Method Article
Swine Models of Aneurysmal Diseases for Training and Research
요약
Description of two different aneurysmal swine models for neuroradiology training courses and research studies. This study provides evidence of the feasibility of these aneurysm porcine model creations and the reproducible methods that are close to the clinical setting.
초록
Large animal models, specifically swine, are widely used to research cardiovascular diseases and therapies, as well as for training purposes. This paper describes two different aneurysmal swine models that may help researchers to study new therapies for aneurysmal diseases. These aneurysmal models are created by surgically adding a pouch of tissue to carotid arteries in swine. When the model is used for research, the pouch must be autologous; for training purposes, a synthetic pouch suffices.
First, the right external jugular vein (EJV) and right common carotid artery (CCA) must be surgically exposed. The EJV is ligated and a vein pouch fashioned from a short segment. This pouch is then sutured to an elliptical arteriotomy performed in the CCA. Animals must be kept heparinized during model creation, and local vasodilators may be used to decrease vasospasms. Once the suture is completed, correct blood flow should be inspected, checking for bleeding from the suture line and vessel patency. Finally, the surgical incision is closed by layers and an angiography performed to image the aneurysmal model.
A simplification of this aneurysmal carotid model that decreases invasiveness and surgical time is the use of a synthetic, rather than venous, pouch. For this purpose, a pouch is tailored in advance with a segment of a polytetrafluoroethylene (PTFE) prosthesis, one end of which is sutured close using polypropylene vascular suture and sterilized prior to surgery. This "sac" is then attached to an arteriotomy performed in the CCA as described.
Although these models do not reproduce many of the physiopathological events related to aneurysm formation, they are hemodynamically similar to the situation found in the clinical setting. Therefore, they can be used for research or training purposes, allowing physicians to learn and practice different endovascular techniques in animal models that are close to the human system.
서문
Intracranial aneurysm (IA) is a severe cerebrovascular disease associated with up to a 50% mortality rate when ruptured. It is a relatively common and potentially lethal condition, with a reported prevalence between 3.6% and 6% in angiographic studies1. The intracranial vessels are abnormally dilated and suffer distension due to multifactorial risk factors, including, but not limited to, smoking, hypertension, excessive alcohol intake, or increasing age. When left untreated, IA can spontaneously rupture, resulting in subarachnoid hemorrhage (SAH) that is responsible for significant morbidity and death2,3,4. Additionally, one third of patients require hospitalization or nursing care, and only 30% of patients with SAH can return to independent living, thus representing a serious disease burden in humans that actually justifies the need for animal experiments5.
Nowadays, patients with high risk of IA rupture and hemorrhage are treated with occlusion mainly by endovascular coiling, microsurgical clipping, or flow diverting stents6,7. The endovascular procedure has been evaluated by the International Subarachnoid Aneurysm Trial (ISAT), demonstrating that coiling is safer, less invasive, and therefore has less significant adverse effects than microsurgical therapy3. For these reasons, the endovascular procedures are the most common techniques used for IA treatment3. Specialized training is required for physicians to perform these minimally invasive procedures correctly8.
Moreover, the development of new devices or therapies for IA treatment needs to be well-established and tested in preclinical studies before their translation to the clinical setting6,9. There are different IA experimental animal models according to the main objective of the research or training purposes. These models have been performed in numerous species, with their limitations and advantages. However, all of them entail artificial induction or surgical creation due to the absence of natural IA in animals2,6,9,10,11,12.
Although no animal model perfectly reproduces the human pathophysiology, small animals, such as rodents, are the most frequently used in IA research studies6. Large species are usually employed for the development of new endovascular devices or training in therapeutic interventions2. Among large animal models, it is common to use swine to research IA disorders and therapies, as well as for training courses. This is because of their ability to tolerate the surgical procedure and their similar vascular diameter and blood flow when compared to human cerebral vessels2,13.
