Method Article
Robotic Radical Cystectomy, Pelvic Lymph Node Dissection, and Intracorporeal Ileal Conduit Urinary Diversion
요약
This paper describes robotic radical cystectomy, pelvic lymph node dissection, and intracorporeal ileal conduit urinary diversion.
초록
The robotic approach to radical cystectomy is compelling because of its oncologic equivalence to open radical cystectomy (ORC), its association with lower surgical blood loss, its potential association with shorter hospital stay after surgery. These factors suggest that the robotic approach to radical cystectomy may be an important component of enhanced recovery programs aimed at reducing surgical morbidity. This paper describes the importance of the cranial placement of robotic trocars, the use of Cadiere forceps for atraumatic bowel grasping, pelvic lymph node dissection (PLND), and utero-enteric anastomoses. Also discussed are steps that are critical for the successful outcome of RARC. In spite of the increased operating times and associated costs and the costs of robotic surgical platforms and equipment, adoption of the robotic technique by bladder cancer surgeons has increased. This paper describes a systematic and reproducible method that details robotic extended pelvic lymph node dissection, cystectomy/cystoprostatectomy, and intracorporeal ileal conduit urinary diversion.
서문
Since the advent of robotic surgery in the USA in 2000, the Da Vinci robot has become increasingly utilized across surgical specialties1. The reasons for this trend are multiple and may include ease of instrumentation with wristed instruments (particularly in small or narrow body cavities), the desire to adopt new technology, and the potential for decreased perioperative morbidity as measured by intraoperative blood loss, post-operative pain, and/or length of inpatient stay after surgery2,3,4,5,6. Radical cystectomy is the standard of care for surgical management of localized muscle invasive bladder cancer (clinical stages cT2-4a, N0, M0)7,8,9. Clinical evidence strongly suggests that oncologic outcomes of open and robotic radical cystectomy are similar10. The impetus to adopt a robotic approach for radical cystectomy is the possibility that a minimally invasive approach may reduce complication rates.
As the morbidity of radical cystectomy is high (90-day overall complication rate of 64% and a 1.5% 30-day mortality rate), reducing cystectomy-associated complications is an urgent clinical need11,12. In fact, the robot-assisted radical cystectomy (RARC) versus ORC in patients with bladder cancer (RAZOR) trial demonstrated that a robotic approach to cystectomy is associated with much lower intraoperative blood loss, lower transfusion rates, and a slightly shorter postoperative length of stay10. It should be noted that RARC with intracorporeal urinary diversion (RARC with ICUD) is a complex procedure with a steep learning curve13,14,15. Accordingly, the objective of this paper is to explicitly detail the smaller component steps to one approach, which when considered individually, are simple and reproducible.
Herein, a systematic approach to robotic radical cystectomy, pelvic lymph node dissection (PLND), and intracorporeal ileal conduit urinary diversion has been described. Institutionally, the decision to perform an extracorporeal versus intracorporeal ileal conduit is surgeon- and patient-dependent. Although not necessary, it is preferable to perform bilateral extended pelvic lymph node dissection (PLND) prior to cystectomy for complete visualization of the external and internal iliac vessels and obturator nerve and vessels during division of the bladder pedicles to prevent inadvertent ligation/division of specific obturator and internal iliac structures. This may help in cases of bulky bladder tumors. Outcomes in three patients have been provided for illustrative purposes.
프로토콜
This protocol and description of representative results abide by the guidelines of the Ohio State University human research ethics committee, and the approval to provide these representative results was obtained from each patient in compliance with the institution's guidelines. The inclusion criteria were patients recommended to undergo surgical management of their bladder cancer. Patients with metastatic disease, comorbidities prohibiting surgical management of their cancer, or cancer determined to be unresectable were excluded.
1. Positioning and induction of anesthesia
- Ensure that preoperative fasting begins at midnight the evening before surgery.
NOTE: More recently, an enhanced recovery after surgery protocol for robotic cystectomy has been implemented, which allows for an oral carbohydrate drink at bedtime and two hours prior to surgery and encourages the use of alvimopan. No additional bowel preparation is prescribed. - Ensure timely administration of preoperative antibiotics and venous thromboembolism prophylaxis.
NOTE: It is preferable to administer 40 mg of subcutaneous enoxaparin for prophylaxis or 5000 units of subcutaneous heparin, if creatinine clearance is less than 30 mL/min. Preferred antibiotics are ampicillin/sulbactam, gentamicin, and diflucan. - Induce general anesthesia.
- Place the orogastric tube.
- Position the patient on the pink pad device, taking care to keep at least one inch of pink pad above the shoulders.
- Position the patient supine with arms tucked if using the Da Vinci Xi robot. Position the patient in dorsal lithotomy if using the Da Vinci Si robot.
- Prepare and drape the patient, then place a 16-18 Fr urethral catheter sterilely.
2. Surgery
- Gaining access and docking the robot
- Make an 8 mm incision 3 cm cranial to the umbilicus.
- Elevating the umbilicus and abdominal wall through the incision with a penetrating towel clamp, insert the Veress needle through the rectus fascia and peritoneum.
- Perform a saline drop test to ensure that access is in the appropriate location.
- Attach a 10 mL Luer lock syringe to the Veress needle with 10 mL of normal saline in the syringe. Aspirate to ensure no blood or fecal content is obtained, and then inject 3-5 mL of saline.
- Remove the syringe and watch for the fluid to drop through the needle into the intraperitoneal cavity, confirming appropriate intraperitoneal access.
- Attach insufflation tubing to the Veress needle, and start CO2 insufflation at a pressure setting of 10 mm Hg, making note of the starting pressure to again confirm appropriate intraperitoneal location.
NOTE: The starting pressure should be less than 8 mg Hg. - Using a 0-degree laparoscope placed through the visual obturator of an 8 mm trocar, insert the trocar into the peritoneum through the previously made 8 mm midline incision.
