Method Article
Demystifying Venous Excess Ultrasound (VExUS): Image Acquisition and Interpretation
요약
Point-of-care ultrasound (POCUS) is often used to assess the hemodynamic circuit and detect the presence of venous congestion. The Venous Excess Ultrasound (VExUS) scoring system was developed to aid clinicians in predicting the impact of venous congestion on organ dysfunction. This article aims to describe VExUS image acquisition and interpretation.
초록
Providers in many medical specialties must accurately assess the hemodynamic circuit to deliver appropriate patient care. Venous congestion is increasingly implicated in a range of multiorgan complications. However, hemodynamic assessment remains challenging because of the complex physiology involved and inconsistent diagnostic accuracy of conventional bedside tools and physical exam maneuvers. While right heart catheterization is regarded as the gold standard for measuring systemic venous pressure, it is invasive and not easily repeatable, and thus, there remains a need for non-invasive alternatives. Even point-of-care ultrasound examinations of the internal jugular vein or inferior vena cava have significant limitations in terms of accuracy of intravascular volume assessment and correlation with central venous pressure. To improve bedside clinicians' accuracy at assessing venous congestion, a protocol was developed and validated that utilizes pulsed-wave (PW) Doppler signals of veins in the liver and kidney to grade the degree of venous congestion present in patients. Although this scoring system, called Venous Excess Ultrasound (VExUS), is being increasingly adopted within certain subspecialties of medicine, such as nephrology and critical care, it remains underutilized in medicine as a whole. This is likely due, at least in part, to knowledge gaps and lack of training in this emerging modality. To address this educational gap, this article will describe VExUS image acquisition and interpretation.
서문
Assessment of the hemodynamic circuit at the bedside is fundamental to the daily care of acutely ill patients. The deleterious effects of fluid overload are increasingly recognized even outside of more obvious clinical syndromes such as heart failure, and there are now multiple studies showing that positive fluid balance is associated with increased mortality1. There is a growing body of evidence that shows that even low levels of venous congestion are associated with organ dysfunction2. Similarly, timely decongestion is associated with improved outcomes3. This multiorgan, dynamic circuit involves the right and left heart, systemic vascular resistance, pulmonary artery pressures, and sequential right-sided venous return, culminating in the vena cava. It is complex, and its accurate assessment remains challenging for bedside clinicians. Clinicians from a variety of specialties make decisions regularly based on this assessment. Conventional bedside tools and physical exam maneuvers, including assessment of jugular venous pressure, are almost always available but remain unreliable4,5,6,7,8,9. Point of care ultrasound (POCUS) is a limited ultrasound examination performed at the bedside and is interpreted by the treating physician to answer focused clinical questions. It is integrated in real-time with the patient's history, physical examination, and other available data to aid in diagnosis and management. Over the last several years, ultrasound has solidified itself as an extension of the physical exam10, improving clinicians' ability to detect venous congestion11,12. Additionally, POCUS can guide decongestive therapy, which can potentially positively affect patient outcomes2,3.
One specific protocol using ultrasound that has been validated to help with hemodynamic assessment is the venous excess ultrasound score, or VExUS. First described by Beaubien-Souligny et al.13 in 2020, this scoring system was originally validated in post-cardiac surgery patients as a reliable predictor of acute kidney injury (AKI). Over the past several years, VExUS has also been shown to help with intravascular volume assessment in multiple other clinical contexts14,15,16,17. VExUS evaluates multiple intra-abdominal veins to screen for sonographic signs associated with congestion. These sonographic signs of congestion appear and progress in incremental fashion as venous congestion worsens, allowing VExUS to both screen for congestion and potentially track its response to therapy over time.
While the individual components of the VExUS exam have long been in use18,19,20, their combination, as well as their use to monitor therapy over time, remain underutilized, partly due to providers' lack of familiarity with how to perform the exam. We believe that this gap in knowledge is one major factor that has prevented wider-scale adoption of VExUS as the primary alternative to gold-standard invasive cardiac monitoring of venous pressures.
To attempt to address this knowledge gap, this article describes an instructional protocol for performing the VExUS exam, which can serve as a step-by-step guide for bedside clinicians. This protocol draws on the collective experience of a group of physicians representing multiple medical specialties (nephrology, critical care, internal medicine, and anesthesiology) from multiple academic medical centers to describe a standardized approach to VExUS image acquisition and interpretation.
프로토콜
All procedures performed in studies involving human participants adhered to the ethical standards of the institutional research committee and the Declaration of Helsinki, including its later amendments or comparable ethical standards. Written informed consent was obtained from the human participants. The scanning technique encompassed transducer selection, machine settings, patient positioning, B-mode scanning, and image acquisition. Patients with unclear volume status, suspected venous congestion, heart failure, acute kidney injury (AKI), and/or chronic kidney disease (CKD) were included in the study, while those with end-stage kidney disease on dialysis, known cirrhosis or portal vein thrombosis, or any condition preventing safe probe usage over the abdomen were excluded. The details of the reagents and equipment used are listed in the Table of Materials.
1. Transducer selection
- Select a low-frequency transducer: the sector array (aka "phased array"; 1-5 MHz) transducer can be selected for its smaller footprint21; the curvilinear transducer (2-5 MHz) can serve as a substitute.
