Method Article
Investigating the Alleviating Effects of Bacillus cereus Administration on Colitis through Gut Microbiota Modulation
요약
Here, we present a protocol to establish the antibiotic-induced pseudo-germ-free dextran sulfate sodium-induced colitis mouse model to investigate the role of gut microbiota in regulating the positive effects of Bacillus cereus on colitis.
초록
Gut microbiota dysbiosis is thought to exert a role in the progression of colitis. However, the precise standards for probiotic administration in alleviating colitis remain undefined. Most analysis methods rely on limited diversity and abundance of gut microorganisms. Therefore, observational studies cannot establish causation. In this study, we applied antibiotic-induced pseudo-germ-free mice to investigate the role of gut microbiota in regulating the probiotic effects of Bacillus cereus (B. cereus) on dextran sulfate sodium (DSS)-induced colitis in mice. This process allows for evaluating the bidirectional regulating effect of B. cereus supplementation on health and provides stable and reproducible results. Here, the detailed protocols for B. cereus cultivation, gavage operation, stool collection, and antibiotic clearance treatment on colitis mice are provided. The optimization methods are also applicable for other chronic inflammatory-associated disorders. The results showed that B. cereus administration decreased body weight loss, colon length shortening, disease activity index, and histopathological scores. However, treatment with antibiotics suppressed the positive effect of B. cereus on colitis. These results indicate that gut microbiota are needed for the alleviating effects of B. cereus on colitis. Therefore, exploring the beneficial effects of probiotics in this research is a promising approach for developing novel treatment strategies for alleviating the symptoms of chronic inflammatory-associated disorders.
서문
Inflammatory bowel diseases (IBDs) are common chronic gastrointestinal inflammatory disorders, including Crohn's disease and ulcerative colitis (UC)1. The current therapeutic avenues for inflammatory colitis are 5-aminosalicylates, corticosteroids, azathioprine, and antibiotics2. Moreover, these drugs have considerable side effects3. Therefore, we should pay more attention to the effect of probiotics on the treatment of colitis.
Alternative therapeutic approaches include probiotic administration, which has been used in animal models and clinical trials. Previous studies have indicated that different probiotic administration, such as Bacillus cereus or Bifidobacterium infantis, could alleviate colitis4,5. In preclinical studies, the effectiveness and safety of probiotics must be investigated in animal models.
Dextran sulfate sodium (DSS) colitis mouse models can help explore the mechanisms of colitis and evaluate the positive effects of probiotics. The DSS inducement results in erosions of the intestinal mucosa, gut barrier dysfunction, and an increase in intestinal epithelial permeability6. Most mouse models for probiotics have mainly highlighted biological effects. However, the mechanism behind the administration of this probiotic is challenging to explore, given the limitation in further verifying the causal relationship between probiotics and the alleviation of colitis. Thus, there is a need to develop a standardized method for investigating the mechanism of probiotics.
Traditionally, these studies require inoculating certain probiotics into germ-free mice7. However, there are some laboratory limitations in using germ-free mice as receptors for probiotics, such as the low immune capacity and expensive germ-free facilities8. In order to avoid these limitations, an alternative DSS-induced colitis model was established using pseudo-germ-free mice. The pseudo-germ-free mouse model was established by the application of antibiotics as previously described9,10.
In this article, we establish pseudo-germ-free mice with an antibiotic cocktail. We also describe in detail the methodology for establishing and evaluating mouse colitis models and investigate the effects of probiotics on alleviating colitis symptoms. The protocol below also provides the methods of probiotic delivery by gavage.
프로토콜
All procedures were carried out in accordance with the Guidelines for Care and Use of Laboratory Animals of Anhui University, China, and all procedures were approved by the Animal Ethics Committee of Anhui University, China (No. IACUC(AHU)-2020-014).