The method of choice for IA animal model creation varies depending on the main objective of each individual research project, such as whether angiographic or histologic endpoints will be evaluated. In this sense, models created by surgical ligation or by adding an autologous pouch of tissue to the CCA are used for IA growth research. Surgical models must be combined with hypertension induction if the primary endpoint of the study is IA rupture. When the model is used for training purposes, the technique can be simplified by using a synthetic pouch sutured onto the CCA without the need for hypertension6.
This paper describes two different aneurysmal swine models that may help researchers to study new therapies or training in endovascular interventions for IA diseases. These aneurysmal models are created by surgically adding a pouch of tissue to the CCA in swine. When the model is used for research, the pouch is autologous, thus providing the ability to study healing of the aneurysm after exclusion without the interference of any exogenous material. For training purposes, a synthetic pouch that recapitulates the endovascular anatomy to reproduce the procedure suffices.
프로토콜
The experiment was approved by the ethical committee of the Jesús Usón Minimally Invasive Surgery Centre, and all procedures were performed according to Spanish Royal Decree 53/2013 and the European regulation (2010/63/EC).
1. Presurgical preparation and anesthesia
- House large white swine weighing 35-40 kg individually, with free access to water and feed once a day. Acclimate for 2 weeks before the date of the intervention, to perform clinical examination and allow time for detecting silent diseases.
- Administer the following oral drugs to prevent thrombotic events during the study: Acetylsalicylic acid (1 g/animal/24 h) and Clopidogrel (75 mg/animal/24 h) from 7 days before model induction until IA therapy.
- Inject ketamine (10 mg/kg) intramuscularly after a 24 h fasting period. Ten min later, induce anesthesia by 1% propofol intravenously (3 mg/kg).
- After endotracheal intubation, use inhaled sevoflurane to maintain anesthesia (3%-4.5% inspiratory fraction). Connect endotracheal tubes to a semiclosed, circular anesthetic circuit attached to a ventilator with a fresh gas flow rate of 0.5-1 L/min.
- To obtain normocapnia (35-45 mmHg CO2), control ventilation by a tidal volume of 8-10 mL/kg. Ensure an adequate intraoperative analgesia by an initial intravenous dose of ketorolac (1 mg/kg) and tramadol (1 mg/kg) combination and a continuous remifentanil infusion (15-18 µg/kg/h).
- Confirm proper animal plane of anesthesia considering unconsciousness (hypnosis), insensitivity to pain, muscle relaxation, and the absence of reflex responses14.
- To prevent dryness while under anesthesia, use a vet ointment on eyes during the surgery procedure.
- Fix the anesthetized animals at the operating table in supine position. Shave the animals´ necks, scrub with povidone-iodine, and drape under sterile conditions.
- Perform all procedures under sterile conditions, using sterile gloves and material.
2. Surgery
- Surgical approach
- Perform a 10 cm long longitudinal skin incision 2-3 cm to the right of the neck´s midline.
- Pouch tailoring
- Autologous pouch
- To expose the EJV, dissect the subcutaneous and fat tissue and perform hemostasis.
- Separate the right sternocephalicus muscle from the connective tissue and retract it with a Weitlaner retractor to facilitate EJV exposure.
- Expose and identify the EJV, which is lateral and deeper than the CCA (Figure 1A).
- Use two bulldog vascular clamps to stop blood flow inside the vessel during the venous segment extraction.
- Isolate a 15-20 mm segment of the EJV to obtain the autologous pouch.
- Ligate the proximal and distal ends of the EJV.
- Flush the extracted vein segment with heparinized saline (5,000 IU/L).
- Check the inside of the excised EJV and select a 7-8 mm long segment where no venous valves are present.
- Fashion this segment into a pouch by closing one end with a 7/0 polypropylene running suture (Figure 1B).
- Keep the pouch immersed in heparinized saline until use.
- Confirm no bleeding from the ligated EJV.
