Method Article
Wireless Telemetry Device Implantation in a Fontan Ovine Model for Continuous and Long-Term Hemodynamic Monitoring
In This Article
Summary
This protocol describes the surgical methodology for implanting a large animal wireless telemetry device to enable continuous and long-term collection of hemodynamic data, including heart rate, arterial blood pressure, inferior and superior vena cava pressures, and cardiac rhythm.
Abstract
While the Fontan procedure drastically improves life expectancy for patients with single ventricle, it is well recognized that the resulting circulation causes significant disease burden long term as a consequence of chronically elevated central venous pressures and decreased cardiac output. Chronic Fontan animal models are a valuable asset to studying the late physiological outcomes associated with this operation and a necessary tool in the evaluation of future devices designed to alleviate Fontan failure. However, previous attempts at the creation of chronic Fontan models have been hindered by poor survival rates. Additionally, effective hemodynamic data collection poses a significant challenge in freely moving animals. To this end, the use of wireless implantable telemetry systems provides a novel solution for real-time and long-term monitoring of cardiovascular data. This protocol describes the methodology for surgical implantation of a wireless telemetry device in a Fontan survival ovine model, facilitating the continuous and ongoing recording of several hemodynamic parameters, including heart rate, arterial blood pressure, and localized pressures in the inferior (IVC) and superior vena cava (SVC). Telemetry devices were implanted with cannulation of either the carotid artery and internal jugular vein or femoral artery and vein, for placement of pressure-sensing catheters in the ascending aorta and SVC or abdominal aorta and IVC, respectively. The use of the wireless telemetry systems enabled close postoperative monitoring following a single-stage Fontan operation, which contributed to improved animal welfare and survival.
Introduction
The development of the Fontan procedure in 1971 led to significant improvements in outcomes for patients with single ventricle1. The purpose of this operation is to separate systemic and pulmonary venous return to the heart, thereby increasing systemic oxygenation and relieving volume load on the systemic ventricle. Since its introduction, numerous modifications have been made to the surgical approach. Currently, total bypass of the right heart is most often achieved through staged reconstruction2,3. Typically, the first stage is performed during the first week of life4. Patients then undergo a second stage, which consists of either the Glenn procedure or hemi-Fontan, to redirect blood flow from the superior vena cava (SVC) to the pulmonary artery (PA)5. This is followed by the Fontan procedure, which involves the creation of an extracardiac conduit or lateral tunnel between the inferior vena cava (IVC) and PA6. Surgical advancements such as those made throughout the history of the Fontan procedure could not have been achieved without the use of animal models7.
While the Fontan procedure drastically improves life expectancy for single ventricle patients, it is well recognized that the resulting circulation, which operates without a subpulmonic pump, causes significant disease burden in the long term as a consequence of chronically elevated central venous pressures (CVP) and decreased cardiac output8,9,10,11,12. Chronic Fontan animal models are a valuable asset to studying the late physiological outcomes associated with this operation13. Active data collection of cardiovascular parameters, such as CVP, heart rate, and other vital signs, to capture the postoperative hemodynamic changes is essential for a comprehensive evaluation of developing pathophysiology. Furthermore, animal models are a necessary tool for testing the capability of novel ventricular assist devices designed to alleviate the hemodynamic shortcomings of the Fontan circulation in vivo14,15,16,17,18,19.
However, effective data collection poses a significant challenge. Invasive catheter-based techniques are limited by their transient nature, associated procedural risks, and the inability to monitor the animal's condition over extended periods. Moreover, previous attempts to create a large animal Fontan model have been hindered by poor survival rates, presumably due to the failure of normal hearts to adapt to the acute establishment of the Fontan circulation7,20. To this end, the use of wireless telemetry systems provides a novel solution for real-time, long-term collection of cardiovascular data in freely moving animals21,22. These devices may also enable close postoperative monitoring, which could lead to improved animal welfare and survival.
Here, we describe the methodology for the successful implantation and use of a wireless telemetry system23 in a chronic Fontan ovine model. This technique provided a robust and reliable means of continuous hemodynamic data collection, enabling the study of venous pressures and other key physiological parameters. Implementation of this technology in preclinical models is critical for advancing our understanding of Fontan physiology and the development of new therapeutic strategies aimed at improving the long-term outcomes of Fontan patients.
Protocol
This experimental protocol was approved by the Institutional Animal Care and Use Committee of the Nationwide Children's Hospital Abigail Wexner Research Institute (AR20-00121). All procedures adhered to the guidelines outlined in the National Institute of Health's Guide for the Use and Care of Laboratory Animals. This research followed the Animal Research: Reporting of In Vivo Experiments guidelines. Dorset sheep with a weight range of 23-38 kg and an age range of 2-12 months were housed in a specific pathogen-free environment with free access to food and water for at least 1 week before surgery. The equipment and reagents used in the study are listed in the Table of Materials.
1. Animal preparation
- Have the sheep undergo evaluation by the veterinary team 1 week prior to surgery to ensure that they can safely undergo anesthesia. Fast healthy sheep and deprive of water for 12 h prior to the surgical procedure.
- Sedate with a combination of ketamine (4 mg/kg) and diazepam (0.5 mg/kg) injected through an internal jugular (IJ) vein.
- Shave sheep according to the planned procedure (detailed below) and over the thigh for electrocautery grounding pad placement. Clean the surgical sites with alcohol.
- Insert an 8-9 mm single-lumen endotracheal tube into the trachea.
- Insert an orogastric tube for decompression of the stomach and rumen.