1. Administration of antibiotics
- Prepare a cocktail of antibiotics by dissolving ampicillin (1 g/L), neomycin sulfate (1 g/L), metronidazole (0.5 g/L), and vancomycin (0.5 g/L) in 1 L of drinking water5.
- After complete dissolution, store the solutions at 4 °C.
- Put the antibiotic cocktail solutions into a water bottle. Place the bottle on top of the mouse cage.
NOTE: Ensure to use brown bottles or wrap the bottles with aluminum foil to protect the antibiotic solutions from light. - Replace the antibiotic cocktail with fresh solutions 2x per week.
- Allow the mice to drink the antibiotic cocktail solutions freely for 4 weeks.
2. Preparation of DSS drinking water and induction of colitis
- To make a 2.5% dextran sulfate sodium (DSS) solution, dissolve 2.5 g of DSS in 100 mL of distilled water11.
- Replace the drinking water with the 2.5% DSS solution in the colitis model mice group. Ensure that the mice in the colitis group have no access to other drinking water. Meanwhile, treat the control mice with distilled water without DSS.
- House male C57BL/6J mice (average body weight, 20 g ± 2 g; aged 7 weeks) under pathogen-free conditions with free access to standard food and water.
NOTE: House a total of five mice per cage under pathogen-free conditions. - Experimental design
NOTE: The animal experiments were designed in two stages. Five mice were included for each group.- First stage: To assess the positive effect of B. cereus on alleviating colitis, randomly assign the mice to the following three groups: control group (control), DSS-induced colitis model (DSS), probiotic B. cereus supplementation for DSS-induced mice (B. cereus).
- Second stage: To explore the role of gut microbiota in regulating the probiotic effects of B. cereus on DSS-induced colitis, randomly assign the mice into the following three groups: ABX (control), ABX (DSS), and ABX (B. cereus + DSS).
- Treat the mice in the ABX (control) group with physiological saline.
- After treatment with antibiotic cocktail solutions for 4 weeks, let the mice in the ABX (DSS) group receive water containing 2.5% DSS for 1 week and normal physiological saline for 2 weeks.
- After treatment with antibiotic cocktail solutions and DSS water, treat the ABX (B. cereus + DSS) group mice daily with B. cereus (2 x 108 cells) in 200 µL of physiological saline using an intragastric cannula for 2 weeks.
- After the administration of the antibiotic cocktail, immediately treat the male C57BL/6 mice with 2.5% DSS solution in drinking water for 7 days.
- Record the bodyweight of the mice before the DSS intervention as the baseline weight.
3. Quantification of B. cereus
- Prepare eight 1.5 mL microcentrifuge tubes filled with 900 µL of sterile normal saline.
- Pipette 100 µL of logarithmic growth B. cereus and dissolve in 900 µL of sterile normal saline.
- Serially dilute B. cereus using tubes.
- Plate 40 µL of each dilution onto the respective LB agar plate labeled with the dilution ratio. Repeat 3x for each dilution ratio.
- Culture the plates in a 37 °C constant temperature incubator for 16 h.
- Select the plates with ~20-70 colonies for counting. Calculate the average bacterial concentration according to the dilution multiple of the selected plates.
4. Preparation of B. cereus for gavage
- After the quantification of B. cereus, collect 1 mL of B. cereus medium containing 1 x 109 CFU and centrifuge at 8,000 x g for 10 min. Then, wash the bacterial cells 2x with sterile water by centrifugation at 8,000 x g for 10 min and remove the supernatant.
- Dilute the concentrated B. cereus with sterile normal saline to a final concentration of 2 x 108 CFU/200 µL4.
- Pipette the dilution up and down repeatedly to acquire a dispersed B. cereus suspension.
- Carry out the quantification of B. cereus concentration by the plate count technique before completion of the gavage method.
5. Application of B. cereus supplements by gavage
- Administer the interventions (2 x 108 CFU probiotics in 200 µL of normal saline) into the mice through oral gavage at a fixed time.
