the disease, which in some patients leads to retinal ischemia with the resulting retinal neovascularization5,6. Cumulative evidence indicates that inflammation plays an important role in the development of the retinopathy7, and leukostasis is considered a subclinical intravascular inflammatory response. Leukostasis occurs in the early stages of diabetes, well before any detectable clinical manifestations have developed8,9,10. The repeated plugging of the retinal vessels by adherent leukocytes over months to years (chronic leukostasis) in diabetes might contribute to the vascular occlusion and degeneration of the capillaries11,12,13. The severity of this leukostasis is of pathologic significance and can be used to monitor the severity of the disease process or to evaluate the efficacy of a therapy in research settings.
To further study the specific effects of the hyperglycemic microenvironment on leukostasis, in vitro models have been designed. Isolated retinal microvascular endothelial cells can be grown and arranged either in 2- or 3-D cultures models (microvasculature-on-a-chip14) to replicate the vascular endothelium (the cell monolayer that paves the lumen of the vessels). However, the interexperimental variation of these models limits their use. The study of leukostasis in human retinal vasculature in vivo is still limited, and therefore, most of the current knowledge on retinal leukostasis is derived from animal models of diabetic retinopathy13,15.
The aim of this report is to describe a standard protocol based on methods described elsewhere16 for the quantification of attached leukocytes to the retinal vasculature as a parameter of leukostasis. This assay can be used to study other vascular diseases that also present leukostasis, such as malignancies3,17,18,19 and some infectious and allergic conditions20. This protocol can be implemented in any basic research laboratory without the need of specialized equipment. In the method summarized below, the vasculature is initially perfused with buffer to remove blood, and then, concanavalin A is perfused into the vasculature where it binds to all exposed cell walls and causes especially bright staining of leukocytes21,22,23. If the perfusion to remove all unbound blood cells is successful, the remaining fluorescently labeled leukocytes that are bound to the vasculature can be manually quantified using any fluorescence microscope on hand.
Protocol
The protocol has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at the University of California Irvine and conforms to governmental regulations regarding the care and use of laboratory animals. There are no stop points in this protocol. The average time per mouse is 30 min.
1. Preparing the perfusion stage
- Warm up the 0.9% saline bag and concanavalin A solution in a 37 oC water bath for 20-30 min before use.
NOTE: Protect concanavalin A from light exposure (cover with foil). - Set up a tray to contain blood and liquids from dripping on the surface where the procedure will take place. On top of the tray, place a heating pad covered with an absorbent bench underpad or any absorbent material.
NOTE: The goal is to avoid the mouse's body losing heat during the procedure, because cooling makes more difficult to remove blood during perfusion.
2. Setting up the pressure infuser
- Connect in series the bag of 0.9% saline, the I.V. catheter set, a 4-way valve stopcock, and the gavage needle.
- Insert the 0.9% saline bag between the netting and the air bladder of the pressure infuser. Hang the saline bag on the hook located on the back of the air bladder. Use the I.V. pole loop to hang the pressure infuser in the I.V. pole.
- Purge the lines and ports of all air bubbles by letting the system open (run) for a couple of minutes and set the flow rate to 18-20 mL/min24. To inflate the pressure infuser air bladder, turn the stopcock handle to point toward the open stopcock vent, then pump the inflation bulb until the pressure gauge indicates the desired pressure. Re-adjust the pressure before perfusing each mouse. To deflate, turn the stopcock handle straight down towards the inflation bulb.
NOTE: If the 0.9% saline bag is new, usually 150 mmHg pressure delivers the desired flow rate; however, the pressure should be adjusted empirically due to variations in pressure infuser brands and over the period of use of the 0.9% saline bag. - Attach a 10 mL syringe filled with warmed concanavalin A solution to the 4-way valve.
NOTE: Protect the syringe from light exposure (cover with foil).
3. Anesthesia
- Deliver anesthesia by intraperitoneal (I.P.) injection of Ketamine:Xylazine; the most widely used dose for mouse surgery/procedure is 100:10 mg/kg body weight25. Assess anesthesia by pedal reflex (firm toe pinch).