- Place additional robotic trocars after making additional transverse 8 mm skin incisions for each trocar: two on the right abdomen at a level 1-2 cm cranial to the umbilicus, 9-10 cm apart. Place one on the left abdomen at the level of the umbilicus also 9-10 cm away from the umbilicus (Figure 1).
- Place two assistant ports: one 12 mm trocar placed in the left lateral abdomen at the level of the umbilicus located 8-10 cm lateral to the left robotic port, and one 5 mm standard assistant trocar placed in the left upper quadrant, triangulated between the camera trocar and the left robotic trocar (cranial to both of these ports).
- Temporarily remove the 12 mm trocar and preplace a 0-Vicryl fascial suture at this site to prepare for fascial closure at the end of the case. Ensure that the ends of this suture are left untied and secured with a hemostat clamp.
- Place the patient in 25-degrees of Trendelenburg, and dock the Da Vinci robot. Dock the Xi robot on the patient's right side and the Si robot between the patient's legs.
- Place the robotic instruments under vision: robotic camera in the midline port (followed by targeting of robotic arms to the pelvis if using the Xi robot), monopolar scissors in the right medial arm, Maryland bipolar graspers in the left arm, and Cadiere grasping forceps in the right lateral arm.
- Initial dissection and pelvic lymphadenectomy
- Release the physiologic adhesions of the sigmoid colon from the left pelvic sidewall distally. Proximally, as the sigmoid colon meets the descending colon, begin to medialize the descending colon by incising the 'white line' of Toldt.
- In the male patient, begin to develop the posterior plane between the rectum and prostate by incising the peritoneum covering the reflection of the anterior rectum and posterior bladder neck. Develop the plane between the prostate and the rectum, taking it as distally as possible.
NOTE: This step combines selective use of electrocautery, sharp tissue division, and blunt spreading as the dissection proceeds distally. - Starting on the left, incise the posterior peritoneum at the root of the sigmoid mesentery, and carry this incision proximally and laterally to expose the left ureter coursing over the ipsilateral common iliac artery toward the bladder.
- Develop a small space posterior to the ureter; insert the Cadiere forceps in this space to retract the ureter anteriorly to expose any vascular attachments to the ureter that can be ligated with bipolar diathermy and subsequent sharp division. In this manner mobilize the ureter proximal to the common iliac artery (3-5 cm) and distally toward the bladder.
- Extended pelvic lymphadenectomy and ureteral ligation
- Starting on the left, divide the peritoneum overlying the external and common iliac artery. Divide the peritoneum lateral to the ipsilateral medial umbilical ligament, and begin to develop the ipsilateral space of Retzius. Join the two peritoneal incisions, and use bipolar diathermy to ligate the ipsilateral vas deferens prior to sharply dividing the vas with scissors.
NOTE: PLND can commence on either the left or the right. The current technique specifies starting on the left because release and mobilization of the sigmoid colon from its physiologic attachments to the left pelvic sidewall also typically reveals the left common/external iliac artery and left ureter, which facilitates starting the lymphadenectomy on that side. - Taking care to preserve the ipsilateral genitofemoral nerve, dissect lymphatics lateral to the external iliac artery off their attachments to the anterior psoas fascia. Divide lymphatic tissue overlying the anterior aspect of the common and external iliac artery.
- Lateral to the external iliac artery and vein, separate lymphatic tissue from the medial border of the psoas and levator muscles of the pelvic sidewall. Use the Maryland bipolar to control perforating vessels.
- Release lymphatic tissue from the medial border of the ipsilateral internal iliac artery.
- As the dissection proceeds distally along the internal iliac artery, note the takeoff of the obliterated umbilical artery (superior vesical artery). Clip the obliterated umbilical artery with a large non-absorbable locking clip placed by the assistant proximally, then ligate with bipolar diathermy distally prior to sharp division.
NOTE: This maneuver, in the midst of the lymphadenectomy, represents the beginning of the division of the ipsilateral bladder pedicle. - Prospectively identify the ipsilateral obturator nerve and vessels prior to removing the external iliac, internal iliac, and obturator lymph node packets. Control vascular attachments with the Maryland bipolar and ligate lymphatic channels with small and large locking clips. During these steps, use the Cadiere forceps to apply medial retraction on the medial umbilical ligament and/or external iliac vessels and/or distal/anterior retraction of the developing lymphatic packets.
- Retract the ureter medially, then remove the common iliac lymphatic tissue from the fossa of Marceille, an area bordered by the psoas laterally, common iliac artery medially, and the obturator nerve posteriorly.
- Remove lymph node packets with a reusable 10 mm specimen bag.
- Repeat steps 2.3.1-2.3.8 on the right to complete the right-sided lymphadenectomy.
- Retract the mesentery of the sigmoid colon (on the right side) anteriorly to expose the root of the sigmoid mesentery, and using selective electrocautery and blunt dissection, develop the pre-sacral space posterior to the sigmoid mesentery, proceeding from right to left.
- Perform pre-sacral lymphadenectomy only after the area between the internal iliac arteries (pre-sacral space) is developed.
- During this step, control small vascular and lymphatic channels with the Maryland bipolar.
- Take care to avoid injury to the right common iliac vein as it lies underneath the right common iliac artery during the pre-sacral dissection.
NOTE: A vascular injury in this location is challenging to control. - Ligate each ureter distally with two large locking clips placed ~5 mm apart. Divide each distal ureter sharply between clips.
- Fold the distal aspect of the left ureter proximally near the window posterior to the sigmoid mesentery. Use the Cadiere forceps to retract the sigmoid anteriorly, and using the distal ureteral clip as a handle, pull the left ureter from left to right posterior to the sigmoid mesentery.
- Starting on the left, divide the peritoneum overlying the external and common iliac artery. Divide the peritoneum lateral to the ipsilateral medial umbilical ligament, and begin to develop the ipsilateral space of Retzius. Join the two peritoneal incisions, and use bipolar diathermy to ligate the ipsilateral vas deferens prior to sharply dividing the vas with scissors.