NOTE: On some machines, the curvilinear probe will de-activate EKG gating, whereas the sector array probe will permit EKG gating. But if EKG gating is available with either the curvilinear or sector array probe, it is preferable to use the curvilinear to maximize visualization of the color Doppler signal.
2. Machine settings
- For all views, set the depth such that the target of the scan will appear in the middle third of the ultrasound screen (the typical setting is between 16-20 cm).
- For all views, set the gain such that the lumen of blood vessels appears anechoic (black), the vessel walls appear hyperechoic (bright), and the surrounding structures appear intermediate between these extremes.
3. Patient and sonographer positioning
- For most of the exam, position the patient supine.
- When imaging the inferior vena cava (IVC), ask the patient to lift their feet onto the bed (i.e., flex their hips) to relax their abdominal muscles and allow for image optimization.
- When imaging the right kidney, reposition the patient in the left lateral recumbent position for better visualization of the right kidney.
- Before scanning, expose the patient's lower chest and abdomen.
- Position the ultrasound machine so that the sonographer's dominant hand can hold the ultrasound probe. This allows for finer manipulation of the ultrasound probe and frees up the non-dominant hand for operating the ultrasound machine. For example, right-handed sonographers should position themselves with the patient on their right side and vice versa.
4. Mode, presets, and setup
- Select two-dimensional (2-D) mode, also called brightness mode (B-mode).
- Select the Cardiac preset.
- Set up EKG gating by plugging the leads into the ultrasound machine and placing the leads in the standard orientation on the patient's skin.
NOTE: The VExUS exam can be performed in either the Cardiac or Abdominal preset, but on many machines, the Abdominal preset will de-activate EKG gating, whereas the Cardiac preset will permit EKG gating. So, if EKG gating is available on a given machine, it is preferable to use whichever preset permits EKG gating.
5. Inferior vena cava (IVC) imaging
- Gel
- Apply gel to the ultrasound probe directly to maximize scanning efficiency prior to acquiring each image.
- Subxiphoid view
- Place the probe beneath the xiphoid process with the indicator pointing cranially (Figure 1).
- Adjust the probe position until the IVC is seen in its maximal anteroposterior diameter (Figure 2).
- While keeping the IVC in the center of the screen, rotate the probe 90 degrees counterclockwise to obtain a short-axis view of the IVC (Figure 3).
- Cine clip acquisition: For machines configured for retrospective image acquisition, click on acquire after step 5.2.3. For machines configured for prospective image acquisition, click on acquire before step 5.2.3.
- Right flank view
- In patients with contraindications to subxiphoid imaging, place the probe along the right anterior axillary line in the body's coronal plane with the probe indicator pointing cranially (Figure 4).
- Adjust the probe position until the IVC is seen in its maximal anteroposterior diameter.
- While keeping the IVC in the center of the screen, rotate the probe 90 degrees counterclockwise to obtain a short-axis view of the IVC.
- Cine clip acquisition: For machines configured for retrospective image acquisition, click on acquire after step 5.3.3. For machines configured for prospective image acquisition, click on acquire before step 5.3.3.
- IVC assessment
- If IVC is >2 cm in anterior-to-posterior maximal diameter (Figure 5), proceed to step 6.
- If IVC is <= 2 cm (Figure 3), VExUS is not indicated. Use clinical judgment or other tools to assess volume status.
6. Hepatic vein Doppler
- Gel
- Apply gel to the ultrasound probe directly to maximize scanning efficiency prior to acquiring each image.
- Right flank view
- Place the probe along the right anterior axillary line in the body's coronal plane with the probe indicator pointing cranially (Figure 4).
- Adjust the probe position until the hepatic vein is visualized emptying into the IVC near the cavo-atrial junction (Figure 6).
- Select the color Doppler mode on the ultrasound machine.
- Move the color box so that the majority of the vessel is within its borders.
- Select PW Doppler mode on the ultrasound machine.
- Move the Doppler gate so that it is located within the lumen of the hepatic vein.
- Activate PW Doppler.
- Ask the patient to hold their breath at end-expiration.
- Allow a full screen of PW Doppler tracing to occur, and then click on Freeze (or equivalent) (Figure 7).
- Click on Acquire (or equivalent) to save still images of flow tracing.
7. Portal vein Doppler
- Gel
- Apply gel to the ultrasound probe directly to maximize scanning efficiency prior to acquiring each image.
- Right flank view
- Place the probe along the right anterior axillary line in the body's coronal plane with the probe indicator pointing cranially (Figure 4).
- Adjust the probe position until the portal vein is visualized (Figure 8).
- Select the color Doppler mode on the ultrasound machine.
- Move the color box so that most of the vessel is within its borders.
- Select PW Doppler mode on the ultrasound machine.
- Move the Doppler gate so that it is located within the lumen of the portal vein.
- Activate PW Doppler.
- Ask the patient to hold their breath at end-expiration.
- Allow a full screen of PW Doppler tracing to occur, and then click on Freeze (or equivalent) (Figure 9).
- Click on Acquire (or equivalent) to save still images of flow tracing.
8. Imaging of renal parenchymal veins
- Gel
- Apply gel to the ultrasound probe directly to maximize scanning efficiency prior to acquiring each image.
- Right flank view
- Place the probe along the right anterior axillary line in the body's coronal plane with the probe indicator pointing cranially (Figure 4).
- Adjust the probe position until the right kidney is seen in the long-axis view.
- Select the color Doppler mode on the ultrasound machine.