NOTE: This dose provides an onset of 4-6 min with a 45-60 min duration of surgical anesthesia. The anesthetic cocktail can be stored at room temperature for a maximum of 2 weeks.
4. Transcardial perfusion and staining with concanavalin A
- Place the mouse on the perfusion stage in the supine position to allow for exposure of the thoracic and abdominal cavity.
- Visually identify the xiphoid process, and with the hemostat in the dominant hand, pin the skin and lock it. Once the hemostat is secured, transfer it to the nondominant hand and lift the skin.
- Use scissors in the dominant hand and cut, in a 90° angle to the spine, a patch of skin to reveal the outer abdominal wall.
- With the xiphoid process and the rib cage now visible, dissect through the abdominal wall bilaterally, taking care to avoid cutting any organs or major vessels.
- With the diaphragm now visible, visualize the heart ventricles and lungs through the diaphragm. Using the tip of the scissors, cut through the diaphragm in one of the flanks, close to the spine, taking care to avoid cutting any organs or major vessels.
NOTE: This "hole" in the diaphragm will equilibrate the negative intrathoracic pressure with the atmospheric pressure, and a pneumothorax will occur collapsing the lungs and retracting the heart, facilitating the dissection of the diaphragm without damaging the lungs or the heart. - Continue dissecting through the ribs and parallel to the lungs to create a chest "flap". Release the hemostat and cut the xiphoid process in the sagittal plane. Gently wide open the xiphoid process manually. Observe the four chambers of the heart.
- With the nondominant hand and using forceps, grasp the heart near its apex. With the dominant hand, hold the gavage needle (attached to the I.V. catheter) and puncture the apex of the heart. To avoid full perforation of the left ventricle or reaching the pulmonary vasculature and then poor perfusion of the systemic vasculature, check the placement of the end of the ball tip of the gavage needle, which should be at the edge of the site of puncture slightly protruding from the heart. Clamp the gavage needle in place using curved mosquito forceps or simply hold it by hand while manipulating the I.V. stopcock.
- Open the stopcock to the 0.9% saline and almost simultaneously, cut open the right ventricle with scissors; perfuse for 2-3 min. During the perfusion time, gently move the needle from side to side and up and down to reduce kinking of the vasculature and increase blood exit from the heart.
- After perfusing with saline, turn the stopcock handle to shut off flow from the saline and allow flow from the syringe to the gavage needle. Perfuse by hand with the concanavalin A solution at a steady state rate. Ensure the 10 mL of concanavalin A solution is dispensed in 30-35 s.
- After perfusing with concanavalin A, turn the valve to shut off flow from the syringe and allow flow from the 0.9% saline to the gavage needle again. Perfuse with the 0.9% saline solution for an additional 2-3 min. Remove the gavage needle from the heart.
NOTE: The concanavalin A suggested in this protocol is conjugated to fluorescein (green); however, concanavalin A attached to other fluorochromes is also available.
5. Enucleation and isolation of fresh retina
- Turn the mouse on its side and, using the nondominant hand, place the index finger and thumb on the superior and inferior eyelids, respectively. Gently retract the eyelids and skin with fingers and proptose the eye, making it partially bulge out of the socket.
- While the eye is proptosed, use curved scissors in the dominant hand and scoop under the eye in a 45° angle. Cut the muscular attachment and optic nerve. Using the same scissors as a spatula, transfer the eye to a small container or directly to the stage of the dissecting microscope.
NOTE: Be careful not to cut off the back of the eye and avoid pulling the eye during this step. - Place the eye on a dental wax to open the globe. Under the dissecting microscope and using the nondominant hand, hold the scleral fold or the muscle remnants still attached externally to the posterior eye with micro-forceps, and orient the eye so that the cornea faces to a side.
NOTE: To prevent the eye from moving/sliding while opening the globe, a piece of wet lint-free tissue can be placed on top of the dental wax. - With one of the sharp corners of a Teflon-coated razor blade, make an incision 1-2 mm behind and parallel to the limbus (cornea-sclera junction). Hold the scleral fold or muscle with the micro-forceps, and draw the blade across the limbus with minimal downward force. Continue to cut with the razor to totally separate the anterior segment (cornea, iris, lens, and vitreous) from the posterior segment (eye cup).