- Prostatic apical dissection and division of remaining bladder pedicles
- Without dividing the urachal ligament that is suspending the bladder to the anterior abdominal wall, develop the space of Retzius, and incise the endopelvic fascia bilaterally.
- Release the pelvic floor muscle fibers from the lateral aspects of the prostate, and tease off lymphofatty tissue from the anterior aspect of the prostate using the Maryland bipolar to control vascular attachments.
- Divide the puboprostatic ligaments, which can be done sharply or through touch application of monopolar electrocautery.
- Ligate the dorsal venous complex (DVC) with a 2-0 V-Loc (barbed absorbable) suture (CT-1 or similar needle). To do this, pass the suture from right to left between the posterior aspect of the DVC and the anterior aspect of the urethra as distally as possible without entrapping fibers of the pubourethralis muscle with the needle. Bring the needle anteriorly through the distal aspects of the cut puboprostatic ligaments (from left to right) and then through the loop at the tail end of the suture.
- Additionally, re-pass the 2-0 V-Loc from right to left through the mid-DVC, and then pass the needle from left to right through the synchondrosis of the pubic symphysis-this pulley system may enhance hemostasis after division of the DVC.
- Sharply divide the DVC, ~3 mm proximal to the ligating suture, which permits full exposure of the urethra and prostatic apex.
- If nerve-sparing is desired and appropriate, incise the lateral prostatic fascia at the mid-prostate bilaterally, and bluntly develop the plane until the neurovascular bundles are released. Continue until that plane joins with the plane posterior to the prostate developed at the beginning of the case.
- Switch the monopolar scissors for the robotic vessel sealer. Using the vessel sealer, divide the remaining bladder pedicles. Take care to stay posterior to the clipped distal ureteral stumps, to ensure a margin-negative resection.
- In nerve-sparing cases, clip the prostatic pedicles with large locking clips, and divide them without thermal energy. Use just one or two large polymer ligating clips to ligate the prostatic pedicle on each side.
- Divide the urachal ligament close to its attachment at the umbilicus with the monopolar scissors.
- Ligate the obliterated umbilical arteries with the Maryland bipolar before dividing them.
- Using a combination of monopolar electrocautery and blunt dissection, separate the bladder from the anterior abdominal wall.
- Circumferentially dissect the specimen, which is now only attached at the urethra. Release the apical attachments of the neurovascular bundles if performing nerve-sparing, as the nerve bundles are medially tethered at the posterolateral aspects of the urethra.
- Remove the foley catheter.
- Ligate the membranous urethra proximally at the prostatic apex. If performing orthotopic neobladder, sharply divide the urethra distal to this, taking care to preserve maximal urethral length. If performing ileal conduit urinary diversion, clip the membranous urethra once more as distally as possible, and divide the urethra in between clips.
- Entrap the specimen in a 12 mm endoscopic specimen bag for subsequent removal.
- Selecting bowel segment for urinary diversion
- Switch instruments: place the Cadiere forceps in the left robotic arm and right-lateral robotic arm and the needle driver in the right-medial robotic arm.
NOTE: The Cadiere forceps can be used to atraumatically grasp the bowel. - Select a 15 cm segment of distal ileum located 20 cm away from the ileocecal valve. Use an umbilical tape cut to 20 cm in length, marked at 5 cm increments, to measure.
- Place seromuscular marking sutures on the bowel with 3-0 silk air knots on the mesenteric aspect of the ileum to mark the distal and proximal ends of the bowel segment that will serve as the ileal conduit.
- Place a suspending suture on the antimesenteric aspect of the distal end of the bowel segment chosen for the ileal conduit using a 3-0 Vicryl suture cut at 20 cm.
- Use the monopolar scissors to pierce the peritoneum of the mesentery, and spread to make two mesenteric windows at the proximal and distal aspects of the chosen bowel segment, staying within 5 mm of the mesenteric aspect of the bowel wall.
- Through each window, insert one end of an Endo-GIA stapler with 80 mm purple loads, clamp and divide the bowel segment. Ensure that the assistant inserts the stapler into the field through the left lateral 12 mm port.
- Using a robotic vessel sealer, divide the mesentery of the isolated ileal conduit segment.
NOTE: It is not necessary to visualize vascular arcades as long as the direction of tissue division is along the axis of the mesenteric vessels, which can be ensured by anterior retraction of the bowel segments with Cadiere forceps. Typically, only two-three applications of the vessel sealer are required on each aspect (proximal and distal) of the ileal conduit. - Ensure the ileal conduit segment is placed caudally.
- Switch instruments: place the Cadiere forceps in the left robotic arm and right-lateral robotic arm and the needle driver in the right-medial robotic arm.
- Ileoileostomy
- Perform ileoileostomy as a side-to-side stapled anastomosis. Using the monopolar scissors, cut along the staple line of the two segments to be anastomosed: start at the antimesenteric aspect, and proceed about halfway into the lumen, exposing enough lumen to expose insert the stapler arms.
- Using the Cadiere forceps, gently slide each of the bowel segments onto one of the stapler arms. Before closing the stapler, use the previously placed 3-0 silk marking sutures to apply traction to turn the mesenteries of the bowel segments away from each other. Close the stapler over the desired tissue by squeezing the ring handle together, and check to ensure appropriate positioning prior to deploying the stapler.
- Deploy the 80 mm stapling system: push the green safety button located above the handles, then slowly squeeze the ring handle unit sequentially until the staple unit is completely fired. Then, open the jaws of the instrument by pulling the black return knobs to their original position.
- To complete the ileoileostomy, use the previously partially divided staple lines as handles to retract the anastomosis anteriorly. Close the remaining open aspect of the bowel, as it is optimally exposed in this way, with one last 80 mm stapler load.
- Reinforce the interior vertex (crotch) of the bowel anastomosis using a 3-0 silk suture.
- Close the mesenteric trap with approximately four 3-0 silk figure of eight sutures.
