Method Article
Establishing a Severe Corneal Inflammation Model in Rats Based on Corneal Epithelium Curettage Combined with Corneal Sutures
* Questi autori hanno contribuito in egual misura
In questo articolo
Riepilogo
We developed a rat model of severe corneal inflammation through corneal epithelium curettage combined with corneal sutures. The study evaluated corneal inflammation patterns, epithelial proliferation, and changes in limbal stem cells under inflammatory conditions.
Abstract
Corneal inflammation, especially severe corneal inflammation, plays a significant role in the development of corneal limbal stem cell dysfunction. Constructing appropriate animal models can help us focus on the effects of severe inflammation on corneal limbal stem cells. A 2 mm rust remover was used to remove the central corneal epithelium of Sprague Dawley (SD) rats to create an injury. Then, the central stroma of the cornea was sutured with nylon sutures to induce persistent inflammation. In this way, a corneal inflammation model with central corneal epithelium abrasion and central stroma suturing was constructed, which induced severe corneal inflammation. The changes in corneal inflammation and the condition of the limbal stem cells at 1, 3, and 7 days post-modeling were observed. On the 3rd day after modeling, the rats' corneal limbus was severely edematous, with obvious neovascularization and local hyperplasia, which are typical signs of limbal stem cell deficiency. By the 7th day, the corneal edema gradually worsened, and the neovascularization continued to increase. Through quantitative reverse transcription polymerase chain reaction (RT-qPCR) and immunofluorescence staining, we found that the corneal epithelial inflammatory factors were significantly upregulated, the corneal epithelial differentiation was abnormal, the corneal epithelial stem cells were significantly reduced, and the cell proliferation and stemness had also decreased. Therefore, this model demonstrates that severe inflammation can induce limbal stem cell damage without directly damaging the limbal stem cells. The model is beneficial for observing the effects of severe inflammation on the biological mechanisms of stem cells and provides an ideal platform for studying the mechanisms of corneal epithelial stem cell dysfunction induced by inflammation.
Introduzione
Limbal stem cell dysfunction (LSCD) is characterized by persistent epithelial defects, corneal vascularization, chronic inflammation, scarring, and conjunctivalization of cornea1,2. It may arise from the depletion or dysfunction of limbal stem cells after severe ocular surface diseases, such as chemical burns, thermal burns, Stevens-Johnson syndrome, and iatrogenic injury caused by ocular surgeries2,3,4. The pathogenic mechanisms of LSCD mainly involve alterations in stem cells and disturbances in the stem cell microenvironment5,6, which affects the homeostasis of the corneal epithelium2,7. There are two primary types of fate changes in limbal stem cells in LSCD: 1) The directional differentiation potential of corneal epithelial stem cells becomes abnormal, leading to their differentiation into skin epithelial cells. This is evidenced by a decrease in the expression of corneal epithelial cell-specific transcription factor Pax6 and cornea-specific keratins Krt12 and Krt3, alongside a significant increase in the expression of skin-related squamous epithelial metaplasia markers Krt10, Krt1, and Sprr1b8; 2) Ocular surface injury directly destroys corneal limbal stem cells, resulting in necrosis or apoptosis, and subsequently causing conjunctival tissue proliferation that covers the cornea9. It has been reported that during the development of LSCD, inflammatory cells such as macrophages, neutrophils, and dendritic cells significantly increase in the corneal epithelial stem cell microenvironment. Correspondingly, cytokines such as interferon-gamma (IFN)-γ, tumor necrosis factor(TNF)-α, interleukin (IL)-1β, and macrophage inflammatory proteins (MIP)-1α/β also show significant elevations10.
Various LSCD models have been established, including those induced by sutures, chemical injuries, and benzalkonium chloride spotting11. Each of these models has its advantages and disadvantages. Chemical injuries can damage the entire ocular surface and directly destroy limbal stem cells12. Benzalkonium chloride functions similarly to chemical injuries but has a relatively slow effect13. The corneal suture model can induce a stable and long-term inflammatory response, but the inflammatory reaction is relatively mild and cannot cause LSCD.
To investigate the role and mechanisms of inflammation in the development of corneal LSCD, an animal model was established that combines curettage of the central corneal epithelium with corneal sutures. This model induces persistent inflammation of the corneal epithelium without directly damaging limbal stem cells.
Protocollo
All procedures conformed to the ARVO guidelines for using animals in ophthalmic and vision research and were approved by the Animal Ethics Committee of Guizhou Medical University (Approval No. 2305192). The rats were housed at the Animal Center of Guizhou Medical University, adhering to relevant animal management regulations.
1. Animal selection
- Use female Sprague-Dawley (SD) rats aged 7-8 weeks. Ensure that there are no ocular surface lesions under slit lamp examination.
- Sterilize the instruments using a rapid sterilizer. Required instruments include corneal rust ring remover, 2 mm size skin sampling marker, needle holders, plate layer knives, ophthalmic surgical scissors, and forceps.
- Anesthetize the rats with 1.25% tribromoethanol via intraperitoneal injection at a dose of 0.3 mL/100 g. Instill one drop of tropicamide eye drops for pupil dilation. Apply proparacaine hydrochloride eye drops.
- Disinfect the skin and hair around the eyes with iodophor.
- Establish the inflammation model.
- Place the rat in the lateral decubitus position. Expose the eyeball under an operating microscope.
- The central cornea was labeled using a 2mm size skin sampling marker, and the labeled central corneal epithelium was scraped using a Corneal rust ring remover.
- Place three 120° corneal sutures using 10-0 nylon wire, needle holder, and ophthalmic surgical scissors and forceps about 3 mm from the limbus, do not penetrate the cornea14,15.
- Apply Ofloxacin eye ointment at the end of the procedure to prevent infection.
- Observe and photograph the cornea on the 1st, 3rd, and 7th day post-operation using a slit lamp magnified 10x lens. Record the symptoms such as corneal epithelial healing, conjunctival hyperemia, corneal edema, limbal hyperemia, and corneal neovascularization.
2. Calculation of the inflammatory index
- Calculate the inflammatory index of the ocular surface after establishing the animal model.
- Score ciliary body congestion as follows: no congestion (0 points); the presence of congestion less than 1 mm (1 point); hyperemia between 1 mm and 2 mm (2 points); hyperemia between 1 mm and 2 mm (3 points).
- Score central corneal edema as follows: no edema (0 points); edema with clear iris visibility (1 point); edema with unclear iris visibility (2 points); edema with the iris not visible (3 points).