- To administer B. cereus, connect a gavage needle (feeding needle size: 20 G, 38 mm) to a 1 mL syringe. Wipe the outside of the gavage needle with 70% ethanol between mice and maintain proper dose application.
- To restrain the mice, pick them up by their tail, and grasp the loose skin of the back firmly with the end of the tail fixed between the handler's last two fingers.
- Keep the mice in a vertical position. Then, insert the gavage needle carefully and gently through the mouth into the esophagus. If no resistance is met in this step, gently push the needle into the stomach.
NOTE: Confirm regular and unimpeded breathing of the mice before drug administration. - Slowly advance the needle and administer the B. cereus solution. Withdraw the needle gently after the completion of the gavage. Release the mouse and return it to its cage.
6. Determination of the disease activity index
NOTE: The disease activity index (DAI) was calculated daily by the mice's body weight loss, fecal occult blood, and stool consistency.
- Measure the bodyweight of the mice daily. Assign a score of 0-4 to each mouse based on the body weight loss percentage (0: 0%, 1: 1%-5%, 2: 6%-10%, 3: 11%-18%, and 4: >18%).
- Collect fresh stool samples from each mouse. Use a cotton swab to stimulate the contraction of the anus to promote defecation. Then, collect the feces samples immediately after defecation in a sterilized centrifuge tube.
- Determine the fecal occult blood by stool occult blood test paper. Collect 10 mg of stool sample on an applicator stick.
- Put the specimen on the test card and apply a thin smear to the card. Open the back flap and apply developer over the smear.
- Then,score for occult blood using stool occult blood test paper within 120 s.
NOTE: Any trace of blue on or at the edge of the smear is positive for occult blood. Fecal occult scores were as follows: 0: None, 1: Hemoccult positive, 2: Slight, 3: Blood traces in stool and 4: Gross bleeding. - Identify the stool consistency by detecting the stool sample11. Assign a score of 0-4 (0: Normal, 1: Loose stools, 2: Semi-formed stools, 3: Liquid stools, and 4: Diarrhea).
7. Tissue collection
- Anesthetize the mice by injecting 10% chloral hydrate (10 mg/kg) at the end of the first and second stages of the experiment. Evaluate the depth of anesthesia in mice by the pinch withdrawal reflex.
- Then, euthanize the mice by cervical dislocation.
- Fix the mice supine on the operating table, and open the peritoneal cavity.
- Separate the entire colon surgically and measure the length with a ruler.
- Then, gently wash the colon with a 5 mL syringe filled with precooled PBS.
8. Assessment of histological damage
- Cut the colon tissue into small fragments, fix them in 4% paraformaldehyde, and embed them in paraffin using a tissue embedding machine.
- Then, section the paraffin-embedded tissues with a microtome to prepare 4 µm thick colonic tissue sections. Dewax and dehydrate the colon sections. Then, stain the colon sections with hematoxylin and eosin (H&E).
NOTE: The stained colon samples are assessed by the investigator with a system for the following measures: degree of inflammatory cell infiltration (None: 0, Mild: 1, Moderate: 2, Severe: 3), mucosal damage (None: 0, Mucous layer: 1, Submucosa: 2, Muscularis and serosa: 3), crypt damage (None: 0, 1/3: 1, 2/3: 2, entire: 3, entire + epithelium loss: 4), range of lesions (%) (0%: 0, 1%-25%: 1, 26%-50%: 2, 51%-75%: 3, 76%-100%: 4). The total histological damage score is calculated for each individual score of the above measures5. - Let two independent experimenters evaluate (blinded) the histological scores of the sample under a light microscope.
결과
B. cereus supplementation and colitis
The acute experimental colitis model was induced by the DSS intervention in drinking water. Figure 1A shows the experimental protocol for B. cereus administration to the colitis mouse model. DSS inducement obviously decreased the body weight (Figure 1B) and colon length. The alleviating effect of B. cereus through oral gavage was investigated on the colitis model mice. Compared with DSS-induced colitis mice, body weight loss and colon length shortening (Figure 1C) were inhibited in the B. cereus-treated mice group.