NOTE: Do not saw back and forth. - Transfer the bisected eye cup to a small Petri dish with PBS.
NOTE: Avoid contact of the retina with the tissue paper (note in step 5.3) since it will tightly stick to the paper and become essentially impossible to recover. - Grab a scleral fold or the remaining muscle on the outside of the sclera with micro-forceps. Completely detach the retina from the sclera by breaking all the connections at the limbus around the perimeter of the eye cup using a micro-spatula. Scoop the retina out from the sclera with the micro-spatula. If the retina is still attached to the sclera by the optic nerve, slip the micro-scissors between the retina and sclera to cut the optic nerve.
- Remove any remnants of vitreous and ciliary muscle in the periphery of the retina. Immediately transfer the isolated retina to a slide with some PBS.
NOTE: Any other retina isolation technique can be used depending on the researcher's preference.
6. Flat mounting of the retina
- Lay out the unfixed retina on a slide with a small amount of PBS. Using the micro-spatula, gently orient the retina with the vitreous side up. If the retina is folded inward, use micro-forceps to hold the edges of the retina while the retina is unfolded using the micro-spatula.
- Make 4-5 radial cuts into the retina so that it lies flat (cloverleaf pattern).
- Using a lint-free tissue, dry up the excess of PBS away from the retina.
NOTE: Do not touch the retina with the tissue; otherwise, the sample will be lost. Coverslipping is desirable to keep the retina flat.
7. Microscopy
NOTE: Any fluorescence microscope with a GFP/FITC (480/530 nm) channel can be used for this step. For this work, we used the referenced microscope with 488 channel and associated software for image acquisition.
- Observe the recently flat-mounted retina under the microscope at 100x magnification (10x objective) and count fluorescently labeled leukocytes (manually) by methodically scanning the entire tissue (right to left or top to bottom).
NOTE: Leukocytes are single fluorescent dots that can display a round or oval shape. They are 12-15 µm in diameter and do not protrude from the retinal capillaries (the structure is completely constrained by the lumen of the vessel). - Acquire representative images with the desired magnification and perform postprocessing of the images with the software of choice (e.g., ImageJ [Fiji]).
- Express the count as leukocytes per retina. Graph the data by mean ± standard deviation.
Results
A well-executed perfusion and staining protocol will show the complete retinal vasculature delineated with concanavalin A (Figure 1). Poor perfusion of the mouse prevents labeling of the entire vascular tree and subsequent analysis of the leukocytes adherent to the lumen (Figure 2), whereas excessive pressure from a rapid squeeze of a syringe (less than 30-35 s) can cause vascular permeability and bursting of the blood vessels (Figure 3). The extravasated concanavalin A can cause mislabeling of external structures leading to confounding quantification of the adherent leukocytes.
Evaluation of the retinal vasculature and quantification of the attached leukocytes by fluorescence microscopy should be done immediately after mounting of the retina (Figure 4). One must be careful when viewing the retina under high magnification because the bends in the capillaries can appear to be labeled leukocytes adherent to the vasculature (Figure 4B, dashed circle). Focusing the microscope up and down while observing the area in question will usually allow differentiation of leukostasis from a vessel diving into the retina. The user can change/choose the fluorophore conjugated to concanavalin A that matches the available fluorescence cube in the microscope to be used. Leukostasis in diabetic retinopathy is not a dramatic event and the researcher should evaluate the whole vascular tree by scanning the entire retina in search of adherent leukocytes. Usually, there are 1-3 leukocytes per retina of non-diabetic animals and 3-12 leukocytes per retina of diabetic animals, and such the minimum sample size to reach statistically significant difference is 10-12 animals per group (Figure 4A,B and Figure 5). Given such phenotype, it is uncommon to find 2 or more leukocytes in close proximity (Figure 4B,C). The same does not hold true for acute models of inflammation such as in the lipopolysaccharide (LPS) challenge where a robust leukostasis phenomenon is observed (Figure 4C and Figure 5). For a detailed protocol of the LPS challenge model refer to 26.