- Insert a single-lumen venous catheter (16-18 G) into the right jugular vein or a lateral saphenous vein for continuous fluid administration, continuous rate infusion (CRI) of propofol, and drug injection as needed.
- Place an arterial line (22-24 G) in an auricular artery for continuous blood pressure monitoring.
- Place a blood pressure cuff on the right front limb for non-invasive blood pressure measurement, a clip on the ear or tongue to monitor oxygen saturation, and electrocardiogram (ECG) leads on all four limbs.
- During the procedure, maintain anesthesia using inhaled isoflurane 1%-3% with 100% O2 and/or propofol CRI (20-45 mg/kg/h).
- Aseptically clean the surgical sites using a chlorhexidine-based prep and drape in the standard sterile fashion.
- Administer cefazolin (25 mg/kg) for antibiotic prophylaxis before incision and re-dose every 4 h during the operation as needed.
- Administer a subcutaneous injection of local anesthetic, such as bupivacaine 0.25%, at all planned incision sites prior to incision.
2. Telemetry device preparation
- Open the telemetry software program and turn on the telemetry device using the magnet switch while it is still sealed in its original packaging.
- Within the software program, click on Hardware located at the top bar and select Edit PhysioTel Digital (CLC) Configuration to assign the telemetry unit to a communication link controller (CLC).
- Once a CLC is selected, its CLC Details page will open. Within this page, click on Search for Implants, which will initiate a search for implant devices that are turned on nearby.
- Click Add to add the telemetry unit to the Implants Selected list. The device will now appear under the Configured Implants list on the CLC Details page. Click Save and Exit.
- Start data acquisition by pressing the Play button next to the name of the telemetry unit in the Sampling Control tab. Graph displaying the live data acquisition will automatically open.
- Remove the device from its exterior packaging and transfer it to its sterile interior packaging onto the operating table.
- Zero the device while it remains in its interior packaging. Wait until measurements from the device have been stable for 30 s and use the stabilized non-pulsatile mean (NPMN) pressure values as the offset.
- Within Subject Setup, select the settings icon next to the parameter that is being zeroed and open the Offsets tab. Enter the offset value obtained from the NPMN measurements into the text box.
- After inputting the offset, check if the NPMN readings are 0 ± 0.1 mmHg. If not, repeat step 2.7 until values are within the desired range.
- Perform steps 2.7-2.9 for both pressure channels.
- Before inserting the pressure-sensing catheters into a blood vessel, tap the tip to identify its corresponding channel. Taps will become apparent in the waveform output.
- Use the catheter corresponding to the left ventricular pressure (LVP) channel for arterial pressure measurement and the blood pressure (BP) channel for venous pressure measurement.
- Within the Standard Attributes tab of the Blood Pressure Analysis Attributes dialog box, set the minimum pulse height to 1 mmHg for the BP channel.
3. Method 1: Femoral artery and vein cannulation
- Shave the sheep in a wide perimeter around the right groin and over the abdomen and chest.
- Position the sheep supine on the operating table with their front limbs secured in flexion using a flexible cloth belt and hind limbs secured in extension using a slipknot tie to allow for access to the groin (Figure 1A).
- Make a 5-cm transverse incision in the right inguinal region centered over the palpable femoral artery, approximately 1 cm below the inguinal crease.
- Using a combination of electrocautery and blunt dissection, dissect through the subcutaneous tissue to the femoral triangle. Locate the femoral vessels by palpating for the arterial pulse.
- Divide between the sartorius and adductor longus muscle along the direction of the muscle fibers to expose the femoral vessels (Figure 1B).
- Using a combination of blunt and sharp dissection, clear the connective tissue from the femoral vessels circumferentially.
- Pass a double-looped 2-0 silk tie around both vessels proximal and distal to the cannulation site for temporary vessel ligation.
- Make a 6-cm transverse incision through the skin in the right lower abdomen, approximately 3 cm above the inguinal crease.
- Using a combination of electrocautery and blunt dissection, dissect through the subcutaneous fat and connective tissue to create a 6 cm x 4 cm pocket superficial to the external oblique.
- Insert the telemetry device into the subcutaneous pocket and secure it in place using a 2-0 silk suture (Figure 1C).
- Tunnel the telemetry device antenna under the subcutaneous tissue and secure it in place using a 2-0 silk suture.
- For placement of the biopotential (ECG) leads, make 1-cm counter incisions in the skin over the mid and lower abdomen, as well as the lower and upper chest. Tunnel subcutaneously to connect these incisions to the device body pocket and guide the ECG leads to their desired location.
- Place the positive electrode in the subcutaneous tissue to the left of the lower sternum. Ensure that the silicone tubing is removed to reveal the tip of the steel wire underneath.
- Place the negative electrode in the subcutaneous tissue to the right of the upper sternum.
- Excess wiring for both leads can be coiled and secured in the subcutaneous location using a 2-0 silk suture.
- Create a subcutaneous tunnel from the lower abdominal device pocket to the inguinal incision and thread the two pressure catheters through.
- Place a purse-string stitch using a 6-0 polypropylene suture around the cannulation site of both the femoral artery and vein, which can be secured using a plastic tourniquet.
- Fill the catheter gel tips with non-compressible, high-viscosity gel to prevent coagulation inside the catheter tips, ensuring no air bubbles.
- Administer a dose of intravenous heparin (100 units/kg) 3 min before cannulation.
- Tighten the proximal and distal 2-0 silk tourniquets around the femoral artery. Carefully incise into the vessel at the center of the purse-string stitch using a #11 blade scalpel and dilate slightly with the tip of a curved hemostat.