- Ureteroileal anastomosis
- Suspend the ileal conduit to the anterior abdominal wall by pulling the previously placed 20cm 3-0 vicryl suture at the distal aspect of the conduit through the abdominal wall using a closure system needle device. Place the needle through the abdominal wall, and press the top of the Carter device to open the jaws of the needle; grab the vicryl suture and secure within the jaws. Pull the suture up through the abdominal wall and secure the suture with a hemostat applied at the skin level.
NOTE: The position for suspension is chosen so that the distal end of the conduit is just distal to the inner aspect of the right lateral robotic trocar. - Open the distal end of the conduit with monopolar electrocautery by cutting along the staple line, starting from the antimesenteric aspect.
- Make two 6 mm enterotomies sharply in the proximal end of the conduit to create recipient sites for each ureter.
NOTE: Both these enterotomies can be made on the medial (camera-facing) aspect of the conduit. - Suspend the left ureter by using the distal clip as a handle, and cut sharply into the lumen of the ureter (~75% transection) and proximally spatulate 10 mm of the ureter.
- Start the ureteroenteric anastomosis using a double-armed 4-0 monocryl suture on a PS-2 reverse cutting needle.
NOTE: The suture is fashioned into a double-armed suture by tying together two 12 cm lengths of suture. - Place the first suture that approximates ureter to bowel in an 'out-to-in' fashion with each needle, and secure with three knots.
NOTE: On the medial aspect of this anastomosis, it is easier to start the running suture 'in-to-out' on the bowel. On the lateral aspect of this anastomosis, it is easier to start the running suture 'in-to-out' on the ureter. Once the anastomosis is halfway complete, begin the process of ureteral stent placement. - Preload a short laparoscopic suction tip with a 6F single J stent that has been lubricated and itself preloaded with a wet guidewire.
NOTE: The laparoscopic suction tip enables the bedside assistant to direct the stent into the anastomosis. - Remove the robotic instrument in the right lateral trocar, and insert the suction tip (which functions as a stent-introducer) into the open/distal end of the ileal conduit, and guide it to the partially complete left ureteral anastomosis.
- Guide the stent into the ureter and the left renal pelvis using the robotic instruments until resistance is met. Partially remove the guidewire to allow the coil of the proximal aspect of the stent to form, and slide the stent proximally another 2-3 cm until resistance is met.
- Use the robotic grasper to secure the stent at the anastomosis internally, remove the guidewire completely. Cut the stent flush with the external aspect of the robotic trocar, and pull the stent through the trocar into the abdomen.
- Complete the running anastomosis over the stent. Use the distal ureteral tissue as a handle until it is no longer needed, then sharply divide it using the assistant's endoscopic scissors. Send the distal ureter as a final ureteral margin.
- Repeat the same procedure for the right ureter.
- Remove all robotic instruments, and undock the robot.
- Ensure that the surgeon stands on the patient's left (for a right-sided ileal conduit, as is typical), and grasps the distal aspect of the ileal conduit with laparoscopic bowel graspers (long D&G) using the robotic camera as a hand-held laparoscope.
- Cut the anchoring vicryl suture short external to the abdominal wall, and pull the remaining suture into the abdomen.
- Suspend the ileal conduit to the anterior abdominal wall by pulling the previously placed 20cm 3-0 vicryl suture at the distal aspect of the conduit through the abdominal wall using a closure system needle device. Place the needle through the abdominal wall, and press the top of the Carter device to open the jaws of the needle; grab the vicryl suture and secure within the jaws. Pull the suture up through the abdominal wall and secure the suture with a hemostat applied at the skin level.
- Stoma maturation and closure
- At a prechosen urostomy site on the right abdominal wall, excise a circular paddle of skin and subcutaneous fat, and clear off the anterior rectus fascia. Make a cruciate incision with electrocautery through the fascia. Spread the rectus muscle fibers in a cranial-caudal axis using a Tonsil/Reinhoff type clamp, then pierce through the anterior peritoneum, and spread just enough to permit externalization of the conduit.
- Through this opening, from the patient's right side, insert a Babcock clamp and grasp the distal end of the ileal conduit, which is 'handed over' from the surgeon on the patient's left using the laparoscopic bowel grasper.
NOTE: A smooth one-step transfer at this step will ensure orthotopic orientation of the conduit. - Using the Babcock clamp, bring the ileal conduit's distal end through the abdominal wall. Carefully externalize the stents without pulling them out of the conduit itself. Secure the conduit in its current position with a Babcock clamp.
- Place a 19 French drain in the pelvis via the left robotic port as an introducer. Using the laparoscopic graspers, place the drain in an 'upside-down-U' configuration such that its distal end is placed in the right obturator fossa, mid portion near the membranous urethra anterior to the rectum, and proximal aspect adjacent to the left obturator fossa.
- Level the operating table, and turn the insufflation off. Remove all robotic trocars.
- Remove the 12 mm port, and tie the pre-placed 0-Vicryl fascial sutures.
- Extend the supraumbilical camera port incision to 4-5 cm, and extract the specimen.
- Mature the stoma by tacking the bowel serosa to the dermis with interrupted 3-0 vicryl sutures in 4 quadrants, everting the distal conduit to create a rosebud by placing triplicate sutures16.
- Use 4-6 additional 3-0 vicryl sutures to close any gaps between the edge of the conduit and skin.
- Secure both stents and the drain with 2-0 nylon sutures.
- Close the fascia of the extraction incision with 0-PDS interrupted sutures. Copiously irrigate all wounds.
- Reapproximate the skin of all surgical wounds with 4-0 suture, and dress with surgical skin glue.
- Considerations in the female patient
NOTE: Variables to consider include surgical history (surgical absence of ovaries and/or uterus), desire for uterine/vaginal organ preservation to optimize postoperative sexual function and/or support for orthotopic neobladder, and feasibility of sparing of the anterior vaginal wall because of tumor location and extent. Positioning can be either dorsal lithotomy to facilitate urethral dissection and specimen removal (even if the Xi robot is used) or supine with the hips rotated laterally such that the knees are rotated laterally and the patient's heels are close together (ensuring all pressure points are carefully padded).- Perform port placement in the same manner as in males.