Figure 1: Representative image of a well perfused and stained retinal vasculature. Wide-field image of the retinal vasculature of a healthy C57Bl/6J mouse (4 months of age) after infusion of concanavalin A-FITC. Concanavalin A-FITC stains the total retinal vascular network uniformly if an optimal perfusion was achieved. After adjusting for brightness and contrast the image was converted to RGB. Scale bar = 100 μm. Please click here to view a larger version of this figure.

Figure 2: Representative image of poor perfusion and staining of the retinal vasculature. Poor perfusion of the animal might lead to limited access of the concanavalin A to the retinal vessels resulting in a deficient staining of the retinal vascular network. Extensive areas of the retina will show lack of staining (dashed rectangle), limiting the visualization of the attached leukocytes to that vasculature. Vessels that received concanavalin A might show attached leukocytes (arrows), but they do not represent the total count of the retina. When this occurs, the sample should be discarded. Deficient stating might also lead to artifictious punctate (dashed circles) due to low-quality microscopy (forced channel to detect some fluorescent signal). After adjusting for brightness and contrast the image was converted to RGB. Scale bar = 50 μm. Please click here to view a larger version of this figure.

Figure 3: Representative image showing bursting of the retinal capillaries. Excessive pressure from a rapid squeeze of a concanavalin A-loading syringe (less than 30-35 s) can cause experimentally induced vascular permeability and bursting of the blood vessels (dashed circles) leading to labeling of external structures or other artifacts (dashed squares). After adjusting for brightness and contrast the image was converted to RGB. Scale bar = 50 μm. Please click here to view a larger version of this figure.
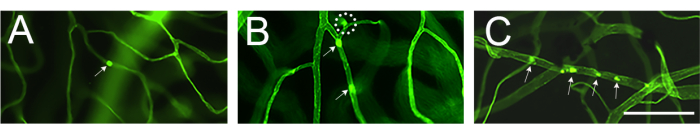
Figure 4: Examples of a bending capillary and leukostasis in nondiabetic, diabetic, and LPS-challenged mice. Examples of retinal leukostasis in C57Bl/6J mouse (4 months of age) after infusion of concanavalin A-FITC. (A) Non-diabetic mice do not show leukostasis, but it is not uncommon to encounter 1-3 leukocytes attached to the retinal vasculature after scanning of the entire retina (arrow). (B) Attached leukocytes to retinal vasculature in diabetic mice (model of subclinical inflammation) might range from 3 to 12 and it is uncommon to find attached leukocytes in close proximity (arrows). The structure enclosed with the dash circle is an example of a bending capillary diving into the retina. (C) Challenging mice with LPS (model of acute inflammation) can be done with each experiment as positive control of leukostasis. In this model, it is common to find several leukocytes attached to the vasculature in close proximity (arrows). After adjusting for brightness and contrast, the image was converted to RGB. Scale bar = 100 μm. Abbreviation: LPS = lipopolysaccharide. Please click here to view a larger version of this figure.

Figure 5: Representative graph to report results. The leukocyte count is expressed per retina and the data are graphed using mean and standard deviation. Abbreviations: N = non-diabetic; D = diabetic; LPS = lipopolysaccharide. Please click here to view a larger version of this figure.
Discussion
Leukostasis in humans refers to symptoms and clinical findings associated with hyperleukocytosis (total leukocytes (WBCs) count >100,000/µL) and is a medical emergency20. The mechanism(s) that lead to leukostasis are under intensive research. To date, the study of leukostasis in humans in vivo is not yet possible and researchers need to rely on animal models to understand this process. Different diseases present leukostasis and having a detailed protocol to visualize the phenomenon ex vivo can be useful for researchers in the laboratory. The protocol presented in this report has been optimized for the study of the retinal vasculature; however, it can be optimized empirically for the study of other suitable vascular beds such as the vasculature in the cremaster muscle27.
When performed correctly, this technique allows the perfusion and labelling of the entire retinal vascular tree and labelling and quantification of leukocytes adhering to the vascular wall. The retina is particularly suited for this method because its vasculature is restricted to defined planes in the tissue (unlike most tissues where the vasculature is less orderly). This method gives good contrast with unstained tissue when using transparent (retina) or very thin tissue (such as cremaster muscle27). Thick tissues or tissues having abundant myelinated nerves or other opaque and pigmented cells are poor sites for evaluation using this method.