- Enlarge the color Doppler box to contain most of the renal cortex (Figure 10).
- Select PW Doppler mode on the ultrasound machine.
- Move the Doppler gate to a location within the renal cortex that has a color Doppler reading.
- Activate PW Doppler.
- Ask the patient to hold their breath at end-expiration.
- Allow a full screen of PW Doppler tracing to occur, and then click on Freeze (or equivalent) (Figure 11).
- Click on Acquire (or equivalent) to save still images of flow tracing.
결과
The first step to the VExUS exam involves imaging the inferior vena cava (IVC) to determine if there are signs of elevated right atrial pressures that would qualify the patient for the remainder of the exam. When imaging the IVC, it is important to view it from both the longitudinal and transverse perspectives to see the vessel in its maximal dimension. If the IVC is greater than 2 cm in its maximal anteroposterior diameter, then the remainder of the examination can be performed.
The next step would be to trace the Doppler flow through the hepatic vein. This is optimally imaged in the portion of the hepatic vein that is closest to the IVC. In patients without venous congestion, the Doppler flow pattern in the hepatic vein most closely resembles a standard central venous tracing, with a systolic and diastolic wave (s and d, equivalent to x and y waves) flowing below midline (i.e., away from the probe, into the IVC). This flow is accompanied by "a" and "v" waves, which represent the atrial kick and the right atrium being full, respectively.
In patients without venous congestion, the hepatic vein systolic flow is generally faster than diastolic flow. As venous congestion increases, the flow of blood out of the hepatic vein into the IVC during systole becomes more impaired. In mild congestion, the systolic flow becomes slower than the diastolic flow. As venous congestion becomes more severe, systolic flow eventually reverses, such that there is backward flow during cardiac systole. Systolic flow reversal correlates with moderate to severe venous congestion (Figure 12).
Next in the VExUS exam is the portal vein Doppler tracing. Between the hepatic and portal veins are the hepatic sinusoids, which act as a bed to absorb the usual flow fluctuations observed in central veins, such as the hepatic and jugular veins. So, venous flow in the portal vein is generally continuous and unidirectional towards the probe. This causes the flow to be measured as positive (i.e., above the baseline) on the Doppler tracing. Normal portal vein flow has a pulsatility index (PI) of less than 30%. The PI is defined as the measurement of the variation in blood flow during the cardiac cycle. It is calculated by dividing the difference between maximum and minimum flow velocities by the maximum flow velocity. As a patient becomes more congested, the flow becomes more pulsatile. Mild congestion has a PI of 30%-49%, and moderate-to-severely abnormal flow is >50% pulsatile (Figure 13).
Finally, the intrarenal vein Doppler flow tracing is obtained. After finding the kidneys, color Doppler is used to locate regions of flow. Then, the pulsed-wave Doppler gate is placed over an area of flow within the renal parenchyma. The small vessels in the renal cortex are typically very close to one another, so it is common to capture both arterial and venous flow simultaneously. The arterial flow will be positive (above the baseline) and can be used to determine the renal resistive index (not part of the VExUS exam). The venous flow will be negative (i.e., below the baseline). The venous flow will be negative and, normally, should appear continuous. As venous congestion increases, intrarenal venous flow will initially become pulsatile and biphasic with systolic and diastolic waves, indicating mild venous congestion. As congestion increases in severity, the systolic flow will eventually cease, leading to pulsatile, monophasic flow during diastole only (Figure 14).
After a patient is ruled into the VExUS exam by having an IVC >2 cm in maximal diameter, the entirety of the exam is performed to give the patient a VExUS grade. Normal tracings are assigned a score of 0, mildly abnormal ones a score of 1, and moderate to severely abnormal ones a score of 2. A patient's VExUS grade is determined by the number of abnormal tracings. A VExUS grade of 1 is given to those with an enlarged IVC and any combination of 0 or 1 scores. A VExUS grade of 2 is given to those with an enlarged IVC and at least one score of 2. A VExUS grade of 3 is given to those with an enlarged IVC and two or more scores of 2. A VExUS grade of 3 is closely associated with the risk of AKI due to venous congestion (Figure 15).

Figure 1: Transducer to the subxiphoid area to visualize the inferior vena cava in the long-axis view. The probe marker is pointing to the patient's head. Please click here to view a larger version of this figure.

Figure 2: Long-axis view of the inferior vena cava. Abbreviations: RA, right atrium; HV, hepatic vein; IVC, inferior vena cava. Please click here to view a larger version of this figure.

Figure 3: Short-axis view of the inferior vena cava. Abbreviations: Ao, aorta; IVC, inferior vena cava. Please click here to view a larger version of this figure.

Figure 4: Transducer to the right anterior axillary line to visualize the hepatic, portal, and intrarenal veins. The probe marker is pointing to the patient's head and hence is not visible in the image. Please click here to view a larger version of this figure.

Figure 5: Short-axis view of the inferior vena cava with an anteroposterior diameter greater than 2.0 cm. Abbreviations: HV, hepatic vein; IVC, inferior vena cava. Please click here to view a larger version of this figure.