- If all pelvic organs are in situ, and anterior pelvic exenteration is planned, identify the infundibulopelvic ligament on each side by retracting the ipsilateral ovary anteriorly and caudally. Develop the vessels that comprise the ligament, and ligate them proximally with a large clip, distally with bipolar diathermy, and divide.
- Isolate the ureters, and perform extended pelvic lymphadenectomy.
NOTE: If the uterus is in situ, whether or not it is spared, it is important to identify and divide the uterine artery on each side. This often originates from the ipsilateral obliterated umbilical artery near its takeoff and crosses anterior to the ureter as it travels medially. - Ligate the uterine arteries with small locking clips or bipolar diathermy.
NOTE: Posterior dissection in females occurs after ureteral mobilization/division and lymphadenectomy, in contradistinction to males. - In the case of anterior pelvic exenteration with en bloc excision of the anterior vaginal wall, insert a lubricated sponge stick into the vagina to easily identify the proximal vaginal cuff. Use monopolar electrocautery to make a 2 cm transverse incision into the vagina over the sponge stick.
- Exchange the monopolar scissors for a robotic vessel sealer, which is used to ligate and divide the anterolateral vaginal wall on each side, proceeding distally toward the bladder neck.
NOTE: This maneuver results in the division of the vascular pedicles of the bladder as well. - Divide the urachus, and develop the space of Retzius to release the bladder from its attachments to the anterior abdominal wall. Divide the endopelvic fascia, and ligate and divide the DVC.
- If a neobladder is planned, circumferentially dissect the urethra, remove the foley catheter, ligate the proximal urethra with a large clip just distal to the bladder neck, and divide the urethra.
- If an ileal conduit is planned, keep the foley catheter in place. Ensure that the bedside surgeon uses a bovie to make an 'upside-down-U' incision anterior and lateral to the urethra externally, and use Metzenbaum scissors to dissect the plane anterior to the urethra.
- Note that the robotic surgeon will be able to see the tips of the Metzenbaum scissors relatively quickly, and then use the monopolar scissors to free the urethra from its remaining attachments with the assurance that the urethra has been removed in totality.
- Ensure that the bedside surgeon clips the foley catheter with a large clip just distal to the urethral meatus and divides it, and the console surgeon subsequently pulls the freed specimen into the pelvis.
- Maintain pneumoperitoneum due to the insufflation system.
- Ensure that the bedside surgeon introduces a 15 mm endoscopic specimen extraction bag through the vaginotomy for the removal of the specimen through the vaginotomy.
- If necessary, mobilize the lateral and proximal flaps of vaginal tissue from the anterior rectal wall.
- Use a 2-0 V-loc to bring the proximal vaginal wall to the anterior/apical vaginal wall, and run this suture proximally until the right aspect of the vaginal closure is complete. Repeat this procedure to close the left side.
- Perform intracorporeal ileal conduit urinary diversion as described above.
NOTE: There is no separate fascial incision or closure in the midline as the specimen has already been extracted. This feature of robotic radical cystectomy in females provides the benefit of eliminating the possibility of fascial dehiscence.
결과
Representative results of the described approach to robotic radical cystectomy, pelvic lymph node dissection, and intracorporeal ileal conduit urinary diversion are presented in Table 1. The three selected patients underwent the procedure by a single surgeon (DS) between December 2019 and June 2020. All procedures were completed on the Da Vinci Xi Robot using the port placement as illustrated in Figure 1. Blood loss was minimal (125 mL or less), and no patients required postoperative transfusion. Adequate lymph node yields (22 to 37 nodes) and negative margins were achieved in all patients. Length of stay varied from 5 to 8 days. There was one patient with complications within 30 days, including a Clavien-Dindo grade III complication of left hydronephrosis secondary to uretero-ileal anastomotic stricture, who needed nephrostomy tube placement.
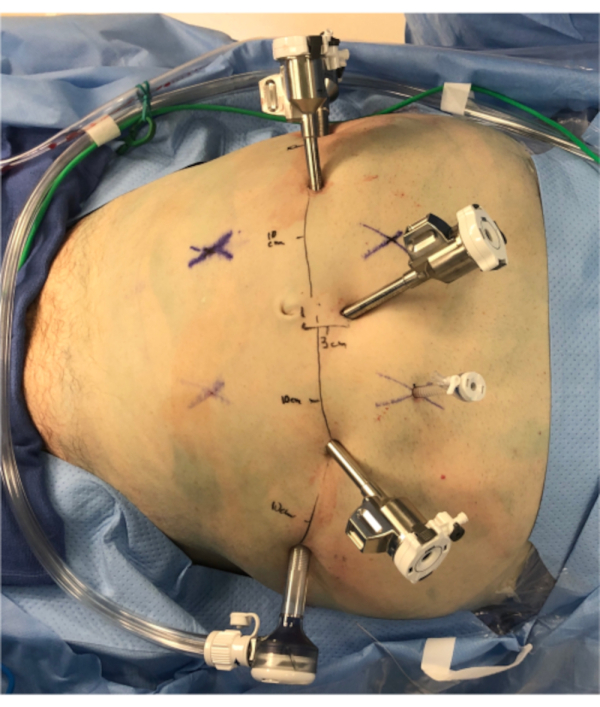
Figure 1: Port placement. This figure demonstrates the appropriate port placement using the Da Vinci Xi docked on the patient's right and the assistant on the patient's left. Photo orientation: caudal is picture left, cranial is picture right, patient left is picture bottom. The left lateral-most trocar is a 12 mm assistant port. The cranial-most trocar in the left upper quadrant is a 5 mm assistant port. All other trocars are Xi 5 mm robotic trocars, spaced 10 cm apart from each other as indicated. Please click here to view a larger version of this figure.