- Score paracentral corneal edema as follows: no edema (0 points); edema with clear iris visibility (1 point); edema with unclear iris visibility (2 points); edema with the iris not visible (3 points).
- Calculate the inflammation index by adding all scores and dividing it by 9.
3. Assessment of corneal neovascularization
- Observe corneal neovascularization with a slit lamp.
- Analyze and process images using slit lamp image processing software16.
- Record the growth of corneal neovascularization at each observation time point, measuring the longest corneal neovascularization towards the central cornea with minimal curvature.
- Grade them as follows: Grade 0: Avascular; Grade 1: Micro (<1 mm); Grade 2: Mild (1 mm to 1.5 mm); Grade 3: Moderate (>1.5 mm to 2 mm); Grade 4: Severe (>2 mm).
4. Analyzing the biology of the model
- Prepare frozen eye specimens.
- Anesthetize the rat with 4-5% isoflurane and euthanize it by cervical dislocation.
- In the lateral decubitus position, lift the rat eyeball outward with micro tweezers and cut the conjunctiva open along the upper and lower eye dome with eye scissors.
- Cut the optic nerve, paying attention to maintaining the integrity of the corneal epithelium.
- Dry the blood stains and water on the eyeball surface with filter paper and put them in the embedding box with optimal cutting temperature (OCT) embedding medium.
- Remove the bubbles in the embedding box with a 200 µL pipette, freeze it using liquid nitrogen, and then save the embedded specimens in the ultra-low temperature refrigerator.
- Prepare frozen sections of the eyeballs.
- Before preparing the frozen section, precool the cryostat to about -28 °C and the specimen holder to -26 °C.
- Remove the frozen specimens from the cold freezer and place them in the cryotome for about 1 h.
- Precool the frozen specimen in a cryotome for 10 min. Mark the specimen with a pencil, fix it on the specimen pad with OCT, and fix them together on the sectioning rack after cooling.
- Adjust the position and direction of the frozen specimen and fix it. First, cut 20 µm sections until the central cornea is seen. Then, obtain 6 µm sections by continuous slicing and collect the sections on the pre-prepared adhesion slides.
- Mark the name, material, section time, name, and number of the tissue on the slide. Place it at room temperature (RT) for about 30 min.
- After drying the sections, put them into a box and keep them in the ultra-low temperature refrigerator for reserve.
- Perform hematoxylin and eosin staining.
- Remove the pre-prepared frozen sections from the ultra-low temperature refrigerator and place them in the fume hood at RT to dry.
- Fix the sections using 4% paraformaldehyde for 15 min at RT. Rinse the specimens with 1x PBS for 5 min.
- Place the specimen sections containing slides on a slide holder and immerse them into the dye cylinder with hematoxylin dye solution at RT for 5 min.
- Slowly rinse the specimen containing slides with tap water to wash off the floating dye, put them into the dye tank with 0.1% hydrochloric acid alcohol, and remove them quickly after 1-2 s.
- Slowly rinse the specimen with tap water for 15 min.
- Remove the specimen from the water and immerse the slides into the eosin dye tank; leave at RT for 3 min.
- Slowly rinse the specimen with tap water, wash away the excess dye, and perform gradient alcohol dehydration using 75% alcohol, 80% alcohol, 95% alcohol, 100% alcohol (one), and 100% alcohol (two). Immsere the sections in each concentration for 2 min.
- Make the specimens transparent for 2 min in xylene (1) and xylene (2) respectively.
- Dry the specimen in the fume hood, seal the sections using a sealing agent, and be careful not to produce bubbles.
- Place the slides in a slide box at RT. Image the sections using a microscope.
- Perform immunofluorescence staining.
- Remove the frozen sections and place them in a box at RT in the fume hood to dry.
- Mark the name of the antibody to be stained in the glass slide blank of the frozen specimen.
- Fix the specimens with 4% paraformaldehyde at RT for 15 min. A small amount of tap water was added to the box to prevent the specimen from drying out.
- Remove the paraformaldehyde fixative and wash the specimen three times with 1x PBS buffer. Be careful not to flush off the specimen.
- Dry the liquid around the tissue section, cover the specimen with cell membrane solution (0.2% TritonX-100), and incubate at RT for 20 min.
- Remove the cell membrane solution and wash the sections three times for 5 min each with 1x PBS buffer.
- Dry the tissue sections, cover the specimen with immunofluorescence blocking solution (2% BSA), and incubate at RT for 1 h.
- Prepare the primary antibody and diluent (1% BSA) during the incubation per the manufacturer's instructions.
- Remove the blocking fluid and dry the specimen with a suction pump. Add the prepared primary antibody, cover the box, and incubate it in a 4 °C refrigerator overnight (incubate for about 12-16 h) away from light.
- Discard the primary antibody and wash the specimen four times for 10 min each in PBS.
- During the cleaning step, prepare the secondary antibody dilution as per the manufacturer's instructions.
- Remove PBS, dry the specimen, and add the prepared secondary antibody to the specimen. Cover the specimen and incubate at RT for 1 h away from light.
- Remove the secondary antibody, wash the specimen 4 times with 1x PBS buffer for 10 min each, and cover the whole slide with 1x PBS buffer.
- Remove PBS, dry the specimen, and seal the sections with a mounting medium containing DAPI, ensuring there are no air bubbles. Wrap the slides in tin foil and keep them at 4 °C away from light.
- Take photos with a laser confocal microscope system or with an upright fluorescence microscope camera system (scan speed: 4 µs/pixel, scanning resolution: 1024 x 1024).
- Single-cell suspension preparation of the rat corneal epithelium
- After euthanasia, remove the eyeballs with tweezers soaked in 75% alcohol and put them into the 1x Hanks' balanced salt solution (HBSS) containing 10% fetal bovine serum (FBS) and 10% penicillin-streptomycin prepared in advance.
- Clean the eyeballs and use 1x HBBS containing 10% FBS and 10% penicillin-streptomycin twice for 5 min each.
- Remove the conjunctiva along the scleral margin; leave the scleral margin at about 1 mm. Again, wash the corneal tissue twice with 1x HBBS containing 10% FBS and 10% penicillin-streptomycin.
- Transfer the clean corneal tissue to a supplemented hormonal epidermal medium (SHEM) containing 2U/mL of Dispase II. Close the seal and place it at 4°C for 14-16 h.
- Have a pair of tweezers, toothless tweezers, an iris recovery device, and a surgical sterilizer ready.
- Under a surgical microscope, gently separate the corneal epithelium along the edge of the cornea.