DAI scores are widely used to evaluate the progression of colitis. DSS intervention markedly increased the DAI scores in the colitis model mice. However, the DAI scores in the B. cereus group were lower (Figure 1D). These data demonstrate that B. cereus supplementation improved DSS-induced colitis.
B. cereus and colonic mucosal injury
H&E staining was used to assess the histopathological damage of colon tissue (Figure 2A). The colon tissue of the colitis model mice presented crypt loss, goblet cell damage, inflammatory cell infiltration, and the loss of epithelium. B. cereus treatment improved DSS-induced histopathological damages.
Moreover, colitis severity was quantitatively assessed according to the histopathological scores (Figure 2B). The histopathological scores of colitis mice were significantly higher than in the control group. However, the histopathological scores in the B. cereus group were significantly lower than in the colitis mice.
Gut microbiota in relation to colitis and B. cereus administration
The role of B. cereus in alleviating colitis was assessed using a pseudo-germ-free mouse model, which avoided interference from complex gut microbiota and environmental factors. To test whether gut microbiota play a role in the alleviating effect of B. cereus on colitis, the pseudo-germ-free mouse model was developed by administering antibiotics for 4 weeks before DSS intervention. Then, B. cereus was translocated into the gut of pseudo-germ-free mice for 2 weeks.
As shown in Figure 3A, mice were randomly assigned into three groups: the ABX (control) group, ABX (DSS) group, and ABX (B. cereus + DSS) group. The present results indicate that the body weight loss, DAI scores, and histopathology scores were higher and the colon length was shorter in the ABX (DSS) group compared with mice in ABX (control) group. The body weight loss (Figure 3B), colon length (Figure 3C), DAI scores (Figure 3D), and histopathology scores (Figure 3E) were not statistically different between the ABX (DSS) and ABX (B. cereus + DSS) groups. The present results indirectly suggest that the gut microbiota plays a key role in the positive effects of B. cereus on colitis.
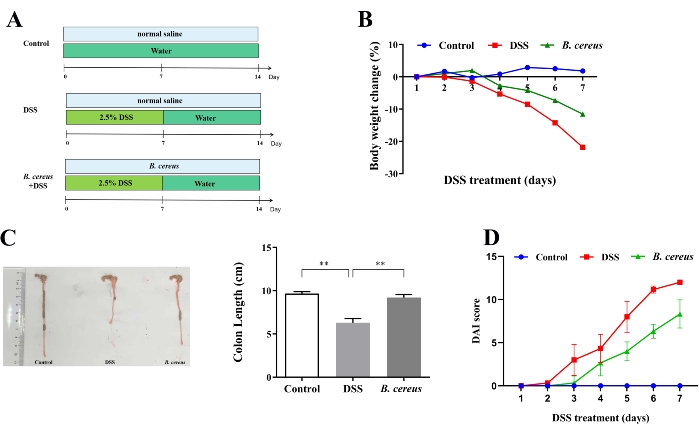
Figure 1: B. cereus administration relieved dextran sulfate sodium (DSS)-induced colitis. (A) Schematic diagram of the experimental procedure. The mice from the DSS and B. cereus groups were administered with 2.5% DSS solutions from day 1 to day 7. The DSS colitis model group and B. cereus group were treated with normal saline and 2 x 108 CFU B. cereus in 200 µL normal saline for 14 days. (B) Changes in mouse body weight. (C) Representative photographs of colons (left) and the quantification of colon length (right). (D) The disease activity index (DAI) scores after DSS administration. Consistent data were obtained from three biologically independent experiments. Data are expressed as mean ± standard deviation. *P < 0.05; **P < 0.01; ***P < 0.001. Please click here to view a larger version of this figure.