- Synthetic pouch
- Cut a 1 cm long segment from a PTFE prosthesis (6-8 mm in diameter, depending on the aneurysmal size that is going to be created).
- Suture one end close using a 6/0 polypropylene vascular suture. Seal this closed part of the prosthesis with vascular glue to avoid bleeding from the suture line.
- Sterilize this synthetic graft prior to surgical aneurysm creation.
- Autologous pouch
- Aneurysm creation surgery
- To expose the CCA, dissect the subcutaneous and fat tissue and perform hemostasis.
- Separate the right sternocephalicus muscle from the surrounding connective tissue and retract it with a Weitlaner retractor to facilitate CCA exposure.
- Identify the CCA. Place 2 silicon vessel loops at the cranial and distal end of the exposed CCA (Figure 2A). Dissect 5 cm of this vessel, removing its adventitia with a dissector (Figure 2B). During this surgical access, take care to avoid injuring the vagus nerve that can lead to Horner´s syndrome.
- Administer vasodilators locally (such as 1-2 mL of nimodipine 10 mg/50 mL) to prevent vasospasms.
- Administer heparin intravenously (150 IU/kg) 5 min before CCA crossclamping.
- Place one bulldog vascular clamp at the caudal dissected part of the CCA and another bulldog vascular clamp 4-5 cm apart at the cranial part of the vessel (Figure 2C).
- Use microscissors to perform an 8 mm elliptical arteriotomy in the CCA between the two bulldog vascular clamps (Figure 2D).
- Use heparinized saline solution (5,000 IU/L) to flush the segment of the CCA intraluminally.
- Suture the autologous or synthetic pouch to the elliptical arteriotomy using a 6/0 polypropylene running suture (Figure 3A,B). Flush the distal and proximal clamps before finishing the pouch suture.
- Protect the vessel and nearby structures with warm, heparinized saline solution during the microsurgical procedure.
- Once the prosthesis or autologous pouch is sutured to the CCA, check there is no bleeding (Figure 3C,D). First, remove the cranial bulldog vascular clamp, and then the caudal one.
- Perform hemostasis by applying pressure with wet swabs if there is some bleeding from the suture line. If required, apply traction to the vessel loops, or replace the bulldog vascular clamps and perform hemostatic stitches at the bleeding site. If necessary, place a piece of hemostatic gelatin sponge around the prosthesis.
- Inspect the CCA pulse cranial to the aneurysmal sac to ensure that correct carotid patency has been recovered.
- Close the surgical incision by layers using 2/0 absorbable sutures and the skin with single stitches with 0 nonabsorbable sutures.
- Administer buprenorphine (10 µg/kg/12 h) intramuscularly during the first 24 h and place a fentanyl transdermic release patch (25 µg/h) after the surgical procedures to achieve postoperative analgesia.
- Decrease the inhaled sevoflurane and increase fresh gas flow rate (20 L/min) to obtain recovery conditions. Remove the endotracheal tube when the animals breathe spontaneously and physiological parameters have been recovered, such as oxygen saturation and heart rate.
NOTE: The fresh gas is a mixture of pure O2 (100%) and medical air (21% O2). Finally, the fraction of inspired oxygen is 50% to 45% (FiO2 = 0.5-0.45). - Do not leave the animals unattended until they have regained sufficient consciousness to maintain sternal recumbence.
- Keep the animals that have undergone surgery isolated from other animals until fully recovered.
3. Angiography test and postoperative phase
- Wait for 24-48 h to avoid damaging the suture line.
- Anesthetize the animal again as described above and shave the groin area. Prepare the zone with povidone-iodine and apply sterile draping.
- Access a femoral artery using the modified Seldinger technique with a 6Fr introducer sheath.
- Insert a 5Fr headhunter catheter through the femoral sheath over a 0.035 in hydrophilic guidewire. Under fluoroscopic guidance, advance this catheter to the origin of the CCA and remove the guidewire.