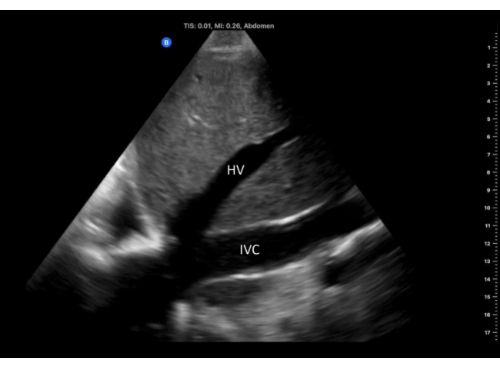
Figure 6: Long-axis view of the IVC with hepatic vein emptying into it, imaged from the right flank window. Abbreviations: HV, hepatic vein; IVC, inferior vena cava. Please click here to view a larger version of this figure.
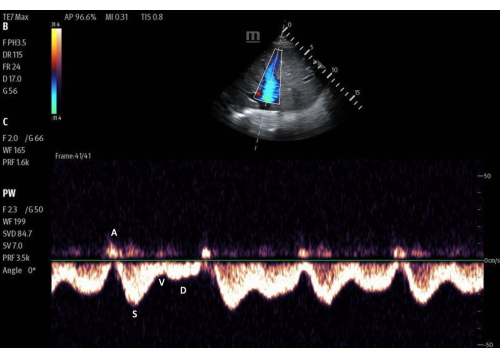
Figure 7: Pulsed-wave Doppler gate within the lumen of the hepatic vein with the flow tracing. Imaged with color Doppler activated. Please click here to view a larger version of this figure.

Figure 8: Long-axis view of the portal vein, imaged from the right flank window. Abbreviations: PV, portal vein. Please click here to view a larger version of this figure.
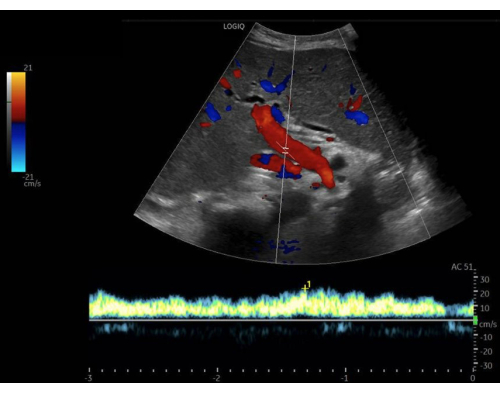
Figure 9: Pulsed-wave Doppler gate within the lumen of the portal vein, with the flow tracing below. Imaged with color Doppler activated. Please click here to view a larger version of this figure.
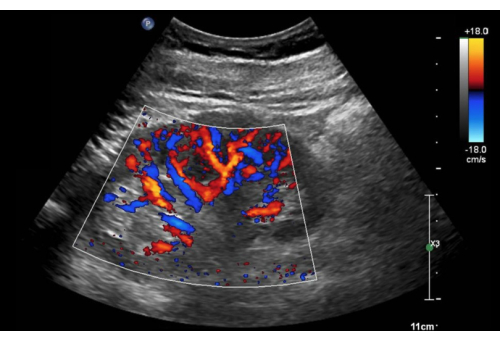
Figure 10: Long-axis view of the right kidney with color Doppler activated. Please click here to view a larger version of this figure.
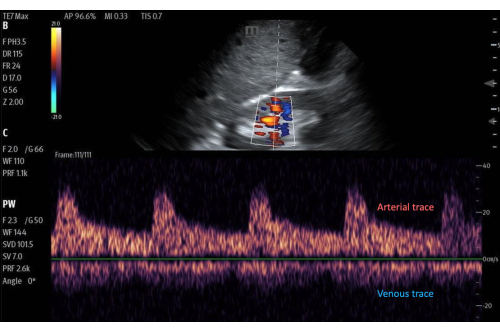
Figure 11: Pulsed-wave Doppler gate on an area of flow within the renal cortex, with the flow tracing below. Imaged with color Doppler activated. Please click here to view a larger version of this figure.
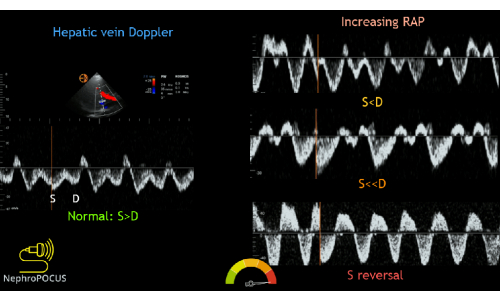
Figure 12: Different phenotypes of the hepatic vein Doppler waveform with varying degrees of congestion. This figure was reused from Koratala, A.22. Please click here to view a larger version of this figure.
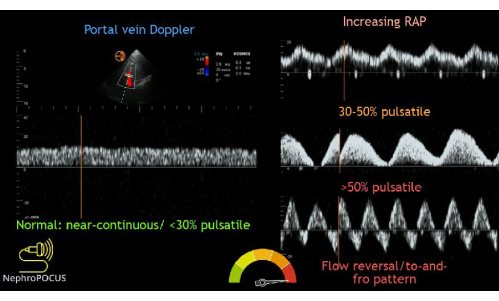
Figure 13: Different phenotypes of the portal vein Doppler waveform with varying degrees of congestion. This figure was reused from Koratala, A.22. Please click here to view a larger version of this figure.