| Variables | Patient 1 | Patient 2 | Patient 3 |
| Age (years) | 74 | 73 | 64 |
| Gender | male | female | male |
| Body Mass Index (kg/m2) | 22.8 | 29 | 27 |
| ASA Class | 3 | 3 | 3 |
| Clinical Stage | cT2 N0M0 | cT1 N0M0 | cTa, Tis N0M0 |
| Neoadjuvant Treatment | Yes | Yes | No* |
| Da Vinci Robot Type | Xi | Xi | Xi |
| Estimated Blood Loss (mL) | 50 | 125 | 100 |
| Intraoperative Transfusion | No | Yes | No |
| Postoperative Transfusion | No | No | No |
| Urinary Diversion | Intracorporeal ileal conduit | Intracorporeal ileal conduit | Intracorporeal ileal conduit |
| Pelvic Lymph Node Dissection | Extended | Extended | Extended |
| Lymph Node Yield | 22 | 37 | 25 |
| Histopathology | Urothelial carcinoma | Urothelial carcinoma with variant histology (squamous, neuroendocrine, small cell) | Urothelial carcinoma |
| Pathologic stage | ypT0N0 | ypT3aN0 | pTisN0 |
| Margin status | Negative | Negative | Negative |
| Length of Stay (days) | 8 | 6 | 5 |
| Time to Flatus (days) | 5 | 4 | 3 |
| 30 Day Complications | None | Hydronephrosis, Clostridium difficile colitis | None |
| Clavien-Dindo Classification | N/A | III | N/A |
Table 1: Representative results. This table demonstrates the baseline clinical characteristics of the patients who underwent surgery, as well as their operative outcomes, pathology results, and perioperative outcomes. *Bacillus Calmette-Guerin unresponsive non-muscle invasive bladder cancer that progressed in immune checkpoint inhibitor clinical trial.
토론
Robotic radical cystectomy was first described in 200317,18. Unlike the widespread adoption of the robotic approach for radical prostatectomy for prostate cancer, less than 20% of radical cystectomies are performed robotically in the USA18. However, as adoption of RARC grows over time, the overwhelming majority of cystectomy cases are performed with a robotic approach at certain centers21. Although intraoperative blood loss, perioperative transfusion rates, and postoperative length of stay tend to be lower among patients with robotic versus ORC, the oncologic outcomes and complications between the two approaches are largely similar5,10,19,20,21. Reasons for the low rate of adoption of the robotic approach to radical cystectomy may include cost, instrument availability, familiarity/comfort of most surgeons with the open technique, and perceived technical difficulties of the procedure, particularly intracorporeal urinary diversion (ICUD).
Hayn et al. evaluated the learning curve for robotic cystectomy and found that 30 patients were needed to obtain lymph node yield of at least 20 and a positive surgical margin rate of 5%, and that 21 patients were required to reach an operative time of 390 min13. It should be noted that the robotic approach is growing in popularity. One tertiary referral center in Sweden that performed their first robotic cystectomy in 2003 has been exclusively performing cystectomy robotically since 201315. Owing to early reports of long operative times and complications associated with ICUD, extracorporeal urinary diversion (ECUD) was the initial approach3,17. ECUD requires a midline incision that converts the procedure to an open one, which may dilute the benefits of a robotic approach (e.g., this exposes the patient to possible wound complications such as dehiscence and infection). It is certainly plausible that ICUD (as compared to ECUD) may be associated with fewer surgical complications, as there is no conventional surgical wound required for ICUD beyond a small extraction incision.
Some studies have found that ICUD is associated with lower gastrointestinal and infectious complication rates3,22,23, although this finding is not universal24. It is noteworthy that clinical trials comparing open and robotic cystectomy did not incorporate ICUD5,10. Thus, the hypothesis that robotic radical cystectomy with ICUD may decrease complications compared to the open technique remains untested. Although urologic oncology surgeons are generally familiar with robotic approaches to pelvic procedures, several steps can be considered 'key' to successful performance in this case. Placement of the robotic trocars more cranially than is necessary for the exenterative portion of the procedure facilitates handling of the bowel segments for ICUD (Figure 1). Using Cadiere forceps instead of conventional Prograsp forceps permits atraumatic bowel grasping during ICUD, as well as when retracting the colon during the dissection posterior to the sigmoid mesentery if performing presacral lymphadenectomy. The Cadiere forceps has adequate retracting function during the cystectomy and lymphadenectomy, which obviates the need for Prograsp forceps. Lymph node dissection can be performed prior to or following radical cystectomy and generally depends on surgeon preference.
However, studies have suggested that lymph node dissection performed prior to radical cystectomy may enhance subsequent visualization of bladder pedicles25,26,27. An alternate approach to the case is to skeletonize and divide the ureters prior to commencing PLND and prior to ligating and dividing the obliterated umbilical arteries as this may minimize inadvertent ureteral injury. Another key point is the holding suture at the distal end of the ileal conduit segment to suspend the conduit anteriorly. This facilitates estimation of the permissible distance of proximal ureteral spatulation, siting, and suturing of the uretero-enteric anastomoses. The Bricker technique is utilized for the uretero-ileal anastomoses. An alternate approach to ureteroenteric anastomosis is to use the Wallace technique, where the spatulated distal ureters are sewn to each other on their posteriomedial aspects and then sewn to the bowel segment to create a larger uretero-enteric anastomosis28. The risk of anastomotic stricture has not been found to differ between the two approaches28,29. Given the lack of evidence to support one approach in particular, surgeon preference generally guides the selection.