- Inject contrast medium (amidotrizoic acid diluted to 50% with a saline solution) through the headhunter catheter to image the CCA with aneurysm model.
- Once the angiography confirms correct aneurysm model creation, use animal models for research or for training endovascular treatment procedures.
- Euthanize the animals when the studies or training courses finish with a lethal intravenous administration of potassium chloride (2 mmol/kg) while under deep anesthesia.
결과
The presented technique has been used for different purposes, namely research into postcoiling aneurysm healing and training in embolization techniques. Venous pouches have been used for testing differential healing using both platinum and bioactive coils. The pouches were sutured as described above and, 24 h after model creation, an angiogram was obtained to document the dimensions and appearance of the aneurysms. Endovascular coil embolization was performed successfully in all the pigs. In each case, the left-side aneurysm was filled with bioactive, and the other side with pure platinum coils (Figure 4A). Animals were followed up for 15 days, when angiography was performed to check aneurismal exclusion. The aneurysms were excluded in all cases except in one case that showed an incomplete embolization.
Synthetic pouches have been used for model creation prior to training courses. A total of 12 aneurysms were created in eight animals (bilateral n = 4, single carotid n = 4). Four animals were subjected to bilateral model creation that was completed successfully in all cases without any complications. No mortality or neurovascular events were observed during model surgery or endovascular procedures 2 days after the aneurysms' creation. The vagus nerve was not injured during surgical procedures, and Horner´s syndrome was not induced in any animal. Additionally, no postoperative bleeding from the sutured prosthesis was observed in any case. CCA angiography was performed 2 days after model creation to check the aneurysm model appearance and patency immediately before proceeding to the model usage for training (Figure 4B). During the training course, aneurysm embolization was successfully achieved in all cases.
A certain degree of aneurysmal neck size variation was observed since running sutures were used. Different neck-to-dome conformations can thus be achieved by controlling this suture and the size of the pouches. The average duration of carotid cross-clamping during aneurysm model surgery with autologous and synthetic pouches was 40 min and 30 min, respectively. The animals tolerated this ischemic time without any complications.

Figure 1: Autologous pouch. (A) EJV identification: this vessel is lateral and deeper than the CCA. (B) Vein pouch: the EJV segment is fashioned into a pouch with a running suture. Abbreviations: EJV = external jugular vein; CCA = common carotid artery. Please click here to view a larger version of this figure.

Figure 2: CCA surgical preparation. (A) CCA identification; (B) CCA dissection. The CCA adventitia is removed with a dissector. (C) CCA clamping: a bulldog vascular clamp is placed at the caudal dissected part of the CCA and another bulldog vascular clamp 4-5 cm apart at the cranial part of the vessel. (D) CCA arteriotomy: an elliptical arteriotomy in the CCA is performed with microscissors between the two bulldog vascular clamps. Abbreviation: CCA = common carotid artery. Please click here to view a larger version of this figure.

Figure 3: Pouch suture. (A) Autologous pouch suturing; (B) Synthetic pouch suturing. The autologous or synthetic pouch is sutured to the elliptical arteriotomy using a 6/0 polypropylene running suture. (C) Autologous pouch is sutured to the CCA. (D) Prosthesis is sutured to the CCA. Bleeding is not checked after the prosthesis or autologous pouch is sutured to the CCA. Abbreviation: CCA = common carotid artery. Please click here to view a larger version of this figure.
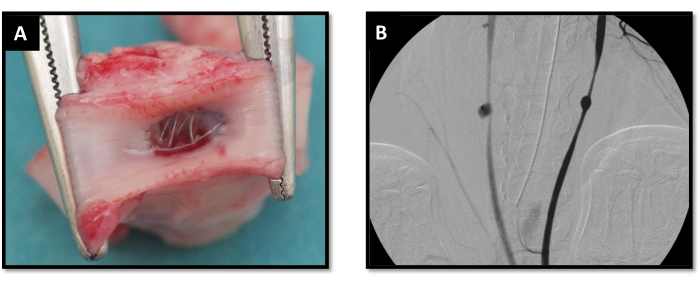
Figure 4: Postoperative phase. (A) Endovascular coil embolization; (B) CCA angiography. It was performed to check the aneurysm model appearance and patency (bilateral aneurysm model). Abbreviation: CCA = common carotid artery. Please click here to view a larger version of this figure.