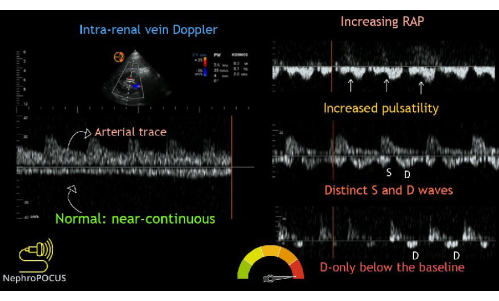
Figure 14: Different phenotypes of the intrarenal vein Doppler waveform with varying degrees of congestion. This figure was reused from Koratala, A.22. Please click here to view a larger version of this figure.

Figure 15: Combination of the various waveforms with a description of the VExUS scoring system. This figure was reused from Koratala, A.22. Please click here to view a larger version of this figure.
토론
Critical steps
VExUS was developed in post-cardiac surgery patients to quantify venous congestion non-invasively, but the utility has expanded for its use to assist in the evaluation of venous congestion and assessment of fluid status in multiple clinical contexts. To perform the exam properly, several critical steps must be considered. First, to maximize the diagnostic yield of the exam, one must consider the requirements of the VExUS exam when selecting a transducer and preset23. Specifically, the yield of the exam is maximized by using a curvilinear probe that permits EKG gating. If a given device's curvilinear probe does not permit EKG gating but compatible EKG wires are available, the next best choice is a phased-array probe with EKG gating. However, if device-compatible EKG wires are simply not available, then a curvilinear probe in either cardiac or abdominal preset can be used.
Second, it is nearly always helpful to visualize the IVC in both the long- and short-axis views. This is necessary to most accurately rule patients in or out of the protocol. The long-axis view of the IVC is notoriously error-prone, especially for less-experienced sonographers2. If the IVC is not visualized in the correct plane, one can underestimate the size of the vessel. To minimize error, visualizing it in the short-axis view can not only show you the maximum diameter reliably, but it can also help differentiate true IVC collapsibility from pseudo-collapsibility (i.e., out-of-plane movement of the vessel11).
Third, when obtaining venous flow tracings, it is important to maintain a stable scanning hand once PW Doppler is activated. Contrary to continuous wave Doppler, PW Doppler uses a "gate" from which it analyzes ultrasound signals from a specific location over time. Once PW Doppler is activated, the image shown to the sonographer is a static image obtained at the time of initiating the PW Doppler mode. If the sonographer or patient moves relative to one another, the location of the gate will change and alter the accuracy of the displayed 2-dimensional image. Thus, it is vital to maintain a stable scanning hand once the target vessel is in view and PW Doppler mode is activated. Additionally, having a patient lie still and hold their breath at end-expiration for a few seconds helps eliminate respiratory variation while PW Doppler is being utilized.
Finally, it is important to note that VExUS exams are not only useful in diagnosing venous congestion but are also helpful in monitoring response to treatment over time24. One of the principal utilities of this scoring system is when it is implemented serially over the course of a hospitalization or treatment course to evaluate the efficacy of the decongestive measures one has implemented.
Modifications and troubleshooting
Two aspects of the VExUS exam that commonly frustrate learners are (1) lack of availability of EKG gating hardware and (2) inability to locate intrarenal vein flow.
Within the VExUS exam, the interpretation of all three extra-cardiac Doppler waveforms is improved by EKG gating. Out of those three waveforms, EKG gating is most essential for the assessment of hepatic vein flow12. The hepatic vein flow tracing contains multiple waves, some above and some below the baseline. Thus, it is often necessary to use EKG gating to identify whether each wave is normal or pathologic and specifically to determine whether the systolic or diastolic velocity is faster. But, in the absence of EKG gating, one can use the non-hepatic vein VExUS data in most cases to draw conclusions about the state of a patient's congestion. Specifically, even if only 75% of the exam is performed (IVC, portal, and intrarenal veins), in many cases, a sufficient determination can be made on the state of congestion that exists in any given patient, especially since only 2 severely abnormal flow patterns indicate the maximum VExUS grade of 3. However, an EKG-free approach is more likely to generate inconclusive VExUS data for two reasons: (1) an EKG-free intrarenal vein Doppler tracing can be challenging to interpret if the intra-renal arterial signal is not prominent and (2) for the portal vein, EKG gating can help to differentiate respiratory versus cardiac pulsatility. For these reasons, the use of EKG gating is preferred whenever possible.
Second, finding the intrarenal vein flow signal can be challenging25. If the kidney is located greater than about 16 cm from the probe, increased attenuation of the ultrasound waves during their journey between the transducer and kidney may cause degradation of the Doppler signal (i.e., lack of color). This can be improved by moving the probe more lateral and posteriorly on the patient's body, bringing the kidney closer to the transducer. If the flow is still not visualized, one can decrease the Doppler scale so that it detects a slower flow. A flow velocity between 12 cm/s and 25 cm/s is typically sufficient to visualize the intrarenal vasculature. Furthermore, one can also increase the Doppler gain to improve the sensitivity to flow, increasing the yield of this portion of the scan. When increasing the gain, one must be wary of the higher likelihood of visualizing an artifact that could be mistaken for flow. Power Doppler mode can also be used, as this is typically better at detecting slower flow. If, after these modifications, a sonographer is still having trouble finding flow in the kidney on the right, they can try the contralateral kidney and implement the same changes on that side.