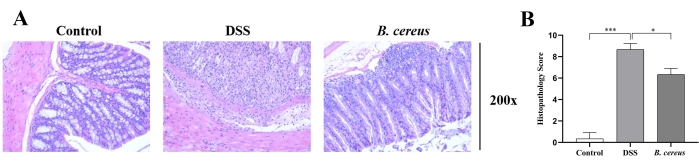
Figure 2: B. cereus treatment relieved colon injury. (A) Representative hematoxylin and eosin-stained histological sections (magnification 200x). (B) Histopathological score analysis of each view. Please click here to view a larger version of this figure.
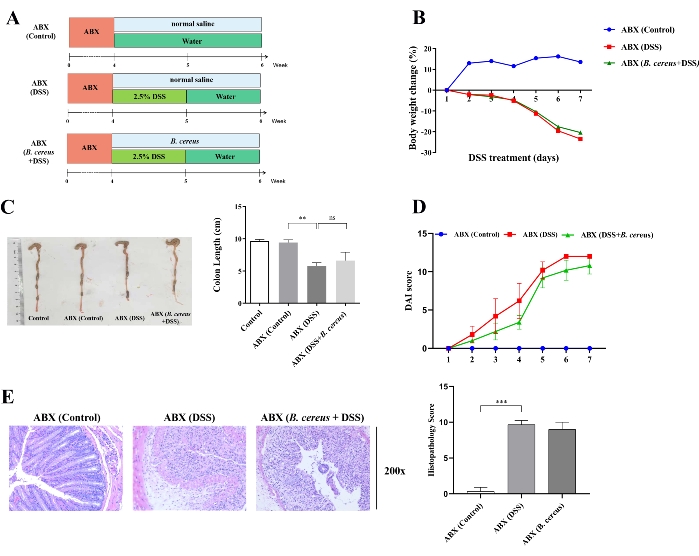
Figure 3: Alleviating effects of B. cereus on colitis were suppressed upon treatment with antibiotics. (A) Mice were induced with antibiotics for 4 weeks to clear the intestinal microbiota before DSS intervention. Then, the mice in the ABX (DSS) and ABX (B. cereus + DSS) groups were administrated with normal saline and 2 x 108 CFU live B. cereus cells in normal saline for 2 weeks, respectively. (B) Bodyweight change during DSS treatment. (C) Representative colon morphology (left) and colon length (right) in ABX-treated mice. (D) Disease activity index scores in ABX-treated mice. (E) H&E stained images (left) and histopathology score (right) in ABX-treated mice. Data are shown as mean ± standard deviation. *P < 0.05; **P < 0.01; ***P < 0.001 (Unpaired t-tests, Kruskal-Wallis test, and one-way analysis of variance, followed by a post hoc test). Please click here to view a larger version of this figure.
토론
In order to use probiotics in clinical studies, it is necessary to evaluate the efficacy and safety of probiotics in animal models. The provided protocols have been previously optimized for evaluating colitis severity. Mice treated with DSS is a model of intestinal inflammation, which mimics the clinical and histological features characteristic of ulcerative colitis12. Acute colitis model mice are characterized by body weight loss, diarrhea, fecal bleeding, and inflammatory cell infiltration13. The disease activity index (DAI) scoring system is employed for qualitative evaluation of the performance of the colitis model. The DAI scores are calculated according to the body weight loss score, stool consistency score, and fecal blood score14. DSS inducement decreased the mice's body weight and colon length and increased the DAI scores. However, the body weight loss, colon length shortening, and DAI scores were significantly decreased in B. cereus-treated colitis mice. Using these experimental methods, it was observed that B. cereus administration alleviated the symptoms of DSS-induced colitis.
The mice's colitis severity was also assessed in this study according to changes in colon length. At the end of the experiment, mice were sacrificed, and the colon was excised. The colon length was measured from the anus to the caecum. The colon tissues were collected and treated in 10% buffered formalin; then, the histopathology scoring system was also used to assess the severity of colitis. The histopathology score of colon injury was performed based on the inflammation score, mucosal damage score, crypt damage score, and range of lesions score15.