토론
There are different techniques to create aneurysm animal models based on the objective of the study. Some aneurysm model protocols include surgical procedures combined with hypertension or hemodynamic stress induction by angiotensin II administration, nephrectomies, or high-salt diet, among others, because the main objective of these studies is aneurysm rupture research. However, in the present study, these conditions are not induced since these animal models are used for neuroradiology training or nonrupture aneurysm research studies, such as new endovascular devices or procedures6.
There are two critical steps in this animal model creation to pay attention to: the CCA dissection and the pouch suture. During CCA dissection, it is common to induce some vagus nerve injury without soft manipulation of this structure15. Furthermore, this step of the surgical procedure could induce vasospasm of the CCA. Adding warm saline solution and waiting several minutes solves this problem. However, incorrect suturing of the pouch to the CCA causes bleeding complications. Care must be taken during surgery to avoid including the carotid posterior wall in the suture, since this would result in CCA stenosis or occlusion. For these reasons, good surgical and microsurgical skills of the surgeons are mandatory for the performance of this surgery9.
The morphology and neck size variabilities allow for the generation of animal models that are close to the clinical setting, since variations in aneurysm morphology are also found in the human patient16. Although swine models are widely used for investigating endovascular devices and training, they have some disadvantages. The main one that can directly affect the described model is the high activation of the coagulation system and its tendency of spontaneous thrombosis in these animals2,17. For this reason, some considerations are important before performing the surgical procedure.
Anticoagulation must be performed by heparin injection just before clamping the CCA. This time point of administration is used to avoid bleeding during prior vessel exposure. Furthermore, it is highly recommended to administer clopidogrel and acetylsalicylic acid 1 week before the study to prevent thrombotic events10. This is a critical point of the methodology since heparin antithrombotic treatment could be performed only by daily injection, a more invasive and less well-tolerated procedure in animals for repeated dosages than oral administration of the antithrombotic drugs. The optimal time to create the aneurysm model is no earlier than 24-48 h prior to the intended use to avoid spontaneous sac thrombosis.
Some methodology modifications of this protocol could be performed. Regarding the postsurgical treatment, the daily intramuscular injection of buprenorphine is effective. However, we combine buprenorphine and fentanyl postoperatively since the fentanyl patch reaches therapeutic concentration 24 h after it is placed. It facilitates the long-term administration of analgesic treatment without more injections after the first one of buprenorphine.
Regarding the prosthetic graft, we create and then sterilize it with formaldehyde at 60 °C. However, a sterile synthetic graft can be used, and the pouch can be prepared under sterile conditions. Another possibility to simplify the protocol entails using carotid ultrasound to document the aneurysm presence and flow. The angiography test is useful because an angiographic image is needed for the training course, but the ultrasound is also a good, less invasive option to verify the model.
This study is not free of limitations. The main one is the absence of pathophysiological and histological features of human IA disease, which is important to consider during research studies. In this case, the histopathological detection has not been performed, which would be required in any long-term research involving the use of this model to investigate the healing of the aneurysmal neck and the efficacy of any therapy or devices. For these research studies, a longer follow-up than 15 days is needed to confirm the efficacy of the devices. Moreover, the cerebral vessels are more difficult to reach by endovascular navigation than the carotid artery, which represents a clear limitation for neurosurgical training courses. However, no current IA animal model in the literature perfectly replicates the IA human disease and all of them are artificially created and are associated with such limitations2,6,9,10. Our results suggest that these two animal models are promising for nonrupture aneurysm research and neuroradiology training. This study provides evidence for the feasibility of these porcine aneurysm model creations, since in the described procedures, no mortality, morbidity, or Horner´s syndrome cases were noted. Therefore, this is a reproducible method to induce aneurysms that contributes to decreasing the number of animals used according to the European legislation of animal welfare guidelines.