Limitations
While VExUS has emerged as a reliable, non-invasive exam to help guide the assessment of the right side of the hemodynamic circuit, it has some important limitations. First, there are many conditions in which VExUS is not validated, including cirrhosis and end-stage kidney disease (ESKD)7. In cirrhosis, there is an alteration in pressures within the liver, due to fibrous tissue, which can alter the ability of the hepatic tissue to serve as a "sponge" that absorbs cardiac pressures. Thus, both hepatic and portal vein flow can be altered. Additionally, there could be hepatic or portal vein thrombi that, again, could lead to misinterpretation of the flow within these vessels. Further, in ESKD, the kidneys become atrophic with decreased blood flow, making interpretation of renal venous flow difficult. Yet despite these limitations, there are case reports demonstrating that VExUS could potentially have value even in patients with cirrhosis26 and/or end-stage kidney disease27, serving as a method to monitor the treatment of venous congestion over time.
Second, it is important to note that VExUS is still a new protocol to estimate venous congestion, and thus, there is some data that suggests that it is not the most reliable or helpful way to estimate venous congestion. In a 2023 observational study published in the Journal of Critical Care, Andrei et al. showed that in a cohort of ICU patients, there was no significant association between VExUS scores and AKI or 28-day mortality28. This was a small cohort; however, the overall prevalence of moderate to severe venous congestion was low. In a group with a higher prevalence of venous congestion, such as cardiorenal syndrome patients, Islas-Rodriguez et al.29 showed that while using VExUS to guide decongestion helped achieve this, it did not increase the probability of kidney function recovery.
Third, there is a lack of consensus about the interpretation of VExUS in patients with pre-existing right ventricular dysfunction and/or significant tricuspid regurgitation. Conceptually, it seems reasonable to use VExUS as a trend monitor in such patients to attempt to differentiate dysfunction versus failure of the right heart circulation. However, we are not aware of any studies to date that have validated this concept.
Fourth, VExUS excludes patients with IVCs that measure less than 2.0 cm in anterior-to-posterior diameter, which may miss venous congestion in patients with small body habitus. In other words, if a 5-foot female and a 7-foot male each have an IVC of 1.9 cm, those two patients are both excluded from further VExUS screening for venous congestion. However, this is at odds with other echocardiographic practices that have increasingly incorporated indexing to body surface area to normalize sonographic measurements of body size30.
Fifth, the VExUS protocol is likely to encounter problems in cases of intra-abdominal hypertension (IAH). In IAH, patients are likely to have a small IVC (<2.0 cm) because high intra-abdominal pressure is likely to extrinsically compress the vessel31. This means most patients with IAH will automatically be excluded from further VExUS evaluation once a small IVC size is detected. However, IAH can be caused by venous congestion, and such congestion would be missed by VExUS due to the automatic exclusion of patients with small IVC caliber. Further, patients with IAH, in general, are likely poor candidates for VExUS. This is because, in IAH, there is extrinsic compression of all intra-abdominal veins, and Doppler waveforms of these veins will reflect a balance between extrinsic compression and intra-mural congestion, making interpretation of Doppler waveforms solely for congestion difficult.
Future directions
The current iteration of the VExUS protocol may evolve with time through multiple avenues. First, the current VExUS protocol includes only a single anterior-posterior measurement of the IVC obtained from a subxiphoid IVC long-axis view. However, this single view can be misleading, and there is evidence that a more robust estimate of right atrial pressure can be achieved by adding an IVC short-axis view to measure the IVC sphericity index: ratio of a lateral-medial to anterior-posterior diameters of the IVC32. Second, the current VExUS protocol only measures maximal IVC diameter and doesn't factor in IVC collapsibility. So, the VExUS protocol currently excludes patients with an IVC that is <=2 cm in diameter who nevertheless have non-collapsible IVCs. Conversely, the current VExUS protocol treats patients with large (>2 cm), collapsible IVC as having some degree of venous congestion. Future research is needed to determine whether IVC collapsibility should be used as a screening criterion for the VExUS exam. Third, femoral vein waveforms can be helpful for those with difficulty holding their breaths. Femoral vein Doppler (FVD) flow should be continuous in normal cases, but as venous congestion increases, the flow becomes increasingly pulsatile, leading to significant flow interruptions. FVD can emerge as a helpful expansion on the current VExUS protocol to allow for the utility of this exam in a larger proportion of patients33. Fourth, there is evidence that there is similar data about venous congestion provided by both measurements of the internal jugular vein and IVC34. Future studies should examine whether jugular vein parameters can substitute for the IVC in VExUS protocol in situations where the IVC is difficult to visualize.
The VExUS protocol is likely to evolve as ultrasound technology broadly integrates more functionality, especially machine learning (ML) and artificial intelligence (AI)35. ML/AI integration into ultrasound hardware and software should be able to automate many aspects of the VExUS protocol that are currently labor-intensive. For example, some existing machines are already able to measure IVC collapsibility automatically and should, in principle, one day also be able to measure IVC sphericity.
Further, it would be highly beneficial for ultrasound machines to offer AI-assisted virtual EKG gating technology, as many point-of-care ultrasound machines currently lack physical EKG cables. This would greatly help clinicians interpret flow patterns in the hepatic vein in the absence of EKG gating capabilities.
Finally, artificial intelligence that obtains the pulsed-wave Doppler tracing of a target vessel automatically can help flatten the already quite steep learning curve that exists for VExUS36. This technology already exists for cardiac output estimation by obtaining the LV outflow tract velocity time integral (LVOT VTI) measurement automatically, so expanding it to the hepatic, portal, and intrarenal vessels is not beyond the realms of possibility at this stage of ultrasound technology.