- Insert the pressure catheter corresponding to the LVP channel and advance it into the abdominal aorta, loosening the proximal silk tourniquet to allow for passage of the catheter. Tighten the purse-string suture and tie it around the catheter.
- Repeat steps 3.20 and 3.21 for femoral vein cannulation using the pressure catheter corresponding to the BP channel and advance it into the abdominal IVC (Figure 1D).
- Confirm that the catheter tips are located appropriately in the IVC and aorta using fluoroscopy.
- Reapproximate the sartorius muscle using a 2-0 absorbable suture.
- Close the skin with deep dermal and subcuticular sutures using 3-0 and 4-0 absorbable sutures, respectively.
4. Method 2: Carotid artery and internal jugular vein cannulation
- Shave the sheep in a wide perimeter around the left neck and down over the chest.
- Position the sheep in right lateral decubitus on the operating table with the left front limb secured in flexion using a slipknot tie to expose the chest (Figure 2A).
- Make a 5-cm longitudinal skin incision above the left carotid artery and IJ vein, approximately 7 cm cranial to the thoracic inlet.
- Using electrocautery, dissect through the subcutaneous fat, connective tissue, and platysma to expose the neck vessels (Figure 2B).
- Using a combination of blunt and sharp dissection, clear the connective tissue from the left carotid artery and IJ vein circumferentially.
- Pass a double-looped 2-0 silk tie around both vessels proximal and distal to the cannulation site for temporary vessel ligation.
- Make a 6-cm longitudinal incision at the base of the left neck between the scapula and cervical spine.
- Using a combination of electrocautery and blunt dissection, dissect through the subcutaneous fat and connective tissue to create a 6 cm x 4 cm pocket extending toward the spine.
- Insert the telemetry device into the subcutaneous pocket and secure it in place using a 2-0 silk suture.
- Tunnel the telemetry device antenna under the subcutaneous tissue and secure it in place using a 2-0 silk suture.
- Make 1-cm counter skin incisions at the base of the neck, as well as the lower left and upper right chest, for placement of the ECG leads. Tunnel subcutaneously to connect these incisions to the device body pocket and guide the ECG leads to their desired location (Figure 2C).
- Place the ECG leads similarly to the steps described above for the femoral implant procedure (section 3).
- Create a subcutaneous tunnel from the lateral device pocket to the medial neck incision and thread the two pressure catheters through. Prep these pressure catheters using gel prior to cannulation, as detailed in the femoral implant procedure.
- Using a 6-0 polypropylene suture, place a purse-string stitch around the site of cannulation on both vessels and secure with a plastic tourniquet.
- Administer a dose of intravenous heparin (100 units/kg) 3 min before cannulation.
- Tighten the proximal and distal 2-0 silk tourniquets around the carotid artery. Carefully incise into the vessel at the center of the purse-string stitch using a #11 blade scalpel and dilate slightly with the tip of a curved hemostat.
- Insert the pressure catheter corresponding to the LVP channel and advance it into the thoracic ascending aorta, loosening the proximal silk tourniquet to allow for passage of the catheter. Tighten the purse-string suture and tie it around the catheter.
- Repeat steps 4.16 and 4.17 for cannulation of the left IJ vein using the pressure catheter corresponding to the BP channel and advance it into the thoracic SVC.
- Confirm the appropriate location of the catheter tips in the thoracic SVC and ascending aorta using fluoroscopy (Figure 2D).
- Reapproximate the platysma muscle using a 2-0 absorbable suture.
- Close the skin with deep dermal and subcuticular sutures using 3-0 and 4-0 absorbable sutures, respectively.
5. Recovery
- Discontinue anesthetics. Remove the orogastric tube and extubate when the sheep is breathing without assistance from the ventilator. This typically occurs after the sheep shows signs of arousal (movement, blinking, response to painful stimuli, jaw tone, chewing).
- Remove the arterial line.
NOTE: Continuous blood pressure monitoring can be provided by the telemetry device if one of its pressure catheters has been placed into the aorta. - Transfer the sheep to an isolated housing unit for recovery. Assist the sheep with staying in sternal recumbency and then eventually with standing.
- Administer intravenous banamine (2.2 mg/kg) and subcutaneous buprenorphine SR (0.03 mg/kg) for postoperative pain.
Results
Surgical outcomes
A total of 13 sheep underwent single-stage Fontan surgery involving total cavopulmonary connection with detachment of both the SVC and IVC from the right atrium, direct end-to-side anastomosis of the SVC to PA, and placement of an extracardiac conduit between the IVC and PA. Sheep underwent this procedure at a mean age of 13.3 ± 7.6 months. Of these, 3 sheep underwent wireless telemetry device implantation with placement of pressure-sensing catheters into the abdominal aorta and IVC; 2 sheep underwent telemetry device implantation with placement of pressure-sensing catheters in the ascending aorta and SVC; and 8 sheep had no telemetry device implanted. No animals experienced any major postoperative complications following telemetry device implantation. Seven out of the 8 sheep (87.5%) with no telemetry device expired within 30 days after the Fontan operation, while only 1 out of the 5 (20.0%) sheep with a telemetry device expired during this postoperative period (Table 1).