In addition to the type of anastomosis, the operative approach has been evaluated with regard to stricture formation. Anderson et al. evaluated 478 consecutive patients who underwent radical cystectomy and found no difference in strictures rates between ORC and RARC with ECUD (8.5% vs 12.6%, p=0.2)30. By comparison, a series of 134 patients who underwent ICUD had an anastomotic stricture rate of only 3%31. A small series of 43 patients had a significantly higher uretero-ileal anastomotic stricture rate in those undergoing ECUD than in those undergoing ICUD (45.5% vs 14.3%, p=0.026)32. Theoretically, there may be an advantage to ICUD in this regard because of the magnified visualization during robotic suturing at this step. The risk of anastomotic stricture can be minimized by gentle ureteral handling, minimizing ureteral mobilization and dissection, preservation of the periureteral tissue, and completion of a tension-free anastomosis. As uretero-enteric strictures may be related to tension at the anastomosis or distal ureteral ischemia, an additional consideration is to visualize ureteral vascularity in vivo robotically using indocyanine green33,34.
Robotic radical cystectomy with extended pelvic lymphadenectomy and intracorporeal urinary diversion is quite feasible in male and female patients with bladder cancer (Table 1)22,23,24,35. Our results are illustrative examples of individual patient case reports, which is distinct from a formal retrospective case series. A limitation of the robotic technique is the need for prolonged Trendelenburg position. An important question for consideration in future prospective comparisons of outcomes between ORC and RARC is how complications differ when the robotic approach also incorporates ICUD. The major advantages of RARC with ICUD, as supported by several retrospective series and prospective trials, are lower blood loss, lower blood transfusion requirements, and potentially lower complication rates5,10,19,20,22. As compared to ORC, RARC has disadvantages as well, owing to the increased operating times (and associated costs) as well as fixed and marginal costs of robotic surgical platforms and equipment. Prospective studies suggest that oncologic outcomes are equivalent between techniques36,37. Compared to other surgical approaches to RARC with ICUD, the steps proposed in this protocol are simple and reproducible; however, whether they will be perceived to be advantageous or not to the individual surgeon will depend on that individual's prior training and preferences.
공개
The authors have nothing conflicts of interest. DS gratefully acknowledges Neema Navai MD and Jay Shah MD for advanced surgical training in these techniques.
감사의 말
No funding or acknowledgments.
자료
| Name | Company | Catalog Number | Comments |
| 19 Fr drain | N/A | N/A | Pelvic drain |
| AirSeal Port | ConMed | IASB12-120 | 12 mm assistant port that keeps stable pneumoperitoneum despite sunctioning |
| Anchor Endo Catch Specimen Bags | ConMed | TRS100SB2 | 10 mm reusable specimen bag for lymph node packets; 12 mm bag for bladder specimen |
| Babcock clamp | N/A | N/A | Used to externalize the ileal conduit |
| Biosyn suture | N/A | N/A | 4-0 suture used to close skin incisions |
| Carter Thomason Needle Device | Cooper Surgical | CTI-1015N | Used for fascial closure and to suspend the ileal conduit to the abdominal wall |
| Da Vinci Xi or Si Robot | Da Vinci | N/A | |
| Endo-GIA Stapler | Medtronic | EGIA30AMT | 80 mm (purple) loads for division of bowel to create ileal conduit |
| Guidewire | N/A | N/A | Used to load the ureteral stents |
| Hem-o-Lok Clip Applier and Clips | Weck | 544995 | Ligation of prostatic pedicle |
| Laparoscopic Suction Tip | N/A | N/A | Used to preload the ureteral stents |
| Luer lock syringe, 10 mL | N/A | N/A | Used to perform saline drop test and to inflate foley balloon. |
| LigaSure Vessel Sealer | Medtronic | Robotic vessel sealer | |
| Monocryl suture | N/A | N/A | 4-0 suture on a PS-2 reverse cutting needle |
| Nylon sutures | N/A | N/A | 2-0, used to secure the drain and ureteral stents to the abdominal wall |
| Robotic cadiere grasping forceps | Da Vinci | 470049 | |
| Robotic maryland bipolar forceps | Da Vinci | 470172 | |
| Robotic monopolar scissors | Da Vinci | 470179 | |
| Silk suture | N/A | N/A | 3-0 silk suture for marking the bowel segment for ileal conduit creation |
| Single J Ureteral stent | N/A | N/A | 6 Fr |
| Symmetric Stratafix Suture | Ethicon | SXPP1A406 | 0 barbed suture |
| Tonsil clamp | N/A | N/A | Used when maturing the stoma |
| Vicryl suture | N/A | N/A | 3-0 vicryl suture cut to 20 cm to be used as a suspending suture for the ileal conduit |
| V-Loc Suture | Covidien | KENDVLOCL0315 | 2-0 on CT-1 needle. Barbed absorbable suture. |
참고문헌
- Fantus, R. J., et al. Facility-level analysis of robot utilization across disciplines in the National Cancer Database. Journal of Robotic Surgery. 13 (2), 293-299 (2019).
- Ghezzi, T. L., Corleta, O. C. 30 years of robotic surgery. World Journal of Surgery. 40 (10), 2550-2557 (2016).
- Ahmed, K., et al. Analysis of intracorporeal compared with extracorporeal urinary diversion after robot-assisted radical cystectomy: results from the International Robotic Cystectomy Consortium. European Urology. 65 (2), 340-347 (2014).
- Narayan, V. M., et al. Radical cystectomy in women: Impact of the robot-assisted versus open approach on surgical outcomes. Urologic Oncology. 38 (4), 247-254 (2020).
- Bochner, B., et al. Comparing open radical cystectomy and robot-assisted laparoscopic radical cystectomy: A randomized clinical trial. European Urology. 67 (6), 104 (2015).
- Novara, G., et al. Systematic review and cumulative analysis of perioperative outcomes and complications after robot-assisted radical cystectomy. European Urology. 67 (3), 376-401 (2015).
- . Treatment of non-metastatic muscle-invasive bladder cancer: AUA/ASCO/ASTRO/SUO guideline Available from: https://www.auanet.org/guidelines/bladder-cancer-non-metastatic-muscle-invasive-guideline (2017)
- . NCCN practice guidelines in oncology: bladder cancer Available from: https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf (2020)
- Parekh, D., et al. Robot-assisted radical cystectomy versus open radical cystectomy in patients with bladder cancer (RAZOR): an open-label, randomised, phase 3, non-inferiority trial. Lancet. 391 (10139), 2525-2536 (2018).