- Wash the corneal epithelium with 1x HBSS containing 10% penicillin-streptomycin for 5 min.
- Transfer the corneal epithelial tissue to a 1.5 mL centrifuge tube containing 200 mL of trypsin and incubate in a 5% CO2, 37 °C incubator. Every 5 min, shake the centrifuge tube three times to digest the corneal epithelial sheet into a single-cell suspension.
- Add an equal amount of SHEM medium to stop the action of the trypsin digestion solution and place it at RT.
- Clone culture of corneal epithelial cells
- Prepare trophoblast cells.
- When the growth density of NIH 3T3 mouse cells reaches 80-90%, discard the cell culture medium. Add pre-prepared 10 mg/mL NIH 3T3 mitomycin culture medium and incubate at 37 °C for 2.5 h.
- Remove the medium, rinse cells with 1x HBSS, replace with new NIH 3T3 cell medium, and continue incubation in a CO2 incubator (5% CO2, 37 °C).
- Prepare the corneal epithelial cell suspension and count the cells according to the required ratio.
- While preparing the corneal epithelial cell suspension, process NIH 3T3 cells treated with mitomycin.
- Discard the supernatant, rinse cells with 1x HBSS, add 1 mL of trypsin-EDTA solution, and incubate at RT for 2-3 min.
- Add an equal amount of supplemental hormonal epithelial medium (SHEM medium: DMEM/F12, 10 ng/mL mouse epidermal growth factor [EGF], 10 µg/mL Insulin-Transferrin-Selenium [ITS], 5% FBS, 1% penicillin-streptomycin, 0.5% dimethyl sulfoxide [DMSO], 0.5 mg/mL hydrocortisone), gently pipette to make a cell suspension, and count the cells.
- Use a 12-well plate and add 0.5 mL of SHEM medium to each well.
- Mix corneal epithelial cells with NIH 3T3 cells at a density of 500 corneal epithelial cells/cm2 and 5 x 105 NIH 3T3 cells/cm2. Add 1 mL of SHEM medium to each well.
- Add 1 mL of the cell mixture to each well and gently pipette to distribute the cells evenly.
- Incubate in an incubator (5% CO2, 37 °C), changing the medium every 2-3 days. Culture for 12-14 days.
- Prepare trophoblast cells.
- Crystal violet staining
- After approximately 12 days, when corneal epithelial clones are about to fuse, remove the culture medium and add 1 mL of 4% paraformaldehyde solution to each well, covering the cells completely. Fix the cells for 15 min at RT.
- Remove 4% paraformaldehyde and wash the wells three times with 1x PBS for 5 min each.
- Remove 1x PBS buffer and add 0.4% crystal violet dye solution to each well. Stain for 30 min at RT.
- Remove or recycle the crystal violet dye solution and wash it three times with 1x PBS for 10 min each.
- Air dry the wells at RT until thoroughly dry.
- Use a regular upright microscope photography system (scan speed: 4 µs/pixel, scanning resolution: 1024 x 1024).
- Extraction of corneal mRNA
- Gather ophthalmic scissors, corneal scissors, toothed forceps, electric corneal epithelial scraper, Trizol, RNase-free 1.5 mL centrifuge tubes, and an ice box.
- Epithelial acquisition
- Anesthetize the rat with 4-5% isoflurane and euthanize it after cervical dislocation. Place the animal in a lateral position. Trim the mouse's whiskers with eye scissors to avoid blocking the view.
- Place the rat under a surgical microscope and adjust it to an appropriate height to focus on the eyeball. Use toothed forceps to gently push and expose the eyeball. Lift the rat's conjunctiva and use a clean electric corneal epithelial scraper to scrape the central 2 mm area of the corneal epithelium.
- Collect the scraped corneal epithelium with clean, toothed forceps sprayed with alcohol. Place the collected tissue into a centrifuge tube containing 1 mL of Trizol.
- Work as quickly as possible to rinse the collected tissue in a 50 mL centrifuge tube containing double-deionized water. Absorb excess water with filter paper. Spray with 75% alcohol and proceed to the next step.
- Lift the rat's conjunctiva again using toothed forceps. Use corneal scissors with the concave side up to cut the conjunctiva along the limbus, leaving about 0.5 mm of white conjunctiva.
- Scrape the corneal epithelium within a 2 mm range at the corneal limbus using the electric scraper. Collect the tissue as described before. Collect the central corneal epithelium from three eyeballs and the limbal epithelium from three eyeballs.
- Place the collected tissues into separate 1 mL Trizol RNA extraction reagent tubes. Perform the entire operation on ice to preserve RNA integrity. Proceed with the next steps immediately or store the samples in an ultra-low temperature freezer at -80 °C for later use.
- Thaw the specimen on ice, use an ultrasonic tissue homogenizer to lyse the cells. Set the ultrasonic homogenizer's power to 25%, time to 2 s per burst, interval time to 5 s, and the total time to 2 min. Let it stand on ice for 10 min.
- Add chloroform (1/5 of the total volume, approximately 200 µL) to each sample, mix thoroughly by inverting for 30 s, and centrifuge at 400 x g for 10 min at 4 °C.
- RNA precipitation
- After centrifugation, the liquid inside the centrifuge tube is layered into three parts. The top clear layer is RNA, the middle white cloudy layer is DNA and proteins, and the bottom layer is Trizol. Carefully pipette about 500 µL of the top clear layer into a new 1.5 mL centrifuge tube, careful not to aspirate the white precipitate in the middle layer. If inadvertently aspirated, repeat centrifugation to collect the clear layer again.
- Add an equal amount of pre-chilled isopropanol to the centrifuge tube containing the clear layer, approximately 500 µL, invert the tube several times to mix thoroughly, then immediately place it in a -80 °C ultra-low temperature freezer for 20-30 min. This step aims to accelerate the precipitation of RNA.
- Take out the sample, and immediately centrifuge at 400 x g for 10 min at 4 °C. A small amount of white, flaky precipitate is seen at the bottom of the centrifuge tube; discard the supernatant.
- Add pre-prepared and ice-cooled 70% ethanol to each tube. Immediately centrifuge at 4 °C in a refrigerated centrifuge at 400 x g for about 10 min; repeat the above steps once to thoroughly wash the precipitate. At this step, pre-sterilize the fume hood with UV light for 15 min.
- After removing the centrifuge tubes and discarding the supernatant, centrifuge again at 4 °C at approximately 250 x g for 2 min. Use a 200 µL pipette tip to carefully aspirate the excess alcohol from the side opposite the precipitate, drying as thoroughly as possible. Place in a pre-UV-sterilized fume hood to dry, air dry the precipitate for about 15 min.