A critical requirement in the procedure is good perfusion of the vasculature. Perfusing while the heart is still pumping facilitates good perfusion and labeling of the microvasculature. Poor vascular perfusion is often due to the use of cold fluids that lead to vasoconstriction, or kinking of the vasculature due to the weight and placement of the perfusion needle or forceps. While perfusing, it is important to pay close attention to the different organs during the procedure since they can provide some clues of the efficiency of the perfusion. For example, if the needle is inserted into the pulmonary vein, the lungs will fill and change color (blanching) immediately when the flow is started, suggesting that the perfusion will be suboptimal due to sequestration of all the liquids in the pulmonary tree. Blanching is observed in the tongue, kidneys, and liver during the perfusion, which might suggest an optimal procedure; however, these organs are downstream of the blood vessels that supply the eyes and therefore, the blanching of the organs guarantees a good perfusion to the body but not always to the retina.
Other potential problems that have been identified that impact the perfusion are: i) air bubbles trapped in the perfusion line that get into the microvessels and prevent perfusion with the fluorescent dye; ii) if the gavage needle inserted into the heart is extended too far into the aortic arch, it is possible to block the right carotid artery, thereby preventing flow of dye into the right retina; and iii) if perfusion is too strong, the resulting increase in intravascular pressure can lead to experimentally induced vascular permeability and might cause some leukocytes to become artifactitiously trapped in the microvasculature. Experimentally induced vascular permeability is visualized under the microscope as a localized burst of extravasated concanavalin A in the surrounding tissue of the vessel, while pathological vascular permeability presents a subtle diffuse pattern. In the first scenario, labeling of external structures or other artifacts might occur potentially confounding the quantification of adherent leukocytes. In this circumstance the observer should rely on the objective indicators that histologically define a leukocyte: shape, size and localization (inside the lumen of a vessel).
Pathological conditions that present acellular (degenerated) capillaries such as diabetic retinopathy do not preclude the use of this technique. Capillaries in early stages of degeneration, those that have lost the endothelium but mural cells are intact, are still patent, while capillaries in late stage of degeneration, although are no longer patent, they stay at their original site (focalized degeneration) and not appreciable changes are observed in the neighboring vessels 28. Both circumstances allow the labeling of the surrounding vasculature and evaluation of leukostasis.
A crucial step in the isolation of the retina is the enucleation of the eye without cutting off the back of the eye. As the optic nerve contains the incoming and outcoming blood vessels that vascularize the retinal layers and neurons, severing the optic nerve too close to its entrance to the eyeball almost guarantees that the retinal vascular tree will collapse, leading to a suboptimal view of the vasculature and the subsequent analysis of the leukostasis phenomenon. Additional attention should be paid to the razor blades used for anterior segment removal of the eye; they should be changed frequently (ideally a razor blade per 1-2 eyes) because dull blades cut poorly and might lead to pulling and tugging of the eye and retina, which in turn will damage the vasculature. When removing the anterior segment of the eye, it is suggested to rotate the eye while cutting at the limbus and remove the lens. The initial removal of the anterior segment of the eye is easier when performed on wet lint free paper; however, the paper must be removed immediately after the removal of the anterior segment, or the freshly isolated retina might attach to the paper and cannot be recovered.
We acknowledge that this protocol has several limitations. First, the initial perfusion may remove leukocytes that are loosely attached to the vasculature, leading the researcher to report an absence of leukostasis when in fact this may not be the case. Including a positive control of leukostasis, such as an acute model of inflammation26, could help to determine that the perfusion was not too harsh by showing that leukostasis is present. And second, it will be difficult for a non-trained observer to determine if the strongly fluorescent structure is in fact the cell of interest and not an artifact. To overcome this issue, the researcher should become familiar with the shape (usually round or oval at the initial attachment to the vasculature) and size (12–15 μm) of the leukocytes29. If there is doubt that the observed structure is or is not a leukocyte, it is advisable do not to include it in the quantification, but the researcher must make the final call once becomes more familiar with the assay. Ideally, automatic quantification using a specific software could facilitate the labor intensive manual quantification of the attached leukocytes to the vasculature, however, up to know, the distinction between leukocytes and artifacts can only be done by the knowledge and experience of the researcher. Developing or optimizing current platforms to quantify leukocytosis in an automatic or semiautomatic manner is an option that should be explored.