In summary, assessing the hemodynamic circuit with POCUS is vital in the management of acutely ill patients37. However, due to a lack of standardized training in image acquisition and interpretation, VExUS remains underutilized. This review presents a framework for VExUS exam image acquisition and interpretation from a group of physicians encompassing a variety of specialties. In turn, this protocol can be used to teach and learn VExUS to improve clinicians' ability to assess venous congestion and monitor its treatment over time.
공개
YSB reports receiving honoraria from the American Society of Anesthesiologists for Editorial Board work on Point-of-Care Ultrasound and from OpenAnesthesia.org for creating educational content related to POCUS. The remaining authors have no disclosures.
감사의 말
None.
자료
| Name | Company | Catalog Number | Comments |
| 5500P Ultrasound System | Philips | HC795143 | Used to obtain a subset of the Figures and Videos |
| Affiniti 70 Ultrasound System | Philips | HC795210 | Used to obtain a subset of the Figures and Videos |
| Curvilinear Transducer (C1-5-D) | GE | 5409287-R | 1-5 MHz, also called the abdominal probe |
| Curvilinear Transducer (C5-1) | Philips | HC989605412041 | 2-5 MHz, also called the abdominal probe |
| Curvilinear Transducer (C5-1) | SonoSite | https://www.sonosite.com/products/ultrasound-transducers/c5-1 | 1-5 MHz, also called the abdominal probe |
| Curvilinear Transducer (C5-2s) | Mindray | https://lysis.cc/products/mindray-c5-2s | 1-5 MHz, also called the abdominal probe |
| Edge 1 Ultrasound Machine | SonoSite | Used to obtain a subset of the Figures and Videos | |
| Handheld Probe (Butterfly iQ3) | Butterfly | https://www.butterflynetwork.com/iq3?srsltid=AfmBOorvY6WqHGbdeWW gtefztEJa8pt_xbwSOc6hQuB2s-Kb0wRlsCLR | Used to obtain a subset of the Figures and Videos |
| LOGIQ P9 Ultrasound System | GE | H42752LS | Used to obtain a subset of the Figures and Videos |
| Lumify Handheld Ultrasound | Philips | Used to obtain a subset of the Figures and Videos | |
| Phased-Array Transducer (3Sc-D) | GE | https://services.gehealthcare.in/gehcstorefront/p/5863286 | 1-5 MHz, also called the cardiac probe |
| Phased-Array Transducer (P4-2s) | Mindray | https://lysis.cc/products/mindray-p4-2s | 1-5 MHz, also called the cardiac probe |
| Phased-Array Transducer (P5-1) | SonoSite | https://www.sonosite.com/in/products/ultrasound-transducers/p5-1 | 1-5 MHz, also called the cardiac probe |
| Phased-Array Transducer (S4-1) | Philips | HC989605389271 | 1-5 MHz, also called the cardiac probe |
| TE7 Max Ultrasound System | Mindray | https://www.mindray.com/na/products/ultrasound/point-of-care/te-series/te-7-max-portable-ultrasound-machine/ | Used to obtain a subset of the Figures and Videos |
참고문헌
- Malbrain, M. L. et al. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther. 46 (5), 361-380 (2014).
- Cardoso, F. S. et al. Positive fluid balance was associated with mortality in patients with acute-on-chronic liver failure: A cohort study. J Crit Care. 63, 238-242 (2021).
- McCallum, W. et al. Rates of in-hospital decongestion and association with mortality and cardiovascular outcomes among patients admitted for acute heart failure. Am J Med. 135 (9), e337-e352 (2022).
- Long, B., Koyfman, A., Gottlieb, M. Diagnosis of acute heart failure in the emergency department: An evidence-based review. West J Emerg Med. 20 (6), 875-884 (2019).
- Wang, C. S., FitzGerald, J. M., Schulzer, M., Mak, E., Ayas, N. T. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA. 294 (15), 1944-1956 (2005).
- Koratala, A., Ronco, C., Kazory, A. Diagnosis of fluid overload: From conventional to contemporary concepts. Cardiorenal Med. 12 (4), 141-154 (2022).
- Chung, H. M., Kluge, R., Schrier, R. W., Anderson, R. J. Clinical assessment of extracellular fluid volume in hyponatremia. Am J Med. 83 (5), 905-908 (1987).
- Zimmerman, J., Morrissey, C., Bughrara, N., Bronshteyn, Y. S. Mistaken identity: Misidentification of other vascular structures as the inferior vena cava and how to avoid it. Diagnostics (Basel). 14 (19), 14192218 (2024).
- Androne, A. S. et al. Relation of unrecognized hypervolemia in chronic heart failure to clinical status, hemodynamics, and patient outcomes. Am J Cardiol. 93 (10), 1254-1259 (2004).
- Lopez Palmero, S. et al. Point-of-Care Ultrasound (POCUS) as an Extension of the physical examination in patients with Bacteremia or Candidemia. J Clin Med. 11 (13), jcm11133636 (2022).
- Turk, M., Robertson, T., Koratala, A. point-of-care ultrasound in diagnosis and management of congestive nephropathy. World J Crit Care Med. 12 (2), 53-62 (2023).
- Koratala, A., Kazory, A. An introduction to point-of-care ultrasound: Laennec to Lichtenstein. Adv Chronic Kidney Dis. 28 (3), 193-199 (2021).