- Prepare the solution by adding 1 mL of RNase inhibitor to 40 mL of Diethyl pyrocarbonate (DEPC) water. Add an appropriate amount of DEPC water/RNase inhibitor mixture, about 20-30 µL, to dissolve the sample. If there is a lot of precipitate, add 30-50 µL. Immediately proceed with reverse transcription PCR.
- Reverse transcription PCR
- Ensure these items are present: PCR machine, RNase-free 1.5 mL centrifuge tubes, 0.2 mL PCR tubes, ice bath or ice box, pipette, and RNase-free tips.
- Measure the RNA concentration using the microvolume spectrophotometer. For each sample, ensure 1 µg of RNA is added to the reaction system.
- The reverse transcription system has a total volume of 20 µL. Calculate the total amount of reagents needed based on the number of samples. Mix according to the ratio specified in the instructions.
- Prepare the reaction system on ice, vortex it, and briefly centrifuge it. Incubate at 42 °C for 15 min and at 90 °C for 30 s.
- Use the cDNA obtained from the reverse transcription process either directly for real-time quantitative PCR analysis or store in a -80 °C refrigerator.
- Real-time quantitative PCR reaction
- Use a real-time quantitative PCR reagent kit17 with a 10 µL reaction system. Use the primer sequences shown in Table 1.
- Add the reaction system described above into a 96-well plate or an eight-strip tube specifically for RT-PCR. Ensure that the samples are well-mixed. Check for bubbles in the tubes before placing them on the PCR machine.
- Amplify the gene using the PCR cycle (95 °C for 5 min), 40 cycles (95 °C for 10 s, 60 °C for 30 s), and complete the data to calculate the 2-ΔΔ Ct value. Process, analyze, and plot the data using appropriate data analysis software.
Risultati
As shown in Figure 1A, through observation with a slit lamp, we found that the healing of the corneal epithelium was impaired. Under normal circumstances, the central corneal epithelium would completely heal within 24-48 h after removal, but in the corneal central epithelium excision combined with a suture model, new blood vessels continued to exist. On the third day after the model was established, significant corneal limbal edema and neovascularization were observed, with typical signs of corneal limbal stem cell deficiency appearing locally. Over time, by the 7th day, healing was still not complete. The corneal inflammation index was assessed using ciliary congestion, paracentral corneal edema, and central corneal edema. Corneal edema gradually worsened, and corneal inflammation (Figure 1A) continued to deteriorate. In addition, corneal neovascularization is an indicator of inflammation. With the progression of inflammation, corneal neovascularization (Figure 1B) and inflammation index (Figure 1C) showed a continuous increase.
We performed cryoembedding on the eye tissues 7 days after model creation. Hematoxylin and eosin staining (Figure 2A) showed thickening of the epithelium at the limbus and central cornea, with the epithelial arrangement losing its normal morphology, indicating atypical hyperplasia. The corneal stroma appeared edematous, with significant infiltration of inflammatory cells. Using PMN and ED1 immunofluorescence staining (Figure 2B), a marked increase in the infiltration of neutrophils and macrophages was observed. The study assessed inflammatory factors using IL-1β (Figure 2C) and MIP-1α (Figure 2D), finding that IL-1β peaked on day 3 and gradually declined thereafter, while MIP-1α continuously increased over time.
Chronic inflammation typically leads to a series of limbal stem cell deficiencies, such as Pax6 downregulation, reduced Krt12 expression, and squamous metaplasia of the corneal epithelium. To verify whether the model developed in this study could induce these changes in the corneal epithelial phenotype, immunofluorescence staining for Krt12 (Figure 3A) and Pax6 (Figure 3B) on the corneal epithelium was performed. Krt12 expression decreased with prolonged inflammation, with only single Krt12-positive cells observed on day 7, and Pax6 expression was significantly reduced in the corneal epithelium. At the mRNA level, the expression levels of normal corneal epithelial markers Krt12 (Figure 3C) and Pax6 (Figure 3D) were significantly reduced on day 7. However, the expression levels of squamous metaplasia markers Krt10 (Figure 3E) and Sprr1b (Figure 3F) were significantly increased on day 7.
We used the proliferation marker Ki67 to stain the corneal epithelium, and the results showed a significant decrease in the number of Ki67-positive corneal epithelial cells by day 7 (Figure 4A). The status of corneal epithelial stem cells was assessed using the corneal limbal stem cell-specific marker ABCG2 (Figure 4B) and the epithelial progenitor cell marker P63 (Figure 4C). ABCG2 and P63 levels were significantly lower than those in the normal group. Additionally, we employed the gold standard for stem cells, the clonal culture method, to assess changes in corneal limbal stem cells during this process. We observed that the clonal formation rate of corneal limbal stem cells (Figure 4D) decreased by day 3 and significantly declined by day 7, with the average area of clones becoming smaller and only a few holoclones visible on day 7. The colony-forming efficiency (CFE) was obtained by counting the cell clones visible in the microscope field of the culture dish. The CFE (Figure 4E) indicated a gradual reduction in the number of corneal limbal stem cells under inflammatory conditions. This suggests that severe inflammation in this model leads to the depletion of corneal limbal epithelial stem cells, and this process does not induce a phenotypic transformation of the corneal limbal stem cells but rather presents issues in differentiation or self-renewal.
The inflammation model used in this study resulted in corneal changes characteristic of LSCD, including extensive infiltration of inflammatory cells into the cornea, dysplasia of the corneal epithelium, and disrupted or reduced expression of Pax6 and Krt12 in the normal corneal epithelial phenotype. Additionally, there was a significant increase in Krt10 mRNA expression. In summary, this experimental model is well-suited for studying the abnormal differentiation of corneal epithelial stem cells induced by inflammation.

Figure 1: Establishment of the inflammation model. To study the effects of severe inflammation on corneal epithelial stem cells, a severe inflammation model was established. (A) After inducing the model, the cornea was observed using slit-lamp photography. Three days after modeling, the rat corneal limbus was severely edematous, with obvious neovascularization and localized hyperplasia, which are typical signs of corneal limbal stem cell deficiency. By the seventh day, corneal edema gradually worsened, and neovascularization continued to grow. With the progression of inflammation, (B) corneal neovascularization and (C) inflammation index showed a continuous increase. *p < 0.05, ****P < 0.0001. Please click here to view a larger version of this figure.