In conclusion, this validated protocol for evaluation of leukostasis might be a useful tool for researchers performing studies on diabetic retinopathy and other ocular and non-ocular vascular diseases and for testing novel therapeutical interventions.
Disclosures
The authors have no conflicts of interest to declare.
Acknowledgements
This work was supported by National Institutes of Health (NIH) Grants R01EY022938, R01EY022938-S1, and K99EY034928. The authors acknowledge services of the CWRU (P30EY11373) and UCI (P30EY034070) Visual Science Research Center Cores, as well as departmental support from an unrestricted grant from Research to Prevent Blindness to the Gavin Herbert Eye Institute at the University of California Irvine.
Materials
| Name | Company | Catalog Number | Comments |
| 10 mL syringe | |||
| 4-way stopcock Luer lock I.V. line valve | Baxter | 2C6204 | |
| Concanavalin A solution | Vector | FL-1001 | Prepare in PBS 1 mg/mL |
| Dissecting tools set | Includes hemostats, scissors and forceps | ||
| FIJI | Software for image processing | ||
| Fluorescence microscope | Nikon | Eclipse Ni | |
| Forceps, Dumont #5, Biological grade tip | Electron Microscopy Sciences (EMS) | 72700-D | |
| Gavage Needle 1.25 mm OD barrel tip x 30 mm | Fine Science | 18060-20 | |
| Halstead Mosquito Forceps | Fisher Scientific | 13-812-10 | |
| I.V. Catheter set with regulating clamp 70 inches | Baxter | 2C5417s | |
| I.V. Pole | |||
| Lint free tissue | Kimpwipes is an option | ||
| Micro dissecting spring scissors, Vannas, 3 mm straight | ROBOZ | RS-5620 | |
| Micro spatula | Fine Science Tools (FST) | 10091-12 | |
| Nikon | NIS-Elements (AR 5.30.03 64-bit) | Software for image acquisition | |
| Petri dish (100 mmx15 mm) | Corning | 351029 | |
| Phosphate buffered saline (PBS) | |||
| Pink dental wax | Electron Microscopy Sciences (EMS) | 72670 | |
| Pressure infuser | Infusurge | 4010 | |
| Razor blades, GEM single edge stainless steel, Teflon coated | Electron Microscopy Sciences (EMS) | 71970 | |
| Saline 0.9%, veterinary grade, 1000 mL | Baxter | 04925-04-10 | |
| Small dissecting scissors, curved blunt end 22 mm | ROBOZ | RS 5983 |
References
- Swystun, L. L., Liaw, P. C. The role of leukocytes in thrombosis. Blood. 128 (6), 753-762 (2016).
- Barouch, F. C., et al. Integrin-mediated neutrophil adhesion and retinal leukostasis in diabetes. Invest Ophthalmol Vis Sci. 41 (5), 1153-1158 (2000).
- Macaron, W., Sargsyan, Z., Short, N. J. Hyperleukocytosis and leukostasis in acute and chronic leukemias. Leuk Lymphoma. 63 (8), 1780-1791 (2022).
- Kempen, J. H., et al. The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol. 122 (4), 552-563 (2004).
- Kohner, E. M. Diabetic retinopathy. Br Med Bull. 45 (1), 148-173 (1989).
- Aouiss, A., Anka Idrissi, D., Kabine, M., Zaid, Y. Update of inflammatory proliferative retinopathy: Ischemia, hypoxia and angiogenesis. Curr Res Transl Med. 67 (2), 62-71 (2019).
- Tsalamandris, S., et al. The role of inflammation in diabetes: Current concepts and future perspectives. Eur Cardiol. 14 (1), 50-59 (2019).
- Adamis, A. P. Is diabetic retinopathy an inflammatory disease. Br J Ophthalmol. 86 (4), 363-365 (2002).