- Shabsigh, A., et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. European Urology. 55 (1), 164-174 (2009).
- Burg, M., et al. Frailty as a predictor of complications after radical cystectomy: A prospective study of various preoperative assessments. Urologic Oncology. 37 (1), 40-47 (2019).
- Hayn, M., et al. The learning curve of robot-assisted radical cystectomy: results from the International Robotic Cystectomy Consortium. European Urology. 58 (2), 197-202 (2010).
- Porreca, A., et al. Robot-assisted radical cystectomy with totally intracorporeal urinary diversion: surgical and early functional outcomes through the learning curve in a single high-volume center. Journal of Robotic Surgery. 14 (2), 261-269 (2020).
- Brassetti, A., et al. Evolution of cystectomy care over an 11-year period in a high-volume tertiary referral centre. BJU International. 121 (5), 752-757 (2018).
- Whitehead, A., Cataldo, P. A. Technical considerations in stoma creation. Clinics in Colon and Rectal Surgery. 30 (3), 162-171 (2017).
- Brown, M., Challacombe, B. Intracorporeal urinary diversion after robot-assisted cystectomy: time to climb the next learning curve. European Urology. 65 (2), 348-349 (2014).
- Chan, K., et al. Robot-assisted radical cystectomy and urinary diversion: technical recommendations from the Pasadena Consensus Panel. European Urology. 67 (3), 423-431 (2015).
- Snow-Lisy, D., et al. Robotic and laparoscopic radical cystectomy for bladder cancer: long-term oncologic outcomes. European Urology. 65 (1), 193-200 (2014).
- Yuh, B., et al. Systematic review and cumulative analysis of oncologic and functional outcomes after robot-assisted radical cystectomy. European Urology. 67 (3), 402-422 (2015).
- Brassetti, A., et al. Long-term oncologic outcomes of robot-assisted radical cystectomy (RARC) with totally intracorporeal urinary diversion (ICUD): a multi-center study. World Journal of Urology. 38 (4), 837-843 (2020).
- Collins, J., Wiklund, N. Totally intracorporeal robot-assisted radical cystectomy: optimizing total outcomes. BJU International. 114 (3), 326-333 (2014).
- Pyun, J., et al. Robot-assisted radical cystectomy with total intracorporeal urinary diversion: comparative analysis with extracorporeal urinary diversion. Journal of Laparoendoscopic and Advanced Surgical Techniques. Part A. 26 (5), 349-355 (2016).
- Lenfant, L., et al. Perioperative outcomes and complications of intracorporeal vs extracorporeal urinary diversion after robot-assisted radical cystectomy for bladder cancer: a real-life, multi-institutional French study. World Journal of Urology. 36 (11), 1711-1718 (2018).
- Ozen, H., et al. Extended pelvic lymph node dissection: before or after radical cystectomy? A multicenter study of the Turkish Society of Urooncology. Korean Journal of Urology. 53 (7), 451-456 (2012).
- Zhu, Z., et al. Effects of performing pelvic lymph node dissection before versus after radical cystectomy. Zhonghua Yi Xue Za Zhi. 93 (32), 2574-2577 (2013).
- Boga, M., Ates, M. Timing of lymphadenectomy during robot-assisted radical cystectomy: before or after cystectomy? Fifteen cases with totally intracorporeal urinary diversions. Videosurgery and Other Miniinvasive Techniques. 15 (1), (2020).
- Davis, N., et al. Bricker versus Wallace anastomosis: A meta-analysis of ureteroenteric stricture rates after ileal conduit urinary diversion. Canadian Urological Association Journal. 9 (5-6), 284-290 (2015).
- Evangelidis, A., et al. Evaluation of ureterointestinal anastomosis: Wallace vs Bricker. Journal of Urology. 175 (5), 1755-1758 (2006).
- Anderson, C., et al. Ureteroenteric anastomotic strictures after radical cystectomy-does operative approach matter. Journal of Urology. 129, 541-547 (2013).
- Tan, W. S., et al. In-depth critical analysis of complications following robot-assisted radical cystectomy with intracorporeal urinary diversion. European Urology Focus. 3 (2-3), 273-279 (2017).
- Carrion, A., et al. Comparison of perioperative outcomes and complications of robot assisted radical cystectomy with extracorporeal vs intracorporeal urinary diversion. Actas Urologicas Espanolas. 43 (6), 277-283 (2019).
- Murthy, P., et al. Robotic radical cystectomy with intracorporeal urinary diversion: beyond the initial experience. Translational Andrology and Urology. 9 (2), 942-948 (2020).
- Tuderti, G., et al. Transnephrostomic indocyanine green-guided robotic ureteral reimplantation for benign ureteroileal strictures after robotic cystectomy and intracorporeal neobladder: step-by-step surgical technique, perioperative and functional outcomes. Journal of Endourology. 33 (10), 823-828 (2019).
- Hussein, A., et al. Outcomes of intracorporeal urinary diversion after robot-assisted radical cystectomy: results from the International Robotic Cystectomy Consortium. Journal of Urology. 199 (5), 1302-1311 (2018).
- Venkatramani, V., et al. Predictors of recurrence, and progression-free and overall survival following open versus robotic radical cystectomy: analysis from the RAZOR trial with a 3-year followup. Journal of Urology. 203 (3), 522-529 (2020).
- Bochner, B., et al. Randomized trial comparing open radical cystectomy and robot-assisted laparoscopic radical cystectomy: oncologic outcomes. European Urology. 74 (4), 465-471 (2018).
재인쇄 및 허가
JoVE'article의 텍스트 или 그림을 다시 사용하시려면 허가 살펴보기
허가 살펴보기This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. 판권 소유