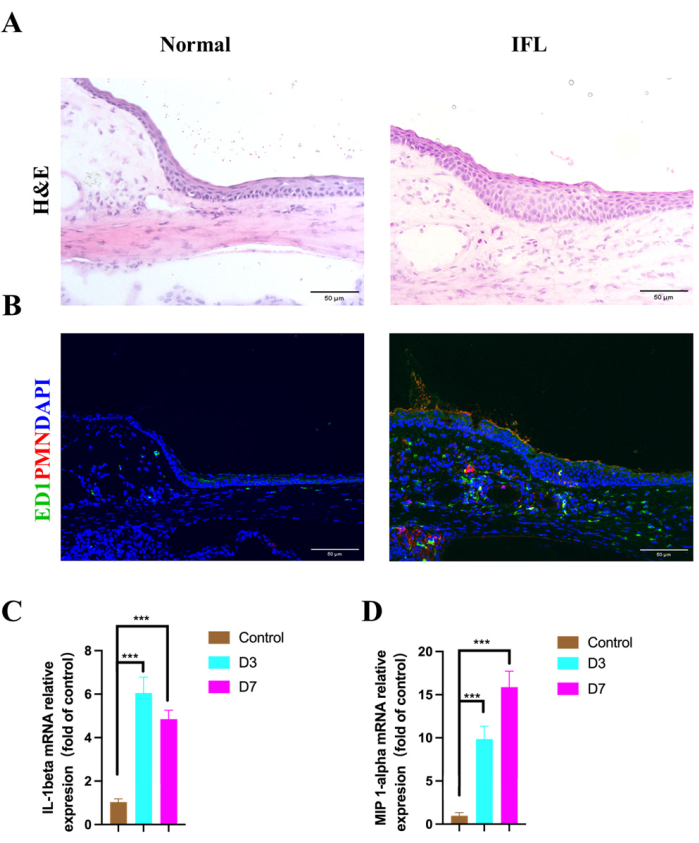
Figure 2: The inflammatory response after modeling. The inflammatory response induced by the model in the cornea was evaluated. (A) Hematoxylin and eosin staining of the eyeball showed that during the inflammatory process, both the limbal and central corneal epithelium thickened, and the corneal epithelial arrangement lost its normal morphology, exhibiting signs of atypical hyperplasia. (B) The macrophage marker ED1 (green) and the neutrophil marker PMN (red) were significantly increased. RT-qPCR showed a significant elevation of corneal epithelial inflammatory factors (C) IL-1β and (D) MIP-1α. Blue represents nuclear staining with DAPI, scale bar = 50 µm; ***p < 0.001. Please click here to view a larger version of this figure.

Figure 3: Phenotypic changes of cells under inflammatory conditions. Immunostaining for (A, red) KRT12 in the limbal epithelium and (B) PAX6. (C) KRT12 mRNA expression levels show a significant reduction as inflammation progresses. (D) PAX6 mRNA expression reveals an abnormal distribution and a marked decrease in the limbal epithelium under inflammatory conditions. Conversely, mRNA expression of (E) Krt10 and the abnormally differentiated corneal epithelial marker (F) Sprr1b significantly increase during inflammation. Blue represents nuclear staining with DAPI, scale bar = 50 µm; **p < 0.01, ***p < 0.001, ****p < 0.0001. Please click here to view a larger version of this figure.
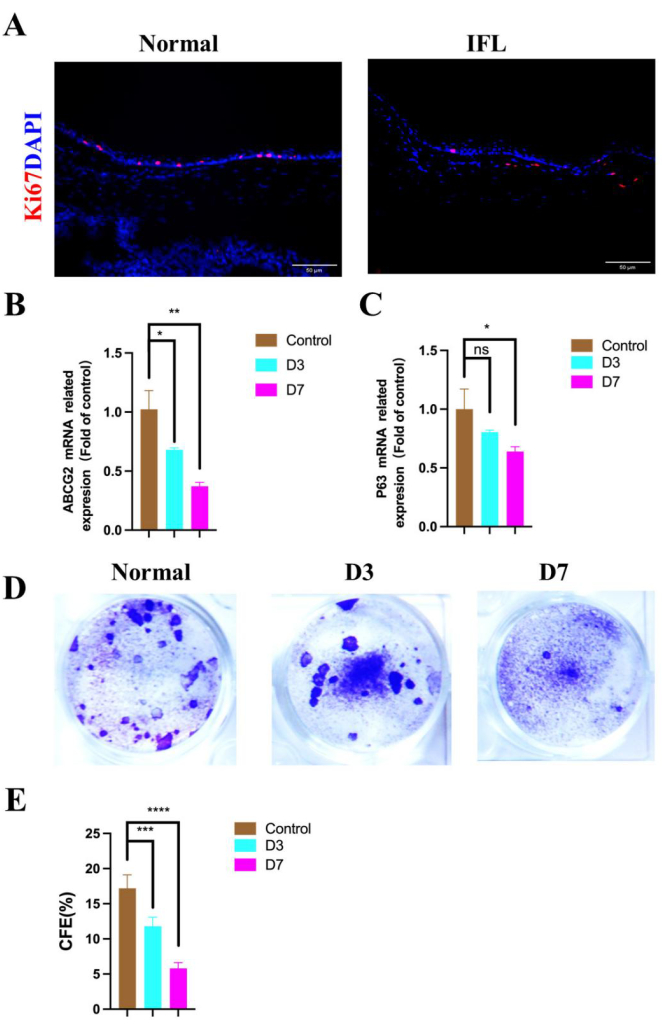
Figure 4: Changes of limbal stem cells under an inflammatory state. Immunofluorescence images of (A, red) Ki67 illustrate changes in proliferating cells following the induction of inflammation. The mRNA expression of the stem cell marker (B) ABCG2 is significantly reduced. The progenitor cell marker (C) p63 is also expressed. (D) Crystal violet staining images and (E) CFE show a decrease in the number of limbal stem cells under inflammatory conditions. Blue indicates nuclei stained with DAPI; scale bar is 50 µm; *p < 0.05, **p < 0.01, ***P < 0.001, ****P < 0.0001. Please click here to view a larger version of this figure.