공개
The authors have no conflicts of interest to disclose.
감사의 말
The study was performed by the ICTS 'NANBIOSIS', more specifically by U-21 (Experimental Operating Rooms), U-22 (Animal Housing), and U-24 (Medical imaging) of the Jesús Usón Minimally Invasive Surgery Centre (JUMISC). This work was funded by the Instituto de Salud Carlos III (CB16/11/00494) and the Consejeria de Economía, Ciencia y Agenda Digital, Junta de Extremadura (GR21201), cofunded by the European Regional Development Fund "A way to make Europe". The authors acknowledge all the work performed by the animal housing, experimental technicians, and Joaquín González for taking photos of the surgical procedure.
자료
| Name | Company | Catalog Number | Comments |
| Acetylsalicylic acid | Sanofi | 700693 | 500 mg tablets |
| Amidotrizoic acid | Bayer Hispania | 914614.6 | Contrast medium 76% |
| Anesthesia Machine | Maquet Clinical Care AB | 6677200 | Maquet Flow-i C20 |
| Bulldog vascular clamp | Dimeda | 12.092.07 | 7.5 cm |
| Buprenorphine | Richter Pharma Ag | 578816 | 0.3 mg/mL |
| Clopidogrel | Sandoz | 704005 | 75 mg tablets |
| Contrast medium | Bayer Hispania | 914614 | Urografin 36% |
| Dissector | Dimeda | 12.421.01 | 21 cm |
| Fentanyl Matrix | Kern Pharma | 664823 | Transdermic release patch 25 µg/h |
| Fluoroscopy equipment | Philips Medical Systems | Veradius Unity | |
| Hemostatic gelatin sponge | Takeda Farmaceutica España, SA | 324459 | Absorbable hemostatic agent. Espongostan |
| Head hunter catheter | Boston Scientific | RF*YB15110M | 5 Fr 100 cm |
| Heparin | Rovi | 641639 | Heparin 5% |
| Hydrophilic guidewire | Terumo | RF*GA35153M | 0.035” 150 cm |
| Introducer sheath | Terumo | RS*B60N10MQ | 6 Fr 10 cm |
| Ketamine | Richter Pharma Ag | 580395 | 100 mg/mL |
| Ketorolac | Laboratorios Normon, S.A. | 603079 | 30 mg/mL |
| Micro-forceps | S&T | JFA-5b (1:1) | Forceps for microsugery |
| Micro-needle holder | S&T | Curved C-14 (Art nº 00088) | Needle holder for microsurgery |
| Microscissors | S&T | Adventitia SAS-15 R-8 (Art nº 00102) | Straight- scissors for microsurgery |
| Needle holder | Dimeda | 24.114.12 | 12 cm |
| Nimodipine | Bayer Hispania, S.L | 641969 | 10 mg/50 mL |
| Povidone-iodine | CV Medica | 193203 | Povidone iodine solution (10%) |
| Propofol | Orion Corporation | 588475 | 10 mg/mL |
| PTFE prosthesis | Maquet | M00201501086B0 | Synthetic prosthesis 6mm |
| Remifentanil | Laboratorios Normon, S.A. | 692295 | 2 mg |
| Scalpel handle | Dimeda | 06.104.00 | 13.5 cm |
| Scissors (Mayo) | Dimeda | 07.164.14 | 14.5 cm |
| Scissors (Metzenbaum) | Dimeda | 07.287.15 | 15 cm |
| Surgical blades | Dimeda | 06.122.00 | 22 |
| Sutures: absorbable suture | Medtronic | GL-123 | 2/0 |
| Sutures: poplypropylene suture | Aragó | 37803 | 6/0 and 7/0 |
| Swabs | Texpol | 1063.01 | 20 x 20 cm |
| Tissue forceps | Dimeda | 10.102.11 /10.120.11 | 11.5 cm |
| Vascular glue | Histoacryl Braun | 1050060 | Tissue adhesive |
| Vessel loops | Braun | B1095218 | 1.5 mm diammeter |
| Weitlaner | Dimeda | 18.670.14 | 14 cm |
참고문헌
- Keedy, A. An overview of intracranial aneurysms. Mcgill Journal of Medicine. 9 (2), 141-146 (2006).