Hemodynamic data collection
Implantation of wireless telemetry systems facilitated continuous and long-term data collection for several cardiovascular parameters, including heart rate, arterial blood pressure, and CVP. This enabled close hemodynamic monitoring of animals undergoing single-stage Fontan surgery before, during, and for numerous days after their operation (Figure 3). Variations in venous pressures were observed minute-to-minute, though overall trends appeared to show an acute increase in both abdominal IVC (Figure 4A) and thoracic SVC (Figure 4B) pressures following the establishment of the Fontan circulation. Some minute-to-minute fluctuations in venous pressures could be attributed to the sheep's activity level and positioning. For instance, pressures in the abdominal IVC were observed to increase consistently when the sheep were resting on their abdomen in recumbency (Figure 5). In one sheep, a catheterization procedure was performed on the same day following device implantation in the neck area with placement of the venous pressure channel in the SVC. This was done to assess for discrepancies between values reported by the telemetry device and those acquired from invasive pressure monitoring, which was viewed as the gold standard. Non-pulsatile mean SVC pressures obtained from the catheterization procedure fluctuated between 2-4 mmHg, with oscillations attributable to changes in intrathoracic pressure along the respiratory cycle (Figure 6). Throughout the procedure, the telemetry device outputted, on average, 43 readings of non-pulsatile mean SVC pressure per minute, with an overall mean SVC pressure of 1.1 ± 3.1 mmHg, indicating minimal offset between device measurements and actual values.

Figure 1: Device implantationwith femoral artery and vein cannulation. For device implantation with femoral artery and vein cannulation, the sheep is positioned supine with hind legs in extension. (A) Preoperative markings indicate the positioning of planned incisions, the device body pocket, and the subcutaneous course of the ECG leads and pressure-sensing catheters to their final location. (B) A subcutaneous pocket measuring approximately 6 cm x 4 cm is created between the subcutaneous tissue and above the external oblique muscle for placement of the telemetry device body. (C) The femoral vessels are exposed following the division of the sartorius muscle. The palpable femoral artery (white arrows) is located medial to the femoral vein (blue arrows). (D) Pressure-sensing catheters are inserted into the femoral artery (white arrow) and vein (blue arrow), then secured into place with a purse-string stitch. Please click here to view a larger version of this figure.
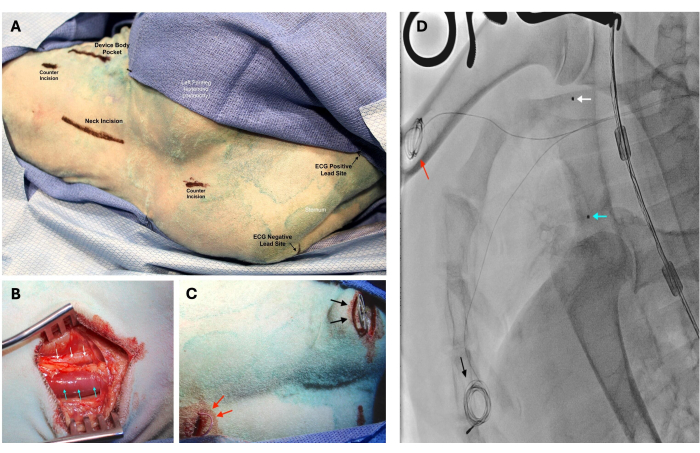
Figure 2: Device implantation with the left carotid artery and internal jugular (IJ) vein cannulation. For device implantation with the left carotid artery and internal jugular (IJ) vein cannulation, the sheep is positioned in right lateral decubitus with its left foreleg extended posteriorly. (A) Preoperative markings indicate the positioning of planned incisions. (B) The left carotid artery (white arrows) and IJ vein (blue arrows) are exposed following the division of the platysma. The carotid artery is located deep and lateral to the IJ vein. (C) Excess wiring of the ECG leads is coiled and then secured in the subcutaneous space. The positive lead is placed left of the inferior aspect of the sternum (black arrows), while the negative lead is placed right of the superior aspect of the sternum (red arrows). (D) Placement of the pressure-sensing catheter tips in the superior vena cava (blue arrow) and ascending aorta (white arrow) was confirmed using fluoroscopy. Positioning of the ECG positive (black arrow) and negative (red arrow) leads can also be seen on X-ray imaging. Please click here to view a larger version of this figure.
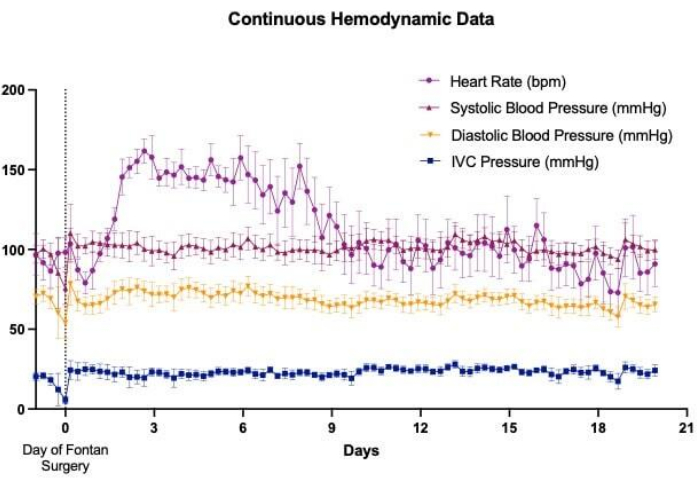
Figure 3: Wireless telemetry device implantation for continuous monitoring. Wireless telemetry device implantation allowed for continuous monitoring of several cardiovascular parameters, including heart rate, arterial pressure, and venous pressure, in Fontan survival ovine models throughout the perioperative period. Hemodynamic trends depicted in this graph are representative of the data that has been collected from pressure-sensing catheters placed within the abdominal aorta and inferior vena cava (IVC). Please click here to view a larger version of this figure.
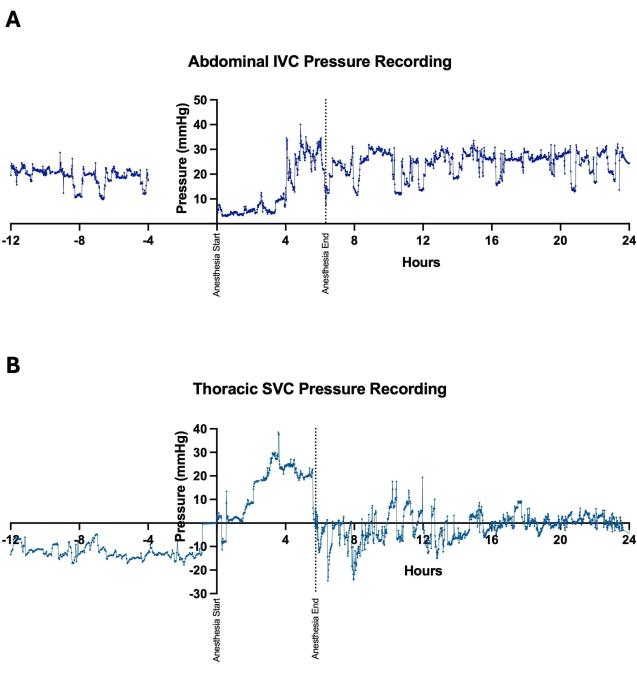
Figure 4: Positioning of pressure-sensing catheters. Pressure-sensing catheters were positioned in either the (A) abdominal inferior vena cava (IVC) or (B) thoracic superior vena cava (SVC) for continuous recording of central venous pressures. Measurements of venous pressures were obtained preoperatively, intraoperatively during the Fontan procedure, and postoperatively to evaluate for trends following the establishment of the Fontan circulation. Minute-to-minute variations in pressure measurements occurred with changes in the animal's positioning and level of activity. Please click here to view a larger version of this figure.
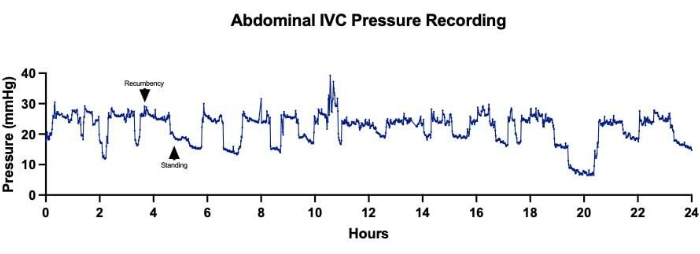
Figure 5: Abdominal inferior vena cava (IVC) pressures. Continuous acquisition of abdominal IVC pressures over a 24-h period showed fluctuations in pressure measurements correlating to changes in the sheep's positioning. Higher average IVC pressures corresponded to times when the sheep was resting in recumbency, while lower average IVC pressures were recorded when the sheep was standing. Please click here to view a larger version of this figure.
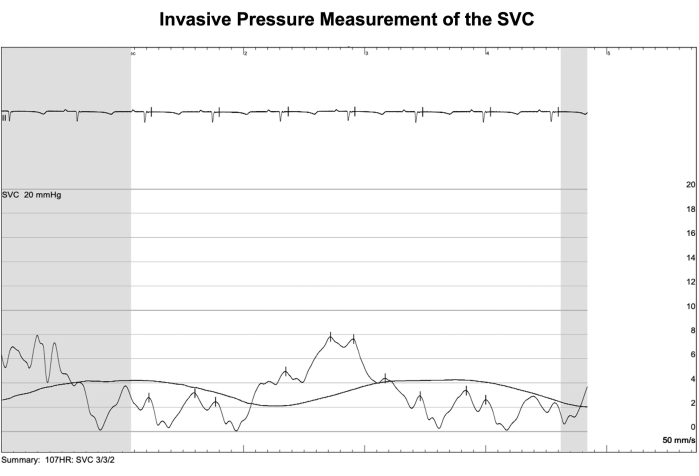
Figure 6: Invasive pressure measurement of the SVC. A catheterization procedure was performed on the same day following device implantation in one sheep to verify superior vena cava (SVC) pressure readings from the telemetry system. Values acquired by this method of invasive pressure monitoring were viewed as the gold standard. Non-pulsatile mean SVC pressures obtained from the catheterization procedure fluctuated between 2-4 mmHg in synchronization with the respiratory cycle. Meanwhile, the average of all non-pulsatile mean SVC pressures collected by the telemetry device during the procedure was 1.1 ± 3.1 mmHg, indicating minimal offset between telemetry readings and actual values. Please click here to view a larger version of this figure.
| Sheep | Sex | Telemetry Device Catheter Placement | Weight at the Time of Fontan Operation (kg) | Age at the Time of Fontan Operation (months) | Perioperative Death |
| 1 | M | None | 45 | 13 | Yes |
| 2 | F | None | 43 | 13 | No |
| 3 | M | None | 46.5 | 25 | Yes |
| 4 | F | None | 46.5 | 19 | Yes |
| 5 | M | None | 50 | 20 | Yes |
| 6 | F | None | 53 | 28 | Yes |
| 7 | M | None | 40.5 | 8 | Yes |
| 8 | M | None | 42 | 10 | Yes |
| 9 | F | Abdominal Aorta and IVC | 33.5 | 3 | No |
| 10 | M | Abdominal Aorta and IVC | 24 | 7 | Yes |
| 11 | M | Abdominal Aorta and IVC | 29 | 8 | No |
| 12 | M | Ascending Aorta and SVC | 37.5 | 13 | No |
| 13 | M | Ascending Aorta and SVC | 39.5 | 6 | No |
Table 1: Surgical outcomes. Thirteen sheep underwent single-stage Fontan surgery, 5 of which had undergone wireless telemetry device implantation 1 month prior. Following the Fontan operation, 7 out of the 8 sheep (87.5%) with no telemetry device expired within 30 days, compared to 1 out of the 5 (20.0%) sheep with a telemetry device.
Discussion
We have developed two surgical methods for the implantation of a wireless telemetry device into an ovine model. The device was successfully implanted in 5 sheep to achieve continuous, long-term monitoring and recording of several cardiovascular parameters, including heart rate, arterial blood pressure, and localized venous pressures from the abdominal IVC and thoracic SVC. All sheep survived the surgery for device implantation without any major complications and went on to undergo a single-stage Fontan operation one month later.
In 2019, Van Puyvelde et al. reported the creation of the first Fontan ovine survival model to study the chronic process of Fontan failure13. However, two-thirds of the animals ultimately did not survive beyond the twentieth postoperative week. Notably, it is extremely difficult to establish an acute Fontan circulation in animals with normal hearts, presumably because they are less adapted than univentricular hearts to this specific physiological state. The utility of telemetry device implantation is consequently two-fold. First, the capacity to closely monitor vitals during the postoperative period may allow for the rapid recognition of and response to signs of cardiovascular decompensation, as well as facilitate the initiation of goal-directed therapies. In our experience, the implantation of wireless telemetry devices in a cohort of sheep undergoing the single-stage Fontan operation contributed to their improved survival. Second, the ability to acquire real-time data in the long term will enable us to identify developing hemodynamic trends associated with Fontan failure.
While we have focused on the creation of a chronic Fontan large animal model, the benefits of wireless telemetry systems can be applied to other endeavors as well, such as the testing and development of novel cavopulmonary assist devices (CPAD) aimed at providing mechanical circulatory support in cases of Fontan failure. Several groups have published large animal studies examining the feasibility and functional capacity of CPADs applied to the Fontan circulation16,17,18,19. However, the majority of these experiments were conducted in acute Fontan models with short-term evaluation of hemodynamic performance using data collection methods that are not viable outside of the operating room. In 2019, Cysyk et al. described the successful implantation of a CPAD in a Fontan ovine survival model14,15. In their study, fluid-filled pressure monitoring lines were placed in the SVC, IVC, PA, and left atrium and brought out through the posterior chest wall to obtain continuous pressure measurements over the 30-day study period. While this method was largely sufficient for the purposes of their study, they did note issues with catheter migration. For long-term data collection greater than 30 days, the use of a wireless telemetry system may prove to be more preferable.
None of the sheep undergoing telemetry device implantation experienced any major complications as a result of the procedure. However, an ECG lead was found to have eroded through the skin of one sheep at the healed incision site several weeks after surgery. This was felt to be related to a pressure injury as sheep typically rest in sternal recumbency with their body weight placed on the anterior sternum over the sites where the ECG leads had been located subcutaneously. Therefore, to avoid ECG lead erosion, ECG leads were positioned lateral, instead of directly over, the sternum in future sheep undergoing device implantation, and no other sheep have since experienced this issue.
Multiple steps are necessary to ensure that pressure readings are as accurate. First, the telemetry device must be zeroed to atmospheric pressure on a flat surface with the catheter tips level with the device body while it is inside its original packaging. Prior to inserting the pressure-sensing catheters into a blood vessel, it is necessary to fill the catheter tips with non-compressible, high-viscosity gel, taking care to ensure there are no air bubbles within the clear gel. Finally, it is important to note the positioning of the device body in relation to the catheter tip, as differences in height may skew the pressure measurements. We opted to place the device body over the lower abdomen or at the base of the neck posterior to the scapula so that it would be at approximately the same level as the catheter tip within the abdominal IVC or thoracic SVC, respectively, when the sheep was upright.
Of note, we also positioned the telemetry device body in a location at least 15 cm away from the region of interest, including the heart, great vessels, and liver, to minimize the amount of artifact it might produce on future magnetic resonance imaging. Lastly, pressure-sensing catheters were inserted into the left carotid artery and IJ vein so that it would be possible to perform future catheterization procedures through the right IJ vein.
Several limitations remain at this time with the use of the wireless telemetry systems as described. Of note, the device implant has a battery lifespan of 84 days. Once implanted, the device battery cannot be recharged or replaced. However, the devices may be turned off and back on during periods of time in which data collection is desired to prolong their use. Additionally, venous pressure measurements were observed to vary depending on the animal's positioning, and it is unclear whether this was due to true changes in intravenous pressure or changes in the positioning of the device body relative to the catheter tips. Moreover, while telemetry device bodies were positioned to be at approximately the same level as their catheter tips in the IVC or SVC when the animals were upright, differences in height remained due to anatomic limitations. For the neck implant, the device body rested higher than the catheter tip in the SVC, and for the groin implant, the device body rested lower than the catheter tip in the IVC. Nonetheless, examination of the overall trends in pressure values may still provide crucial insight into any hemodynamic changes that may arise. Further analysis of the long-term data collected by these telemetry systems will be necessary to achieve a fuller understanding of chronic Fontan physiology and the mechanisms of Fontan failure. Additional catheterization procedures performed postoperatively will also be needed to verify the accuracy of the telemetry output and identify possible sensor drift over time. Finally, displacement of device catheter tips as a result of somatic growth is a potential concern, especially when telemetry units are implanted in younger animals. The location of the radiopaque catheter tips can be ascertained and confirmed during postoperative catheterization procedures.