| Primer name | Primer sequence | |||
| TNF-a | Forward | 5'-TCAGTTCCATGGCCCAGAC-3' | ||
| Reverse | 5'-GTTGTCTTTGAGATCCATGCCATT-3' | |||
| IL-1β | Forward | 5'-CCTCGTCCTAAGTCACTCGC-3' | ||
| Reverse | 5'-GGCTGGTTCCACTAGGCTTT-3' | |||
| Pax6 | Forward | 5'-GTGTTCAGTGCAGAGCCTTC-3' | ||
| Reverse | 5'-TTCACCGTTGCTGTTCACTG-3' | |||
| Krt12 | Forward | 5'-AGCTAACGCGGAACTGGAAA-3' | ||
| Reverse | 5'-CTCTCCGCTCTTGGTGAGGT-3' | |||
| Krt10 | Forward | 5'-TCCGGGATCTGGAAGAGTCAA-3' | ||
| Reverse | 5'-TTGGGTAAGCTTTGCTAAGTGGAA-3' | |||
| Sprr1b | Forward | 5'-CCATCCCAAGGCACCTGAG-3' | ||
| Reverse | 5'-TGCTGGTATGGTGATGGAGT-3' | |||
| Actin | Forward | 5'-CACCCGCGAGTACAACCTTC-3' | ||
| Reverse | 5'-CCCATACCCACCATCACACC-3' | |||
Table 1: Sequence of the primers used for quantitative RT-PCR.
Discussione
Inflammation plays a key role in the development of limbal stem cell deficiency18, but its specific mechanisms and effects require further exploration. To address this issue, we induced LSCD by scraping the central corneal epithelium and suturing the central corneal stroma to cause severe corneal inflammation. We observed the evolution of corneal inflammation and the status of the limbal stem cells on days 1, 3, and 7 post-modeling. On the third day post-modeling, significant edema of the rat limbus, marked neovascularization, and increased local tissue proliferation were observed, which are typical characteristics of limbal stem cell deficiency. By day 7, corneal edema had further intensified, and the number of new blood vessels continued to increase. Through RT-qPCR and immunofluorescence staining techniques, we observed that on day 7 post-modeling, the expression levels of macrophage marker ED1, neutrophil marker PMN, and central corneal epithelial inflammatory factors MIP-1α and IL-1β were significantly elevated. During the progression of inflammation, the immunostaining imaging and related mRNA expression levels of corneal epithelial cell-specific transcription factor Pax6 and corneal-specific keratin Krt12 were significantly reduced. The mRNA expression of skin-related squamous epithelial metaplasia marker Krt10 and corneal epithelial marker Sprr1b increased during the progression of inflammation, indicating abnormal differentiation. The mRNA expression of stem cell marker ABCG2 significantly decreased. Precursor cell marker p63 was also expressed. Crystal violet staining images and CFE showed that under inflammatory conditions, the number of limbal stem cells decreased. A comprehensive analysis indicated that seven days post-modeling, inflammatory factors in the corneal epithelium significantly increased, differentiation of the corneal epithelium was abnormal, the number of corneal epithelial stem cells significantly decreased, and cellular proliferation capacity and stem cell characteristics declined.
This model induces corneal inflammation without directly damaging the limbal epithelial stem cells, thereby promoting research into the effects and mechanisms of corneal inflammation on limbal epithelial stem cells. In epithelial tissues, including the corneal epithelium, stem cell reprogramming manifests as epithelial transdifferentiation. This process is closely related to inflammation and is characterized by abnormal differentiation induced by various cytokines, growth factors, and other soluble factors in the microenvironment19,20,21,22. In an inflammatory state, especially in long-term persistent inflammatory states, such as dry eye syndrome and other ocular surface diseases23,24,26, the corneal epithelium can be reprogrammed into skin epithelium, a condition known as squamous metaplasia. This transition involves the weakening or loss of corneal epithelial-specific markers Krt12, which are replaced by skin-specific markers Krt10 and Sprr1b27,28. The model described in this protocol exhibits similar phenomena. Initially, limbal stem cells are activated, and then, under sustained inflammation, corneal epithelial squamous metaplasia occurs. We observed typical squamous epithelial cells in the corneal epithelium, characterized by reduced expression of Krt12 and Pax6 and increased expression of Krt10 and Sprr1b (markers of squamous metaplasia). Early inflammation enhanced the proliferation of the corneal epithelium, while later stages saw a decline in proliferation. Consistent with what we found in our studies on LSCD caused by Stevens-Johnson syndrome or congenital aniridia, the limbal stem cell markers ABCG2 and the gold standard for assessing stem cells, the colony-forming efficiency, were significantly reduced. However, the clonal cells retained the ability to differentiate into mature corneal epithelial cells. In previous studies, squamous metaplasia of the corneal epithelium was observed in air-liquid interface cultures, but the corneal epithelial stem cells still exhibited the potential to differentiate into a normal corneal epithelial phenotype and increased proliferative capacity. This is very similar to our current research29.
Therefore, the research model developed in this protocol confirms that severe inflammation can lead to damage to limbal stem cells without directly damaging the limbal stem cell niche. This finding is very beneficial for observing the effects of severe inflammation on the biological mechanisms of limbal stem cells and provides an ideal experimental platform for studying the mechanisms of LSCD caused by corneal inflammation.
However, this model has its limitations. Anesthetized rats undergo mechanical scraping of the corneal epithelium followed by corneal suturing, both of which are invasive procedures with high risks of intraoperative and postoperative infection30. Moreover, the rat cornea's thinness makes it prone to perforation during suturing, requiring high operator proficiency.
Therefore, we must pay attention to the following points during the procedure: (i) cleanliness and disinfection: All instruments must be sterilized before surgery. Antibiotic eye drops should be administered the day before and on the day of the procedure to reduce the risk of infection. Since the corneal suture is a foreign body that can cause inflammation and infection, strict aseptic technique must be followed, and antibiotic eye drops should be continued for three days postoperatively. (ii) Handling the rat cornea: The rat cornea is relatively thin and prone to perforation during suturing. Use toothed forceps to hold the conjunctiva on the opposite side of the suture site to stabilize the eyeball and facilitate the operation. Place the suture approximately 1.5-2 mm from the corneal limbus, where the cornea is thicker and less prone to perforation while avoiding damage to the limbal stem cells. (iii) Corneal epithelium removal: The corneal trephine and corneal epithelial scraper can quickly remove the corneal epithelium and is easy to operate, but it is quite sharp and may damage the stroma or cause perforation. Control the intensity of force during removal to prevent perforation. To ensure uniformity in the removal area, use a circular markerto mark the area before removing the corneal epithelium.
Divulgazioni
The authors have nothing to disclose.