DSS-induced colitis mice showed colonic mucosal injury as evidenced by goblet cell depletion, epithelial erosion, and inflammatory cell infiltration16. Furthermore, these histological damages in mice with colitis were associated with clinical features, such as body weight loss, colon shortening, and rectal bleeding. However, the histological appearance of the colon was ameliorated obviously by B. cereus administration.
Then, the experimental protocols for oral administration of antibiotics to mice were applied to evaluate the role of gut microbiota in improving colitis. Antibiotic-mediated depletion of gut microbiota in animals is an alternative to germ-free mice to investigate gut microbiota-dependent effects17. The pseudo-germ-free mice offer a more amenable and cost-effective mice model for studying the mechanism. The antibiotic cocktail protocol included ampicillin, vancomycin, neomycin, and metronidazole, which provide bactericidal activity against a full spectrum of bacteria9. Our results indicated that the alleviating role of B. cereus against colitis depended on the gut microbiota, as proved by antibiotic treatment.
In summary, we have described protocols in detail for analyzing tissue damage during DSS colitis and the oral antibiotic treatment of mice for evaluating the role of probiotics in improving colitis. The evidence obtained from DSS-induced colitis model mouse experiments supports the establishment of exploring the protective effects of probiotics. In addition, pseudo-germ-free mice are cost-effective and reliable for manipulating the gut microbiota and investigating the role of gut microbiotics in colitis. It is important to emphasize that these experimental protocols need to be further optimized and can be adjusted depending on the different experimental purposes.
공개
The authors have nothing to disclose.
감사의 말
This study was supported at least in part by the National Natural Science Foundation of China (K.S., grant No. 32000081), the Natural Science Foundation of Anhui Province (K.S., grant No. 1908085QC120), the Open Project Program of State Key Laboratory of Food Science and Technology, Jiangnan University (K.S., grant No.SKLF-KF-201920), the Natural Science Foundation of Anhui Higher Education Institutions of China (K.S., grant No. KJ2019A0040), the Doctoral Scientific Research Foundation of Anhui University (K.S., grant No. J01003316 ), the National Natural Science Foundation of China (Y.W., grant Nos. 31770066, 31470218), the Open fund for Discipline Construction, Institute of Physical Science and Information Technology of Anhui University (Y.W.), and the Outstanding Talents Program of Anhui University, China (Y.W.).
자료
| Name | Company | Catalog Number | Comments |
| 0.01 M PBS (powder, pH7.2–7.4) | Solarbio | P1010-2L | |
| Absolute ethyl alcohol | Hushi | 64-17-5 | |
| Acid alcohol fsat differentiation solution | Beyotime | No.C0163S | |
| Agar | Sangon Biotech | 9002-18-0 | |
| Ampicillin | Solarbio | 69-53-4 | Store at 2–8 °C |
| Anaerobic incubator | Long Yue | LA1-3T | |
| Dextran sulfate sodium salt colitis grade | MP Biomedicals | 160110 | |
| Electrothermal incubator | SANFA | HP-050A | |
| General purpose tissue fixator | biosharp | BL539A | |
| Glycerinum | Hushi | 56-81-5 | |
| Hematoxylin and eosin staining kit | Beyotime | No.C0105 | |
| Kisser's Mounting Medium | Beyotime | No.C0181 | |
| Metronidazole | Solarbio | 443-48-1 | Store at 2–8 °C |
| Neomyein sulfate | Solarbio | 1405-10-3 | Store at 2–8 °C |
| Oscillating incubator | Shanghai Zhichu | ZQLY-180S | |
| Sodium chloride | Sangon Biotech | 7647-14-5 | |
| Stool occult blood test paper | Baso | BA2020B | |
| Tryptone | OXOID | 2285856 | |
| Vancomycin | Solarbio | 1404-93-9 | Store at 2–8 °C |
| Xylene | Hushi | 1330-20-7 | |
| Yeast extract | Sangon Biotech | 8013-01-2 |
참고문헌
- Pittayanon, R., et al. Differences in gut microbiota in patients with vs without inflammatory bowel diseases: A systematic review. Gastroenterology. 158 (4), 960-946 (2020).