- Beaubien-Souligny, W. et al. Quantifying systemic congestion with Point-Of-Care ultrasound: Development of the venous excess ultrasound grading system. Ultrasound J. 12 (1), 16 (2020).
- Yamamoto, M. et al. Prognostic impact of changes in intrarenal venous flow pattern in patients with heart failure. J Card Fail. 27 (1), 20-28 (2021).
- Ohara, H. et al. Renal venous stasis index reflects renal congestion and predicts adverse outcomes in patients with heart failure. Front Cardiovasc Med. 9, 772466 (2022).
- Spiegel, R. et al. The use of venous Doppler to predict adverse kidney events in a general ICU cohort. Crit Care. 24 (1), 615 (2020).
- Rola, P. et al. Clinical applications of the venous excess ultrasound (VExUS) score: Conceptual review and case series. Ultrasound J. 13 (1), 32 (2021).
- Beaubien-Souligny, W. et al. Alterations in portal vein flow and intrarenal venous flow are associated with acute kidney injury after cardiac surgery: A prospective observational cohort study. J Am Heart Assoc. 7 (19), e009961 (2018).
- Reynolds, T., Appleton, C. P. Doppler flow velocity patterns of the superior vena cava, inferior vena cava, hepatic vein, coronary sinus, and atrial septal defect: a guide for the echocardiographer. J Am Soc Echocardiogr. 4 (5), 503-512 (1991).
- Iida, N. et al. Clinical Implications of Intrarenal Hemodynamic Evaluation by Doppler ultrasonography in heart failure. JACC Heart Fail. 4 (8), 674-682 (2016).
- Hoffman, M., Convissar, D. L., Meng, M. L., Montgomery, S., Bronshteyn, Y. S. Image acquisition method for the sonographic assessment of the inferior vena cava. J Vis Exp. 191, e64790, (2023).
- Koratala, A. VExUS flash cards., <https://nephropocus.com/2021/10/05/vexus-flash-cards/> (2021).
- Koratala, A., Romero-Gonzalez, G., Soliman-Aboumarie, H., Kazory, A. Unlocking the potential of VExUS in assessing venous congestion: The art of doing it right. Cardiorenal Med. 14 (1), 350-374 (2024).
- Kanitkar, S., Soni, K., Vaishnav, B. Venous excess ultrasound for fluid assessment in complex cardiac patients with acute kidney injury. Cureus. 16 (8), e66003 (2024).
- Koratala, A., Reisinger, N. Venous excess Doppler ultrasound for the nephrologist: Pearls and pitfalls. Kidney Med. 4 (7), 100482 (2022).
- Koratala, A., Taleb Abdellah, A., Reisinger, N. Nephrologist-performed point-of-care venous excess Doppler ultrasound (VExUS) in the management of acute kidney injury. J Ultrasound. 26 (1), 301-306 (2023).
- Koratala, A., Ibrahim, M., Gudlawar, S. VExUS to guide ultrafiltration in hemodialysis: Exploring a novel dimension of Point of Care ultrasound. POCUS J. 9 (1), 16-19 (2024).
- Andrei, S., Bahr, P. A., Nguyen, M., Bouhemad, B., Guinot, P. G. Prevalence of systemic venous congestion assessed by Venous Excess Ultrasound Grading System (VExUS) and association with acute kidney injury in a general ICU cohort: A prospective multicentric study. Crit Care. 27 (1), 224 (2023).
- Islas-Rodriguez, J. P. et al. Effect on kidney function recovery guiding decongestion with vexus in patients with cardiorenal syndrome 1: A randomized control trial. Cardiorenal Med. 14 (1), 1-11 (2024).
- Lang, R. M. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 28 (1), 1-39 e14 (2015).
- Bauman, Z. et al. Inferior vena cava collapsibility loses correlation with internal jugular vein collapsibility during increased thoracic or intra-abdominal pressure. J Ultrasound. 18 (4), 343-348 (2015).
- Seo, Y. et al. Estimation of central venous pressure using the ratio of short to long diameter from cross-sectional images of the inferior vena cava. J Am Soc Echocardiogr. 30 (5), 461-467 (2017).
- Koratala, A., Argaiz, E. R. Femoral vein Doppler for guiding ultrafiltration in end-stage renal disease: A novel addition to bedside ultrasound. CASE. 8 (10), 475-483 (2024).
- Kumar, A., Bharti, A. K., Hussain, M., Kumar, S., Kumar, A. Correlation of internal jugular vein and inferior vena cava collapsibility index with direct central venous pressure measurement in critically-ill patients: An observational study. Indian J Crit Care Med. 28 (6), 595-600 (2024).
- Kim, Y. H. Artificial intelligence in medical ultrasonography: Driving on an unpaved road. Ultrasonography. 40 (3), 313-317 (2021).
- Argaiz, E. R. VExUS Nexus: Bedside assessment of venous congestion. Adv Chronic Kidney Dis. 28 (3), 252-261 (2021).
- Rola, P., Haycock, K., Spiegel, R., Beaubien-Souligny, W., Denault, A. VExUS: Common misconceptions, clinical use and future directions. Ultrasound J. 16 (1), 49 (2024).
재인쇄 및 허가
JoVE'article의 텍스트 или 그림을 다시 사용하시려면 허가 살펴보기
허가 살펴보기This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. 판권 소유