Riconoscimenti
This study was supported in part by the Guizhou Provincial Science and Technology Projects (QKHJC-ZK[2024]ZD043), Fujian Provincial Science Fund for Distinguished Young Scholars (2023J06053 [to S.O]); the Natural Science Foundation of China (No.82101084 [to S.O.] and China Scholarship Council (CSC, 202306310049 [to Y.W.]).
Materiali
| Name | Company | Catalog Number | Comments |
| 10-0 suture line | Alcon, Inc., USA | 8065698001 | |
| 2 mm corneal trephine | Suzhou XVI Vision Company, China | http://www.66vision.com/ | |
| 20 μ L, 100 μ L, 1 mL pipette gun heads, cell culture plate culture plates, centrifuge tubes | Guangzhou Jiete Biological Co., Ltd., China | https://www.jetbiofil.com/index.html | |
| -20/-80 °C fridge | Qingdao Haier Company, China | https://www.haier.com/cn/ | |
| A 10-mL syringe | Zhejiang Condelai Medical Device Co., Ltd., China | https://en.kdlchina.com/zhejiang/25.html | |
| Adhere to the slide | ShiTai Company | 188105 | |
| Amylene alcohol | Shanghai. Macklin Biochemical, China | http://www.macklin.cn/products/ | |
| Anti-Cytokertin | Abcam, United Kingdom | ab76318 | |
| Anti-Keratin 12 antibody EPR17882 | Abcam, United Kingdom | ab185627 | |
| Anti-PAX6 antibody | Abcam, United Kingdom | ab195045 | |
| Anti-stripping slides, cover slips | Jiangsu Shitai Experimental Equipment Co., Ltd., China | https://cn.citotest.com/ | |
| Autoclave | Hirayama, Japan | https://hirayama.com.cn/about/ | |
| Avertin | Shanghai Aladdin Biochemical Technology, China | https://www.aladdin-e.com/zh_cn/A111225.html | |
| Chloroform | Shanghai Biological Engineering Co., Ltd., China | https://www.siobp.com/web | |
| CO2 Oven incubator, ultrasonic crusher, centrifuge | Thermo Fisher Technologies Inc., USA | https://www.thermofisher.cn/cn/zh/home.html | |
| Confocal microscope | Shanghai Qinxiang Scientific Instrument Co., Ltd., China | https://www.clinx.cn/about | |
| Corneal epithelial forceps | Suzhou XVI Vision Company, China | 53418D | |
| DAPI | Vector. Inc., USA | H-1000 | |
| DEPC water | Shanghai Biological Engineering Co., Ltd., China | https://www.siobp.com/web | |
| Dicuro eye cream (ofloxacin eye cream) | Santian Pharmaceutical Corporation, Japan | https://www.santen.com/asia | |
| D-KSFM, culture medium, double antibody, and PBS | Thermo Fisher Scientific, Inc., USA | https://www.thermofisher.cn/cn/zh/home.html | |
| ED1 | AbD Serote c, United Kingdom | MCA341GA | |
| Electronic balance | Shanghai Ohaus Biotechnology Company, China | http://shbio.com/company | |
| Enclosure of the membrane | Parafilm, Inc., USA | https://www.sigmaaldrich.cn | |
| Fluorescein sodium test strip was used in ophthalmology | Tianjin Jingming New Technology Development Co., Ltd., China | https://www.eworldtrade.com/c/jingmingtechnological/ | |
| Fluorescent inverted phase-contrast micrographic system | TE-2000U, Nikon, Japan | https://www.microscopyu.com/museum/eclipse-te2000-inverted-microscope | |
| Frozen table-top centrifuge | Eppendorf, Germany | https://www.eppendorf.sh.cn | |
| Glass sheet frame | Xiamen Tianjing Biotechnology Co., Ltd., China | http://www.tagene.net/ | |
| H-1200 with DAPI sealagent | Xiamen Juin Biotechnology Co., Ltd., China | https://www.bosonbio.com.cn/ | |
| HE staining kit | Auragene, China | http://jushengwu.com/a048/ | |
| Hematoxylin-eosin dye solution kit | AURAGEN, United States | P032IH | |
| High and low precision electronic balance | Sdolis, Germany | https://www.solisinverters.com/global | |
| Isopropanol | Shanghai Sinopharm Chemical Reagents Co., Ltd., China | https://en.reagent.com.cn/ | |
| Ki-67 antibody | Abcam, United Kingdom | ab16667 | |
| liquid nitrogen | Xiamen Yidong Gas Co., Ltd., China | https://www.china.tdk.com.cn/tdk_chn_en/tdk_xiamen/ | |
| Microhand holder | Suzhou XVI Vision Company, China | http://www.66vision.com/ | |
| Multiformaldehyde powder | Sigm, America | https://www.sigmaaldrich.cn | |
| Normal temperature centrifuge 46 | Eppendorf, Germany | https://www.thermofisher.cn | |
| OCT | Shanghai Maokang Biotechnology Co., Ltd., China | 4583 | |
| Ordinary biological micrographic system | Eclipse 50i, Nikon, Japan | https://www.microscope.healthcare.nikon.com/zh_CN/products/upright-microscopes/eclipse-ni-series | |
| PBS | Shanghai Anjin Biotechnology Co., Ltd, China | SH30256.01 | |
| PCR, and the reactor apparatus | Biometra Thermocycler, Germany | https://www.analytik-jena.com/products/life-science/pcr-qpcr-thermal-cycler/thermal-cycler-pcr/biometra-tone-series/ | |
| Pipettes of various specifications | Eppendorf Company, Germany | https://www.eppendorf.com/cn-zh/ | |
| Primers for Rt-PCR | Shanghai Bio-Tech Co., Ltd. | ||
| Promeaine hydrochloride 0.5% eye drops | Alcon, Inc., USA | https://www.alcon.com/about-us | |
| Rat PMN antibody | Fitzgerald, United States | 20R-PR020 | |
| Real time fluorescence quantitative PCR | Applied Biosystems, United States | https://www.thermofisher.cn | |
| Reverse transcription kit | TAKARA, Japan | https://www.takarabiomed.com.cn/ | |
| Slit lamp | TOPCON, Japan | https://topconchina.cn/ | |
| Smooth forceps | Suzhou XVI Vision Company, China | http://www.66vision.com/ | |
| Specimen-box | Lambolid (Fuzhou) Biotechnology Co., Ltd., China | http://chuangdianbio.com/ | |
| Superclean bench | New Plus Pi Art High Technology Company Limited, Singapore | https://www.hi-p.com/ | |
| Toothed forceps | Suzhou XVI Vision Company, China | http://www.66vision.com/ | |
| Toppicamide eye drops (Medol) | Alcon, Inc., USA | https://www.alcon.com/about-us | |
| Trace nucleic acid protein concentration tester | Thermo Fisher Technologies Inc., USA | https://www.thermofisher.cn/cn/zh/home.html | |
| Trizol | Invitrogen The United States | https://www.fishersci.com/us/en/brands/IIAM0WMR/invitrogen.html | |
| Zeiss, with a surgical microscope | Carl ZEIS, Germany | https://www.zeiss.com/corporate/en/about-zeiss.html |
Riferimenti
- Ang, L. P., Tan, D. T. Ocular surface stem cells and disease: Current concepts and clinical applications. Ann Acad Med Singap. 33 (5), 576-580 (2004).