- Kuehn, F., Hodin, R. A. Impact of modern drug therapy on surgery: Ulcerative colitis. Visceral Medicine. 34 (6), 426-431 (2018).
- Harmand, P. -. O., Solassol, J. Thiopurine drugs in the treatment of ulcerative colitis: Identification of a novel deleterious mutation in TPMT. Genes. 11 (10), 1212 (2020).
- Sheng, K., et al. Synbiotic supplementation containing Bifidobacterium infantis and xylooligosaccharides alleviates dextran sulfate sodium-induced ulcerative colitis. Food & Function. 11 (5), 3964-3974 (2020).
- Sheng, K., et al. Probiotic Bacillus cereus alleviates dextran sulfate sodium-induced colitis in mice through improvement of the intestinal barrier function, anti-inflammation, and gut microbiota modulation. Journal of Agricultural and Food Chemistry. 69 (49), 14810-14823 (2021).
- Wei, L., et al. PRKAR2A deficiency protects mice from experimental colitis by increasing IFN-stimulated gene expression and modulating the intestinal microbiota. Mucosal Immunology. 14 (6), 1282-1294 (2021).
- Souza, E. L. S., et al. Beneficial effects resulting from oral administration of Escherichia coli Nissle 1917 on a chronic colitis model. Beneficial Microbes. 11 (8), 779-790 (2020).
- Trinder, M., Daisley, B. A., Dube, J. S., Reid, G. Drosophila melanogaster as a high-throughput model for host-microbiota interactions. Frontiers in Microbiology. 8, 751 (2017).
- Liang, W., et al. Colonization potential to reconstitute a microbe community in pseudo germ-free mice after fecal microbe transplant from equol producer. Frontiers in Microbiology. 11, 1221 (2020).
- Hernandez-Chirlaque, C., et al. Germ-free and antibiotic-treated mice are highly susceptible to epithelial injury in DSS colitis. Journal of Crohns & Colitis. 10 (11), 1324-1335 (2016).
- Sheng, K., et al. Grape seed proanthocyanidin extract ameliorates dextran sulfate sodium-induced colitis through intestinal barrier improvement, oxidative stress reduction, and inflammatory cytokines and gut microbiota modulation. Food & Function. 11 (9), 7817-7829 (2020).
- Xu, X., et al. Histological and ultrastructural changes of the colon in dextran sodium sulfate-induced mouse colitis. Experimental and Therapeutic Medicine. 20 (3), 1987-1994 (2020).
- Zheng, L., et al. Jianpi Qingchang decoction alleviates ulcerative colitis by inhibiting nuclear factor-kappa B activation. World Journal of Gastroenterology. 23 (7), 1180-1188 (2017).
- Gao, X., et al. Symptoms of anxiety/depression is associated with more aggressive inflammatory bowel disease. Scientific Reports. 11, 1440 (2021).
- Thippeswamy, B. S., et al. Protective effect of embelin against acetic acid induced ulcerative colitis in rats. European Journal of Pharmacology. 654 (1), 100-105 (2011).
- Sakai, S., et al. Astaxanthin, a xanthophyll carotenoid, prevents development of dextran sulphate sodium-induced murine colitis. Journal of Clinical Biochemistry and Nutrition. 64 (1), 66-72 (2019).
- Li, S., et al. Role of gut microbiota in the anti-colitic effects of anthocyanin-containing potatoes. Molecular Nutrition & Food Research. 65 (24), 2100152 (2021).
재인쇄 및 허가
JoVE'article의 텍스트 или 그림을 다시 사용하시려면 허가 살펴보기
허가 살펴보기This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. 판권 소유