- Joussen, A. M., et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 18 (12), 1450-1452 (2004).
- Serra, A. M., et al. CD11b+ bone marrow-derived monocytes are the major leukocyte subset responsible for retinal capillary leukostasis in experimental diabetes in mouse and express high levels of CCR5 in the circulation. Am J Pathol. 181 (2), 719-727 (2012).
- Kinukawa, Y., Shimura, M., Tamai, M. Quantifying leukocyte dynamics and plugging in retinal microcirculation of streptozotosin-induced diabetic rats. Curr Eye Res. 18 (1), 49-55 (1999).
- Linsenmeier, R. A., et al. Retinal hypoxia in long-term diabetic cats. Invest Ophthalmol Vis Sci. 39 (9), 1647-1657 (1998).
- Herdade, A. S., et al. Effects of diabetes on microcirculation and leukostasis in retinal and non-ocular tissues: Implications for diabetic retinopathy. Biomolecules. 10 (11), 1583 (2020).
- Oakley, J., et al. Incorporating hemoglobin levels to map leukostasis risk in acute leukemia using microvasculature-on-chip technologies. Blood. 136 (Suppl 1), 9-10 (2020).
- Miyamoto, K., et al. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc Natl Acad Sci U S A. 96 (19), 10836-10841 (1999).
- Veenstra, A., et al. Diabetic retinopathy: Retina-specific methods for maintenance of diabetic rodents and evaluation of vascular histopathology and molecular abnormalities. Curr Protoc Mouse Biol. 5 (3), 247-270 (2015).
- Lester, T. J., Johnson, J. W., Cuttner, J. Pulmonary leukostasis as the single worst prognostic factor in patients with acute myelocytic leukemia and hyperleukocytosis. Am J Med. 79 (1), 43-48 (1985).
- Porcu, P., et al. Hyperleukocytic leukemias and leukostasis: a review of pathophysiology, clinical presentation and management. Leuk Lymphoma. 39 (1-2), 1-18 (2000).
- Giammarco, S., et al. Hyperleukocytosis and leukostasis: management of a medical emergency. Expert Rev Hematol. 10 (2), 147-154 (2017).
- Mank, V., Azhar, W., Brown, K. Leukocytosis. StatPearls. , (2024).
- Bernhard, W., Avrameas, S. Ultrastructural visualization of cellular carbohydrate components by means of concanavalin A. Exp Cell Res. 64 (1), 232-236 (1971).
- Oliver, J. M., Zurier, R. B., Berlin, R. D. Concanavalin a cap formation on polymorphonuclear leukocytes of normal and beige (chediak-higashi) mice. Nature. 253 (5491), 471-473 (1975).
- Pink, J. R., Hoessli, D., Tartakoff, A., Hooghe, R. Characterisation of Concanavalin A-binding glycoproteins from mouse splenic leukocytes by two-dimensional electrophoresis: preferential binding of incompletely glycosylated forms of H-2 antigen to the lectin. Mol Immunol. 20 (4), 491-497 (1983).
- Janssen, B., Debets, J., Leenders, P., Smits, J. Chronic measurement of cardiac output in conscious mice. Am J Physiol Regul Integr Comp Physiol. 282 (3), R928-R935 (2002).
- Flecknell, P. A., Flecknell, P. A. Anaesthesia of common laboratory species: Special considerations. Laboratory Animal Anaesthesia. , 181-241 (2009).
- Seemann, S., Zohles, F., Lupp, A. Comprehensive comparison of three different animal models for systemic inflammation. J Biomed Sci. 24 (1), 60 (2017).
- Lessieur, E. M., et al. ICAM-1 on the luminal surface of endothelial cells is induced to a greater extent in mouse retina than in other tissues in diabetes. Diabetologia. 65 (10), 1734-1744 (2022).
- Kuwabara, T., Cogan, D. G. Studies of retinal vascular patterns: I. Normal architecture. Arch Ophthalmol. 64, 904-911 (1960).
- Tigner, A., Ibrahim, S. A., Murray, I. V. Histology, white blood cell. StatPearls. , (2024).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved