Method Article
Radial Endobronchial Ultrasound and Electromagnetic Navigation Bronchoscopy with Fluoroscopy for the Diagnosis of Peripheral Lung Lesions
要約
Diagnosing small lung tumors is quite difficult using a bronchoscope alone. Electromagnetic navigation bronchoscopy is used to locate the lesion, similar to the Global Positioning System. Radial endobronchial ultrasound and fluoroscopy confirm the correct location and monitor the sampling.
要約
Diagnosing lung cancer using a flexible bronchoscope is a safe procedure with a very low risk of complications. Bronchoscopy has high diagnostic accuracy for endobronchial lesions, but it falls short when sampling peripheral lesions. Therefore, several modalities have been invented to guide the bronchoscope to the lesion and confirm the location of the tumor before tissue sampling.
Fluoroscopy is used during bronchoscopy to provide a 2D X-ray image of the thorax during the procedure. The bronchoscope and tools will be visible, as well as lesions if larger than 2.0-2.5 cm. Radial endobronchial ultrasound (rEBUS) consists of an ultrasound probe, small enough to be inserted into the working channel of the bronchoscope. The ultrasound probe is used to differentiate between consolidated tissue, such as tumor tissue, and normal air-filled lung parenchyma. Electromagnetic navigation bronchoscopy (ENB) creates a 3D model of the bronchial tree from computed tomography (CT) scans of the patient. Prior to the bronchoscopy, a route from the trachea to the lesion is planned, to create real-time guidance of the bronchoscope to the lesion during the procedure, similar to the Global Positioning System. The aim of this article is to describe a stepwise approach to performing bronchoscopy with rEBUS and fluoroscopy, bronchoscopy with ENB, rEBUS, and fluoroscopy. In the discussion section, the pros and cons of each modality will be discussed.
概要
Lung cancer is one of the most common cancer types worldwide and the leading cause of cancer-related deaths1. Screening for lung cancer with low-dose computed tomography (CT) has therefore been suggested to diagnose patients before symptoms occur2. Low stages are often detected as small lung lesions or nodules. From one of the largest screening studies conducted in the Netherlands, we know that these lesions are often located in the outer 2/3 of the lung parenchyma and are thereby defined as peripheral lung cancers3,4. To determine whether a lesion is malignant, a tissue sample is required. This can be obtained in several different ways such as surgical excision biopsy, trans-thoracic needle biopsy, or endoscopic with a bronchoscope5,6, the latter having a lower risk of complications compared to surgery and the trans-thoracic approach and a preferable method for diagnosing an increasing elderly population with considerable comorbidities. The diagnostic yield, however, is still lower than the other modalities5.
The bronchoscope allows for visual inspection of the trachea and the main bronchi, but when the bronchi branch into segments and subsegments, locating one small lesion is comparable to finding a needle in a haystack. Therefore, several additional modalities have been developed to guide the bronchoscope to the lesion and confirm the location of a tumor before tissue sampling7. The purpose of these modalities is to increase the diagnostic yield of endoscopic tissue sampling and expand the reach of the bronchoscope towards the pleura, where trans-thoracic needle biopsies are otherwise performed8,9.
Fluoroscopy using a C-arm provides a 2D X-ray image of the thorax during bronchoscopy. It can be used to visualize the position of the bronchoscope and the forceps for trans-bronchial biopsies (TBB) to avoid sampling the pleura and the vascular structures of the intermediate 1/3 of the lung parenchyma when performing random TBBs. When diagnosing lung cancer, fluoroscopy can be used to guide the scope to an "approximate" location of the lesion. Lesions are usually visible on fluoroscopy when the diameter is around 2-2.5 cm or above10. The drawback of fluoroscopy is the 2D image properties, which makes it impossible to know whether the scope is in front, behind, or the center of the lesion11. However, fluoroscopy is also used to confirm that the biopsy tools are in the desired location during sampling if the presence of a tumor has been confirmed with radial endobronchial ultrasound (rEBUS)12.
rEBUS was first described in 1992 by Hürter et al. and is increasingly used in diagnostic workup of peripheral lung lesions13. This modality utilizes the fact that the air-filled lung tissue does not conduct ultrasound waves, whereas denser tissue will appear as a consolidation when scanned using an ultrasound probe. rEBUS is comprised of a circular and rotating ultrasound probe, an ultrasound driving unit, and a guide sheath used to protect the probe while ensuring the correct position of the biopsy tools14. rEBUS can be used on its own or together with other modalities such as electromagnetic navigation bronchoscopy (ENB)15,16,17.
ENB is used to locate a peripheral lung lesion18. The system uses a software program and a CT scan from the patient. A virtual model of the patient´s airways is generated from the CT scan and the operator designs a route from the trachea to the lesion. An electromagnetic field is then created around the patient´s chest and the software synchronizes this field with the virtual field generated from the CT scan, thus assisting the operator to follow the preplanned route during the bronchoscopy, similar to Global Positioning System technology. ENB does not provide real-time confirmation of tumor location. ENB can be combined with fluoroscopy and rEBUS19,20. Virtual Navigation Bronchoscopy (VBN) is the predecessor to ENB and consists of software for creating the virtual model of the bronchial tree along with a route to the lesion. The system does not include real-time navigation, but the route can be displayed during the bronchoscopy21,22. New systems incorporate VBN with fluoroscopy but the use of VBN will not be described in the following protocol23.
ENB systems
Currently, two companies produce systems for ENB, the SPiN system from Olympus and the superDimension system and the ILLUMISITE both sold by Medtronic. The protocol will describe a procedure using the superDimension system, which currently has the most publications. However many steps of the procedure are interchangeable.
The following protocol will describe how to perform rEBUS under fluoroscopy and ENB + rEBUS under fluoroscopy in a clinical setting. The procedures can easily be performed under conscious sedation and general anesthesia. The protocol will not describe any methods for sedation. In the discussion section, the pros and cons of each procedure will be presented.
プロトコル
The protocol in this article describes standard clinical practice. No permission from the ethical committee was needed. Images in the protocol contain no information which can be used to indentify patients.
1. Radial endobronchial ultrasound
- Preparing for the procedure
- Examine the CT scan to check for bronchus signs and the location of the lesion before the procedure.
- Calibrate the ultrasound probe, guide sheath, and biopsy tools of choice prior to the examination, ensuring that the tool will reach the same position as the ultrasound probe when inserted into the guide sheath.
- Perform a systematic examination of the bronchial tree and segments as described in "Systematic bronchoscopy: The 4 landmark approach"24.
- Place the C-arm over the patient and adjust until the lesion is visible in the image.
- Insert the tip of the bronchoscope in the segment or subsegment where the lesion is most likely present.
- Advance the guide sheath and probe under fluoroscopy guidance until the metallic guide sheet tip is adjacent to or in the lesion.
- Advance and activate the probe. Now an ultrasound image will appear on the monitor.
- If the probe is surrounded by a tumor or dense tissue, a concentric consolidation will appear (see Figure 1A). Look for grey and homogeneous consolidation with a hyperechoic border toward the normal lung tissue.
- If the probe is placed adjacent to the lesion, the image will be eccentric (see Figure 1B).
- If the probe is placed in normal lung tissue, only a scattered image of air will appear (see Figure 1C).
- With lesser dense lesions such as ground glass lesions, inflammatory tissue, or atelectasis, a more heterogeneous and less defined image will be presented (see Figure 1D). This is caused by air (hyperechoic) or fluid (hypoechoic) trapped in the bronchi.
- If a consolidation matching the lesion on the CT scan does not appear, adjust the guide sheath and probe until the correct placement is achieved.
- Save the fluoroscopy image of the position where the ultrasound probe provides the best consolidation (will be used in step 1.3.2).

Figure 1: Radial EBUS ultrasound images. (A) Concentric consolidation, (B) Eccentric consolidation, (C) Air-scattered ultrasound image, (D) Irregular consolidation. Abbreviation: EBUS = endobronchial ultrasound. Please click here to view a larger version of this figure.
- Tissue sampling
- Remove the ultrasound probe from the guide sheath and insert forceps or other sampling tool of choice.
- Compare the placement of the forceps to the placement of the ultrasound probe on the C-arm screen, ensuring the sampling is performed in the correct location (see Figure 2). Verify the placement of the forceps, especially if the patient is coughing. Collect a minimum of 10-15 samples using forceps and confirm the correct location by removing the forceps and inserting the ultrasound probe every four to five samples.
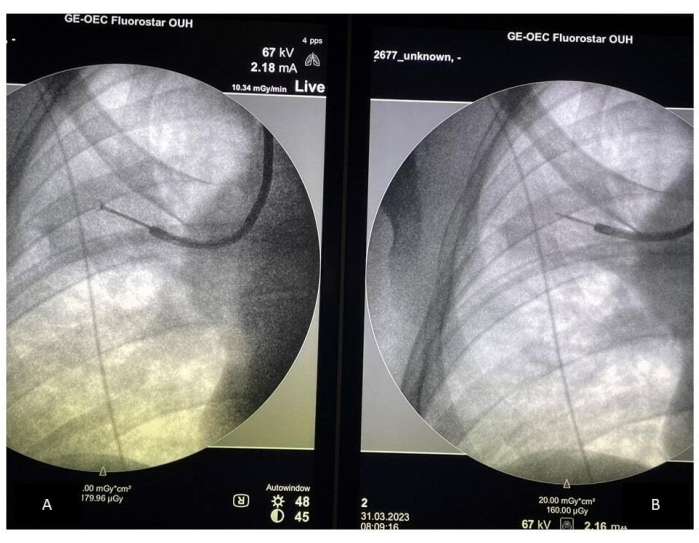
Figure 2: Fluoroscopy-guided sampling. (A) Placement of the forceps during sampling; (B) Placement of the rEBUS probe. Abbreviation: rEBUS = radial endobronchial ultrasound. Please click here to view a larger version of this figure.
2. Electromagnetic navigation bronchoscopy
NOTE: The following procedure is based on the superDimension system by Medtronic.
- The planning phase
- Before the procedure, evaluate the CT images. Ensure that the image is of high quality, preferably with slices no thicker than 1.5 mm, and recorded during inspiration to fully expand the airways revealing bronchi leading to the lesion25.
- Use the software to create a 3D model of the bronchial tree, where the route to the lesion is planned, starting at the lesion and moving toward the trachea.
NOTE: If a pathway to the lesion cannot be established based on the scans, ENB is not the correct modality for the patient.
- Registration
- Place the patient on a board. Place the three sensors on the thorax to compensate for movements during respiration.
- Begin the procedure with a systematic bronchoscopy24.
- Insert the extended working channel (EWC) and locatable guide into the working channel of the bronchoscope until the tip of the locatable guide is visible.
- Lock the locatable guide.
- Press Start registration on the machine or use the foot pedal.
- Insert the bronchoscope stepwise in each lobar bronchus, starting contralaterally and ending in the lobe with the lesion. The position and movements are registered by the system, and the information is used to match the registration of the patient´s airways to the 3D model of the bronchial tree created based on the CT scan. Start the registration on the opposite side from the lesion and finish closest to the target.
- When the system shows that all lobes are registered, press Review registration to see if the registration points match the virtual 3D model. If there is a mismatch, repeat steps 2.2.5-2.2.7 (see Figure 3D).
- Navigation
- Press the foot pedal to begin the navigation. The software will demonstrate a route to the target. Use the central navigation in the larger airways, displaying the video image from the camera along an image of the route in the 3D model of the airways (see Figure 3A).
- Follow the preplanned route until the scope cannot be advanced further.
- Unlock the EWC and locatable guide.
- Switch from central navigation to peripheral navigation by pressing the foot pedal.
NOTE: Peripheral navigation is used when advancing the EWC. During this part, the 3D model of the airways is visible alongside the CT images from the scan. The target is marked with a green ball and a reticle, which provides direction and distance from the target (see Figure 3B). - Keep the target on the right side while ensuring that the route is illuminated ("right and bright"). Advance the EWC, ensuring the route to the bronchi appears open on the CT images.
NOTE: The EWC is maneuverable and can be turned in the desired direction (X, Y, and Z axes) to reach the target. - Once the target is in the center of the reticle, with a distance of 0.4-0.9 mm from the target, the navigation is complete.
NOTE: It is recommended that the locatable guide is not 0.0 mm from the distance, since this can cause the biopsy tool to extend beyond the target. - Lock the EWC in position and retract the locatable guide from the scope.

Figure 3: Electromagnetic navigation bronchoscopic navigation. (A) Central navigation, (B) Peripheral navigation, (C) Review registration with good alignment, (D) CT to body divergence. Abbreviation: CT = computed tomography. Please click here to view a larger version of this figure.
3. Fluoroscopy, rEBUS, and tissue sampling
NOTE: Once the locatable guide is retracted, fluoroscopy can be used without disturbing the electromagnetic field.
- Place the C-arm over the patient and adjust until the lesion is visible in the image.
- Confirm the correct position of the EWC using fluoroscopy and by inserting the rEBUS probe as described in step 1.2.5. If the EWC is not in the lesion under fluoroscopy or a consolidation does not appear using rEBUS, the position must be adjusted as described in steps 1.2.4-1.2.6.
NOTE: CT-to-body divergence occurs when the alignment between the constructed 3D model and the real-time registration points is skewed; the marked target will not be at the location of the lesion. - When the correct location is reached and confirmed, perform tissue sampling as described in steps 1.3.1-1.3.2.
結果
The described technique facilitates the sampling of peripheral lung lesions. Radial EBUS and fluoroscopy will aid the bronchoscopist in confirming the presence of a lesion before sampling the tumor (see Figure 1 and Figure 2). By adding ENB, the bronchoscopist is guided to the correct spot instead of searching for the lesion. The planning phase provides the bronchoscopist with a route to the lesion, real-time guidance to the lesion with the navigation system, confirmation of the presence of a tumor by rEBUS, and tissue sampling guided by fluoroscopy (see Figure 3). Radial EBUS and ENB can be used together or separately. The success of the procedure is often reported as diagnostic yield = diagnostic procedures divided by the total number of procedures. Reported yields in the literature vary from 47% to 83% for the combination of ENB and rEBUS16,26. Complications most often reported are mild bleeding or pneumothorax, however, commonly reported under 2%27.
ディスカッション
This article presents a practical approach for performing rEBUS and ENB with fluoroscopy. The following discussion is the opinion of the authors and is based on practical clinical experience from two centers.
Tips and tricks
rEBUS
Before the procedures the Chest CT sectional walker app can be used to check in which segment the lesion is located14. However, since the anatomy of patients differs, the lesion may be located in another segment than displayed on the app.
It is possible to use larger (2.8 mm working channel) or smaller bronchoscopes (2.0 mm working channel) for this procedure. The smaller scopes are easier to place in the upper lobes and advance to lesions where the bronchi are curved at a sharp angle, for example, behind the heart. Smaller sheaths and forceps for the smaller scopes exist, but if the scope is placed close to the lesion, the sheath can be removed and large forceps can be used instead28. This will sometimes produce better samples.
ENB
Sometimes more than one route to the target is available in the planning phase. It is possible to upload both so that if one fails, it is possible to try the other planned routes before aborting the procedure.
The EWC is curved to ease the placement into the upper lobes and move around sharp corners. They come in different angles ranging from 0° to 190°. The more curved probes are suitable for routes with sharp angles.
After registration, the overlap between the 3D model and the actual registration point is presented in the review registration box (Figure 3C). If the alignment between the constructed 3D model and the real-time registration points is skewed, the marked target will not be at the location of the lesion. This is called CT-to-body divergence25. If this occurs, registration can be repeated; otherwise, ENB may not be a suitable procedure (see Figure 3D).
Selection of patients
Since peripheral lesions can be sampled by surgical excision biopsy, trans thoracic biopsy, and bronchoscopic biopsy, one of the phycian's challenges is to choose the right procedure for each patient. For mediastinal staging, an extensive number of studies have been published29,30. There are guidelines from large societies, and training programs with an assessment of competencies exist4,31. The situation is quite different for peripheral lesions.
The guideline on nodules management from the British Thoracic Society actually states that if the risk of malignancy is above 70%, surgical excision biopsy or non-surgical treatment should be considered6. However, there are no guidelines stating when bronchoscopy should be the first choice and when trans thoracic biopsy should be chosen.
Studies have demonstrated that lesion size below 2 cm, placement in the lower lobes, and lack of bronchus sign are associated with lower bronchoscopic diagnostic yield26,32.
At our centers, the general practice is to choose the procedure with the lowest risk of complications on the condition that obtaining a diagnosis seems feasible. For lesions with broad pleural contact, trans thoracic ultrasound-guided biopsies have demonstrated high diagnostic yields and a low risk of complications and are performed when possible33. Otherwise, transthoracic biopsy is only performed if bronchoscopy is unlikely to be successful.
Pros and cons of rEBUS and ENB
Radial EBUS can be used together with all other equipment and on all patients who are able to undergo bronchoscopy, which is a huge advantage of this modality. The probe can be used with smaller scopes, which is quite helpful when the bronchi to the lesion are crooked34. If the lesion is not visible on fluoroscopy, navigating can be quite difficult. This can explain the lower yield compared to ENB + rEBUS, most recently published in an RCT study, especially for nodules not visible on fluoroscopy16.
A large systematic review from 2020 demonstrated a slightly higher pooled diagnostic yield for ENB than for rEBUS, and two RCT studies have shown a higher diagnostic yield for ENB+ rEBUS than for rEBUS alone16,19,35. The navigation system, as well as the mobility of the EWC, allows for the scope to reach distal parts of the airways. The planning phase will demonstrate particularly tricky passages of the bronchi preparing the bronchoscopist for the procedure.
This modality also has some drawbacks, which must be considered when planning a procedure. The system does not have real-time confirmation of tumor location, which can be accommodated using rEBUS and fluoroscopy, but especially in the lower lobes near the diaphragm, CT to body divergence can be limiting for performing the procedure36,37. The Medtronic systems are only compatible with a bronchoscope with a 2.8 mm working channel. Hence, thin or ultrathin scopes cannot be used. This may be a disadvantage since the thinner scopes are easier to maneuver than the EWC. ENB is not recommended for patients with an implantable cardioverter defibrillator unit or pacemaker. Furthermore, patients with severe obesity or thorax deformations will not fit properly onto the electromagnetic board resulting in poor alignment or no alignment at all.
Finally, yet importantly, an ENB system can cost around 10 times the amount of the equipment for rEBUS. Furthermore, the price for the single-use EWC/LG for ENB is approximately 7 times the price of the singe use guide sheaths used for rEBUS. The ultrasound probes for rEBUS can be used around 60 times before a replacement is needed.
None of the modalities allow for real-time tool in lesion confirmation; however, the newer system from Medtronic incorporates a fluoroscopic digital tomosynthesis image for continuous image-guided sampling38. The complication rates are low for both modalities35.