- Thompson, J. W., et al. In vivo cerebral aneurysm models. Neurosurgical Focus. 47 (1), 20 (2019).
- Diaz, O., Rangel-Castilla, L. Endovascular treatment of intracranial aneurysms. Handbook of Clinical Neurology. 136, 1303-1309 (2016).
- Texakalidis, P., et al. Aneurysm formation, growth, and rupture: the biology and physics of cerebral aneurysms. World Neurosurgery. 130, 277-284 (2019).
- Petridis, A. K., et al. Aneurysmal subarachnoid hemorrhage: diagnosis and treatment. Deutsches Ärzteblatt International. 114 (13), 226-236 (2017).
- Strange, F., Gruter, B. E., Fandino, J., Marbacher, S. Preclinical intracranial aneurysm models: A systematic review. Brain Sciences. 10 (3), 134 (2020).
- Diana, F., et al. Microsurgical clipping versus newer endovascular techniques in treatment of unruptured anterior communicating artery-complex aneurysms: a meta-analysis and systematic review. Neurosurgical Review. , (2021).
- Fernández-portales, J., et al. Modelos animales en el aprendizaje de cardiología intervencionista. Revista Española de Cardiologia Suplementos. 13, 40-46 (2013).
- Boillat, G., et al. Creation of two saccular elastase-digested aneurysms with different hemodynamics in one rabbit. Journal of Visualized Experiments. 170, 62518 (2021).
- Marbacher, S., Strange, F., Frösén, J., Fandino, J. Preclinical extracranial aneurysm models for the study and treatment of brain aneurysms: A systematic review. Journal of Cerebral Blood Flow and Metabolism. 40 (5), 922-938 (2020).
- Murayama, Y., et al. Ion implantation and protein coating of detachable coils for endovascular treatment of cerebral aneurysms: concepts and preliminary results in swine models. Neurosurgery. 40 (6), 1233-1244 (1997).
- da Silva Júnior, S. L., et al. Stable experimental model of carotid artery saccular aneurysm in swine using the internal jugular vein. Revista do Colegio Brasileiro de Cirurgioes. 40, 130-136 (2013).
- Crisóstomo, V., et al. Common swine models of cardiovascular disease for research and training. Lab Animal. 45 (2), 67-74 (2016).
- Cobo, A. A., et al. Anesthesia protocols used to create ischemia reperfusion myocardial infarcts in swine. Journal of the American Association for Laboratory Animal Science. 59 (5), 478-487 (2020).
- Musk, G. C., King, M., He, B. Horner Syndrome in 2 Pigs (Sus scrofa) after Vascular Grafting of the Carotid Artery and Jugular Vein. Comparative Medicine. 67 (6), 518-523 (2017).
- Mocco, J., et al. Aneurysm morphology and prediction of rupture: An international study of unruptured intracranial aneurysms analysis. Neurosurgery. 82 (4), 491-495 (2018).
- Lee, D., et al. Thrombus organization and healing in the swine experimental aneurysm model. Part I. A histological and molecular analysis. Journal of Neurosurgery. 107 (1), 94-108 (2007).
재인쇄 및 허가
JoVE'article의 텍스트 или 그림을 다시 사용하시려면 허가 살펴보기
허가 살펴보기This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. 판권 소유