- Mao, Y., et al. Downregulation of p38 MAPK signaling pathway ameliorates tissue-engineered corneal epithelium. Tissue Eng Part A. 28 (23-24), 977-989 (2022).
- Sprogyte, L., Park, M., Di Girolamo, N. Pathogenesis of alkali injury-induced limbal stem cell deficiency: A literature survey of animal models. Cells. 12 (9), 1294 (2023).
- He, X., et al. Lacrimal gland microenvironment changes after obstruction of lacrimal gland ducts. Invest Ophthalmol Vis Sci. 63 (3), 15 (2022).
- Le, Q., Chauhan, T., Deng, S. X. Diagnostic criteria for limbal stem cell deficiency before surgical intervention- A systematic literature review and analysis. Surv Ophthalmol. 65 (1), 32-40 (2020).
- Li, W., Hayashida, Y., Chen, Y. T., Tseng, S. C. Niche regulation of corneal epithelial stem cells at the limbus. Cell Res. 17 (1), 26-36 (2007).
- Ou, S. K., et al. The role of ectodysplasin a on the ocular surface homeostasis. Int J Mol Sci. 23 (24), 12 (2022).
- Lin, S., et al. Limbal stem cell dysfunction induced by severe dry eye via activation of the p38 MAPK signaling pathway. Am J Pathol. 193 (11), 1863-1878 (2023).
- Jiang, G. J., Li, B. B., Fan, T. J. Timolol induces necroptosis, apoptosis and senescence concentration-dependently in rabbit limbal stem cells in vitro. Life Sci. 277, 119453 (2021).
- Castro-Munozledo, F. Review: Corneal epithelial stem cells, their niche and wound healing. Mol Vis. 19, 1600-1613 (2013).
- Atalay, E., et al. Animal models for limbal stem cell deficiency: A critical narrative literature review. Ophthalmol Ther. 13 (3), 671-696 (2024).
- Delic, N. C., Cai, J. R., Watson, S. L., Downie, L. E., Di Girolamo, N. Evaluating the clinical translational relevance of animal models for limbal stem cell deficiency: A systematic review. Ocul Surf. 23, 169-183 (2022).
- Lin, Z., et al. A mouse model of limbal stem cell deficiency induced by topical medication with the preservative benzalkonium chloride. Invest Ophthalmol Vis Sci. 54 (9), 6314-6325 (2013).
- Ye, J., et al. Biomimetic nanocomplex based corneal neovascularization theranostics. J Control Release. 374, 50-60 (2024).
- Zhu, H. M., et al. A synergistic therapy with antioxidant and anti-VEGF: Toward its safe and effective elimination for corneal neovascularization. Adv Healthc Mater. 13 (5), 10 (2024).
- Yu, J. W., et al. In vivo assembly drug delivery strategy based on ultra-small nanoparticles: Toward high drug permeation and accumulation for CNV treatment. Chem Eng J. 450, 137968 (2022).
- Wu, H., et al. A SU6668 pure nanoparticle-based eyedrops: Toward its high drug accumulation and long-time treatment for corneal neovascularization. J Nanobiotechnol. 22 (1), 290 (2024).
- Long, Q., et al. A novel tissue-engineered corneal epithelium based on ultra-thin amniotic membrane and mesenchymal stem cells. Sci Rep. 14 (1), 17407 (2024).
- Giroux, V., Rustgi, A. K. Metaplasia: Tissue injury adaptation and a precursor to the dysplasia-cancer sequence. Nat Rev Cancer. 17 (10), 594-604 (2017).
- Herfs, M., Hubert, P., Delvenne, P. Epithelial metaplasia: Adult stem cell reprogramming and (pre)neoplastic transformation mediated by inflammation. Trends Mol Med. 15 (6), 245-253 (2009).
- Slack, J. M. Metaplasia and transdifferentiation: From pure biology to the clinic. Nat Rev Mol Cell Biol. 8 (5), 369-378 (2007).
- Slack, J. M., Tosh, D. Transdifferentiation and metaplasia--switching cell types. Curr Opin Genet Dev. 11 (5), 581-586 (2001).
- Wang, S., et al. Impact of chronic smoking on meibomian gland dysfunction. PLoS One. 11 (12), e0168763 (2016).
- Wu, Y., et al. Evolution of therapeutic strategy based on oxidant-antioxidant balance for fuchs endothelial corneal dystrophy. Ocul Surf. 34, 247-261 (2024).
- Li, Y., et al. Meibomian gland alteration in patients with systemic lupus erythematosus. Lupus. 31 (4), 407-414 (2022).
- Zhang, Y., et al. Histopathology-based diagnosis of Mooren's ulcer concealed beneath the pterygium on eye. J Histotechnol. 45 (4), 195-201 (2022).
- Tseng, S. C., Hatchell, D., Tierney, N., Huang, A. J., Sun, T. T. Expression of specific keratin markers by rabbit corneal, conjunctival, and esophageal epithelia during vitamin A deficiency. J Cell Biol. 99 (6), 2279-2286 (1984).
- Espana, E. M., et al. Characterization of corneal pannus removed from patients with total limbal stem cell deficiency. Invest Ophthalmol Vis Sci. 45 (9), 2961-2966 (2004).
- Li, W., et al. Air exposure induced squamous metaplasia of human limbal epithelium. Invest Ophthalmol Vis Sci. 49 (1), 154-162 (2008).
- Marino, G. K., et al. Epithelial basement membrane injury and regeneration modulates corneal fibrosis after pseudomonas corneal ulcers in rabbits. Exp Eye Res. 161, 101-105 (2017).
Ristampe e Autorizzazioni
Richiedi autorizzazione per utilizzare il testo o le figure di questo articolo JoVE
Richiedi AutorizzazioneEsplora altri articoli
This article has been published
Video Coming Soon