Wireless telemetry systems allow for the long-term collection of continuous hemodynamic data in real-time from freely moving large animal models. Surgical implantation of these devices with the placement of pressure-sensing catheters in the IVC and SVC, as well as the abdominal and ascending aorta, is safe and feasible.
Disclosures
This project was funded by the Additional Ventures Cures Collaborative, Palo Alto, California.
Acknowledgements
We appreciate the dedicated veterinarian staff at the Animal Research Core. We also wish to express our gratitude to Mary Walker, DVM, MS, for her invaluable expertise and vigilant care throughout the study.
Materials
| Name | Company | Catalog Number | Comments |
| 0.9% Sodium Chloride solution | Baxter Healthcare Corporation | Pharmacy | Intraoperative fluid resuscitation and wound rinse |
| 16 G intravenous catheter | BD | 382259 | For fluid and drug administration |
| 22 G intravascular catheter | BD | 381423 | For arterial blood pressure monitoring |
| 70% isopropyl alcohol | Aspen Vet | 11795782 | Topical cleaning solution |
| ACT cartridge | Abbot Diagnostics | 03P86-25 | Activated clotting time |
| Backhaus towel clamp | Medline | MDS1411111 | To affix sterile drape |
| Banamine | Hospira Pharmaceuticals | Pharmacy | Postoperative pain control: concentration 50 mg/mL, dose 2.2 mg/kg |
| Blood pressure cuff | Royal Philips | 9.89803E+11 | Non-invasive blood pressure monitoring |
| Bupivacaine hydrochloride | Hospira Pharmaceuticals | Pharmacy | Local anesthetic: concentration 2.5 mg/mL, dose 2.5 mg/kg |
| Buprenorphine | Hospira Pharmaceuticals | Pharmacy | Postoperative pain control: concentration 0.3 mg/mL, dose 0.03 mg/kg |
| Castroviejo needle holder | Medline | MDS0750386 | Needle holder when suturing blood vessels |
| Cautery cleaner pad | Cardinal Health | 300-2SS | To clean cautery pencil tip |
| Cautery pencil | Medline | ESRK3002L | For dissection using electrocautery |
| Cefazolin | Hospira Pharmaceuticals | Pharmacy | Antibiotic prophylaxis |
| Cetacaine | Cetylite | 220 | Topical anesthetic spray for intubation |
| Chloraprep | BD | 930825 | Topical antiseptic |
| Debakey atraumatic forceps | Medline | MDS1130630F | For tissue handling |
| Diazepam | Hospira Pharmaceuticals | Pharmacy | Sedative: concentration 5 mg/mL, dose 0.5 mg/kg |
| ECG leads | 3M | 2570 | ECG monitoring |
| Endotracheal tube, size 8-9 | Covidien | 86452, 86114, or 86454 | To secure airway |
| Hartmann hemostatic forceps | Medline | MDS1221109 | To clamp blood vessels and hold small sutures |
| Heparin | Hospira Pharmaceuticals | Pharmacy | Anticoagulant: 1,000 USP units/mL |
| Pressure transducer kit | Edwards Lifesciences | VSYPX12N | For arterial blood pressure monitoring |
| Pulse oximeter lingual clip | Nellcor | PO736 | For pulse oximetry |
| Isoflurane | Baxter Healthcare Corporation | Pharmacy | Anesthetic: dose 1-3% |
| Kantrowitz forcep (right angle) | Medline | MDS1243528 | For blunt dissection around blood vessels |
| Ketamine | Hospira Pharmaceuticals | Pharmacy | Sedative: concentration 100 mg/mL, dose 4 mg/kg |
| Laparotomy drape | Medline | DYNJP3008 | Sterile drape |
| Lubricating jelly | Medline | MDS0322273Z | Endotracheal tube lubricant |
| Mayo Hegar needle holder | Medline | MDS2418420F | Needle holder when suturing soft tissue |
| Mayo scissors | Medline | MDS0816121 | To cut suture |
| Metzenbaum curved scissors | Medline | MDS3223226 | For sharp dissection |
| Needles and syringes | Cardinal Health | 309604 | For intravenous and subcutaneous drug administration |
| Optixcare | Aventix | OPX-4252 | Corneal lubricant |
| Perma-Hand silk suture | Ethicon | C016D | For blood vessel ligation and attachment of the telemetry device subcutaneously |
| PhysioTel Digital wireless telemetry device | Data Sciences International | L21 model | Wireless telemetry device implant |
| Pierce microforceps | Medline | MDG384908 | Small needle handling |
| Plastic tourniquet and suture snare | Medtronic | 79013 | To facilitate hemostasis during vessel cannulation |
| Pressure bag | Carefusion | 64-10029 | For arterial blood pressure monitoring |
| Prolene 6-0 suture | Ethicon | 8307H | Purse string stitch for vessel cannulation |
| Propofol | Fresenius Kabi | Pharmacy | Anesthetic: concentration 10 mg/mL, dose 20-45 mg/kg/h |
| Scalpel #10 blade | Medline | MDS15310 | For skin incisions |
| Scalpel #11 blade | Medline | CISION11CS | For incision into blood vessels |
| Schnidt tonsil forceps | Medline | MDS5018719 | For blunt dissection through subcutaneous tissue |
| SoftCarry stretcher | Four Flags Over Aspen | SSTR-4 | For animal transportation |
| Sterile disposable OR towel | Medline | MDT2168201 | Sterile drape |
| Sterile bowl | LSL Industries | 5232 | To hold saline solution |
| Sterile cotton X-ray detectable gauze sponge | Medline | NON21430LF | Fluid absorption |
| Orogastric tube | Jorgensen Lab, Inc. | J0348R | For stomach and rumen decompression |
| T-port | Medline | DYNDTN0001 | Intravenous catheter tubing connector |
| Urine drainage bag | Covidien | 3512 | Connects to orogastric tube to collect gastric fluids |
| Veterinary trocar with stylet | Braintree Scientific, Inc. | TRO-STY 7B-12 | To guide telemetry wires through subcutaneous tissue |
| Vicryl 2-0 suture | Ethicon | VCPB269H | Closure of subcutaneous soft tissue |
| Vicryl 3-0 suture | Ethicon | VCPB416H | Closure of deep dermal layer |
| Vicryl 4-0 suture | Ethicon | J494H | Closer of subcuticular layer |
| Warming blanket | Jorgensen Lab, Inc. | J1034B | To maintain animal's body temperature |
| Weitlander retractor | Teleflex Medical | 165358 | For wound retraction |
| Yankauer bulb tip suction | Medline | DYND50138 | Sterile waste management |
References
- Fontan, F. Baudet, E. Surgical repair of tricuspid atresia. Thorax. 26 (3), 240-248 (1971).