If there is a large variation
This article describes the method for combining the procedures. Only a few studies have actually compared ENB to ENB and rEBUS, and while the only RCT study showed a higher diagnostic yield when combining the procedures, it is unclear whether rEBUS improves the yield of ENB19,39. If both modalities are available, they are quite easy to combine but when acquiring new equipment, the lack of solid evidence should be taken into account.
Not many RCT studies in interventional bronchoscopy have been conducted and both prospective and retrospective studies are quite heterogeneous, both in setup and results17,26,40,41,42.
A peripheral lesion is defined as a lesion located in the outer 2/3 of the chest in the ERS guidelines on mediastinal staging from 20154. However, a survey study from 2017 demonstrated great variety in how tumor placement was defined among interventional pulmonologists and thoracic surgeons43. If the is a large variation in the selection of patients for procedures and studies it is quite difficult to compare the outcomes. With an increase in elderly or fragile patients, improving the endoscopic yield is of great importance. Future studies comparing endoscopic modalities are needed.
rEBUS and ENB are two valuable tools for safely diagnosing peripheral lung lesions. They can be performed separately or together. There are advantages and drawbacks to both modalities. The literature on endoscopic diagnostics of peripheral lung lesions is heterogeneous. As such currently, no guidelines for choosing modalities exist.
開示事項
Medtronic has kindly lent ENB equipment to the Simulation Center at Odense University Hospital, for a study conducted by A. Juul. Medtronic has not been a part of writing this article
謝辞
The authors would like to thank all the bronchoscopists at the Department of Respiratory Medicine, Odense University Hospital, for providing images for the article.
資料
| Name | Company | Catalog Number | Comments |
| Bronchoschope | Olympus | ||
| Edge Extended working channel | Medtronic | ||
| Edge locatable guide | Medtronic | ||
| Guide sheath kit | Olympus | ||
| OEC fluorostar | GE healthcare | C-arm for fluoroscopy | |
| Probe Driving Unit | Olympus | ||
| Radial EBUS probes | Olympus | ||
| superDimension | Medtronic | Navigation system |
参考文献
- Ferlay, J., et al. Cancer statistics for the year 2020: an overview. Int J Cancer. , (2021).
- Adams, S. J., et al. Lung cancer screening. Lancet. 401 (10374), 390-408 (2022).
- Horeweg, N., et al. Characteristics of lung cancers detected by computer tomography screening in the randomized NELSON trial. Am J Respir Crit Care Med. 187 (8), 848-854 (2013).
- Vilmann, P., et al. Combined endobronchial and oesophageal endosonography for the diagnosis and staging of lung cancer. Eur Resp J. 46 (1), 40-60 (2015).
- Schreiber, G., McCrory, D. C. Performance characteristics of different modalities for diagnosis of suspected lung cancer: summary of published evidence. Chest. 123, 115-128 (2003).
- Callister, M. E. J., et al. British Thoracic Society guidelines for the investigation and management of pulmonary nodules: accredited by NICE. Thorax. 70, (2015).
- Shulman, L., Ost, D. Advances in bronchoscopic diagnosis of lung cancer. Curr Opin Pulm Med. 13 (4), 271-277 (2007).
- Eberhardt, R., Gompelmann, D., Herth, F. J. Electromagnetic navigation in lung cancer: research update. Expert Rev Respir Med. 3 (5), 469-473 (2009).
- Han, Y., et al. Diagnosis of small pulmonary lesions by transbronchial lung biopsy with radial endobronchial ultrasound and virtual bronchoscopic navigation versus CT-guided transthoracic needle biopsy: A systematic review and meta-analysis. PLoS One. 13 (1), 0191590 (2018).
- Deng, C., et al. Small lung lesions invisible under fluoroscopy are located accurately by three-dimensional localization technique on chest wall surface and performed bronchoscopy procedures to increase diagnostic yields. BMC Pulm Med. 16 (1), 166 (2016).
- Sánchez-Font, A., et al. Endobronchial ultrasound for the diagnosis of peripheral pulmonary lesions. A controlled study with fluoroscopy. Arch Bronconeumol. 50 (5), 166-171 (2014).
- Tanner, N. T., et al. Standard bronchoscopy with fluoroscopy vs thin bronchoscopy and radial endobronchial ultrasound for biopsy of pulmonary lesions: a multicenter, prospective, randomized trial. Chest. 154 (5), 1035-1043 (2018).
- Hürter, T., Hanrath, P. Endobronchial sonography: feasibility and preliminary results. Thorax. 47 (7), 565-567 (1992).
- Zhang, L., Wu, H., Wang, G. Endobronchial ultrasonography using a guide sheath technique for diagnosis of peripheral pulmonary lesions. Endosc Ultrasound. 6 (5), 292-299 (2017).
- Song, J. Y., et al. Efficacy of combining multiple electromagnetic navigation bronchoscopy modalities for diagnosing lung nodules. J Clin Med. 11 (24), 7341 (2022).
- Zheng, X., et al. A novel electromagnetic navigation bronchoscopy system for the diagnosis of peripheral pulmonary nodules: a randomized clinical trial. Ann Am Thorac Soc. 19 (10), 1730-1739 (2022).
- Sainz Zuñiga, P. V., Vakil, E., Molina, S., Bassett, R. L., Ost, D. E. Sensitivity of radial endobronchial ultrasound-guided bronchoscopy for lung cancer in patients with peripheral pulmonary lesions: an updated meta-analysis. Chest. 157 (4), 994-1011 (2020).
- Criner, G. J., et al. Interventional bronchoscopy. Am J Respir Crit Care Med. 202 (1), 29-50 (2020).
- Eberhardt, R., Anantham, D., Ernst, A., Feller-Kopman, D., Herth, F. Multimodality bronchoscopic diagnosis of peripheral lung lesions: a randomized controlled trial. Am J Respir Crit Care Med. 176 (1), 36-41 (2007).
- Folch, E. E., et al. Electromagnetic navigation bronchoscopy for peripheral pulmonary lesions: one-year results of the prospective, multicenter NAVIGATE study. J Thorac Oncol. 14 (3), 445-458 (2019).
- Asano, F., et al. A virtual bronchoscopic navigation system for pulmonary peripheral lesions. Chest. 130 (2), 559-566 (2006).
- Asano, F., et al. Virtual bronchoscopic navigation without X-ray fluoroscopy to diagnose peripheral pulmonary lesions: a randomized trial. BMC Pulm Med. 17 (1), 184 (2017).
- Tsai, Y. M., Kuo, Y. S., Lin, K. H., Chen, Y. Y., Huang, T. W. Diagnostic performance of electromagnetic navigation versus virtual navigation bronchoscopy-guided biopsy for pulmonary lesions in a single institution: potential role of artificial intelligence for navigation planning. Diagnostics (Basel). 13 (6), 1124 (2023).
- Cold, K. M., Vamadevan, A., Nielsen, A. O., Konge, L., Clementsen, P. F. Systematic bronchoscopy: the four landmarks approach). J Vis Exp. (196), (2023).
- Pritchett, M. A., Bhadra, K., Calcutt, M., Folch, E. Virtual or reality: Divergence between preprocedural computed tomography scans and lung anatomy during guided bronchoscopy. J Thorac Dis. 12 (4), 1595-1611 (2020).
- Ost, D. E., et al. Diagnostic yield and complications of bronchoscopy for peripheral lung lesions. Results of the AQuIRE Registry. Am J Resp Crit Care Med. 193 (1), 68-77 (2016).
- McGuire, A. L., Myers, R., Grant, K., Lam, S., Yee, J. The diagnostic accuracy and sensitivity for malignancy of radial-endobronchial ultrasound and electromagnetic navigation bronchoscopy for sampling of peripheral pulmonary lesions: Systematic review and meta-analysis. J Bronchology Interv Pulmonol. 27 (2), 106-121 (2020).
- Oki, M., et al. Guide sheath versus non-guide sheath method for endobronchial ultrasound-guided biopsy of peripheral pulmonary lesions: a multicentre randomised trial. Eur Respir J. 59 (5), 2101678 (2022).
- Korevaar, D. A., et al. Added value of combined endobronchial and oesophageal endosonography for mediastinal nodal staging in lung cancer: a systematic review and meta-analysis. Lancet Respir Med. 4 (12), 960-968 (2016).
- Micames, C. G., McCrory, D. C., Pavey, D. A., Jowell, P. S., Gress, F. G. Endoscopic ultrasound-guided fine-needle aspiration for non-small cell lung cancer staging: A systematic review and metaanalysis. Chest. 131 (2), 539-548 (2007).
- Farr, A., et al. Endobronchial ultrasound: launch of an ERS structured training programme. Breathe. 12 (3), 217 (2016).
- Bellinger, C., Poon, R., Dotson, T., Sharma, D. Lesion characteristics affecting yield of electromagnetic navigational bronchoscopy. Respir Med. 180, 106357 (2021).
- Laursen, C. B., et al. Ultrasound-guided lung biopsy in the hands of respiratory physicians: diagnostic yield and complications in 215 consecutive patients in 3 centers. J Bronchology Interv Pulmonol. 23 (3), 220-228 (2016).
- Oki, M., et al. Value of adding ultrathin bronchoscopy to thin bronchoscopy for peripheral pulmonary lesions: A multicentre prospective study. Respirology. 28 (2), 152-158 (2023).
- McGuire, A. L., Myers, R., Grant, K., Lam, S., Yee, J. The diagnostic accuracy and sensitivity for malignancy of radial-endobronchial ultrasound and electromagnetic navigation bronchoscopy for sampling of peripheral pulmonary lesions: Systematic review and meta-analysis. J Bronchology Interv Pulmonol. 27 (2), 106-121 (2020).
- Folch, E. E., et al. Electromagnetic navigation bronchoscopy for peripheral pulmonary lesions: one-year results of the prospective, multicenter NAVIGATE study. J Thorac Oncol. 14 (3), 445-458 (2019).
- Pritchett, M. A., Bhadra, K., Calcutt, M., Folch, E. Virtual or reality: divergence between preprocedural computed tomography scans and lung anatomy during guided bronchoscopy. J Thorac Dis. 12 (4), 1595-1611 (2020).
- Dunn, B. K., et al. Evaluation of electromagnetic navigational bronchoscopy using tomosynthesis-assisted visualization, intraprocedural positional correction and continuous guidance for evaluation of peripheral pulmonary nodules. J Bronchology Interv Pulmonol. 30 (1), 16-23 (2023).
- Juul, A. D., et al. Does the addition of radial endobronchial ultrasound improve the diagnostic yield of electromagnetic navigation bronchoscopy? A systematic review. Respiration. 101 (9), 869-877 (2022).
- Silvestri, G. A., et al. An evaluation of diagnostic yield from bronchoscopy: the impact of clinical/radiographic factors, procedure type, and degree of suspicion for cancer. Chest. 157 (6), 1656-1664 (2020).
- Silvestri, G. A., et al. A bronchial genomic classifier for the diagnostic evaluation of lung cancer. N Engl J Med. 373 (3), 243-251 (2015).
- Rozman, A., Zuccatosta, L., Gasparini, S. Dancing in the dark. Respiration. 101 (9), 814-815 (2022).
- Casal, R. F., et al. What exactly is a centrally located lung tumor? Results of an online survey. Ann Am Thorac Soc. 14 (1), 118-123 (2016).
転載および許可
このJoVE論文のテキスト又は図を再利用するための許可を申請します
許可を申請This article has been published
Video Coming Soon
Copyright © 2023 MyJoVE Corporation. All rights reserved