- Attanavanich, S., Limsuwan, A., Vanichkul, S., Lertsithichai, P., Ngodngamthaweesuk, M. Single-stage versus two-stage modified fontan procedure. Asian Cardiovasc Thorac Ann. 15 (4), 327-331 (2007).
- Bove, E. L. Lloyd, T. R. Staged reconstruction for hypoplastic left heart syndrome. Contemporary results. Ann Surg. 224 (3), 387-394; discussion 394-385 (1996).
- Iskander, C. et al. Comparison of morbidity and mortality outcomes between hybrid palliation and norwood palliation procedures for hypoplastic left heart syndrome: Meta-analysis and systematic review. J Clin Med. 13 (14), 4244 (2024).
- Salik, I., Mehta, B., Ambati, S. Bidirectional Glenn Procedure or Hemi-Fontan. Statpearls, Treasure Island, FL (2024).
- Daley, M. D'udekem, Y. The optimal Fontan operation: Lateral tunnel or extracardiac conduit? J Thorac Cardiovasc Surg. 162 (6), 1825-1834 (2021).
- Jalal, Z. et al. Role and applications of experimental animal models of Fontan circulation. J Clin Med. 13 (9), 2601 (2024).
- Al Balushi, A. Mackie, A. S. Protein-losing enteropathy following Fontan palliation. Can J Cardiol. 35 (12), 1857-1860 (2019).
- Emamaullee, J. et al. Fontan-associated liver disease: Screening, management, and transplant considerations. Circulation. 142 (6), 591-604 (2020).
- Mazza, G. A., Gribaudo, E., Agnoletti, G. The pathophysiology and complications of Fontan circulation. Acta Biomed. 92 (5), e2021260 (2021).
- Schwartz, I., Mccracken, C. E., Petit, C. J., Sachdeva, R. Late outcomes after the Fontan procedure in patients with single ventricle: A meta-analysis. Heart. 104 (18), 1508-1514 (2018).
- Zafar, F. et al. Long-term kidney function after the Fontan operation: Jacc review topic of the week. J Am Coll Cardiol. 76 (3), 334-341 (2020).
- Van Puyvelde, J. et al. Creation of the Fontan circulation in sheep: A survival model. Interact Cardiovasc Thorac Surg. 29 (1), 15-21 (2019).
- Cysyk, J. et al. Chronic in vivo test of a right heart replacement blood pump for failed Fontan circulation. ASAIO J. 65 (6), 593-600 (2019).
- Cysyk, J. P. et al. Miniaturized Fontan circulation assist device: Chronic in vivo evaluation. ASAIO J. 67 (11), 1240--1249 (2021).
- D'udekem, Y. et al. Validating the concept of mechanical circulatory support with a rotary blood pump in the inferior vena cava in an ovine Fontan model. Bioengineering (Basel). 11 (6), 594 (2024).
- Granegger, M. et al. Feasibility of an animal model for cavopulmonary support with a double-outflow pump. ASAIO J. 69 (7), 673-680 (2023).
- Wei, X. et al. Mechanical circulatory support of a univentricular Fontan circulation with a continuous axial-flow pump in a piglet model. ASAIO J. 61 (2), 196-201 (2015).
- Zhu, J. et al. Cavopulmonary support with a microaxial pump for the failing Fontan physiology. ASAIO J. 61 (1), 49-54 (2015).
- Kelly, J. M. et al. Investigation of a chronic single-stage sheep Fontan model. JTCVS Open. 21, 268-278 (2024).
- Anderson, N. H. et al. Telemetry for cardiovascular monitoring in a pharmacological study: New approaches to data analysis. Hypertension. 33 (1 Pt 2), 248-255 (1999).
- Kearney, K., Appleby, C., Kieper, J., Atterson, P. Comparative analysis of data sciences international PhysioTel™ D70 and PhysioTel™ digital telemetry platforms. J Pharmacol Toxicol Methods. 81, 364-365 (2016).
- Physiotel digital l series. At <https://www.datasci.com/products/implantable-telemetry/large-animal/physiotel-digital-l > (2024).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved