Method Article
A Mouse Model of Mechanotransduction-driven, Human-like Hypertrophic Scarring
* Questi autori hanno contribuito in egual misura
In questo articolo
Riepilogo
This protocol will explain how to establish a hypertrophic scarring murine model that increases mechanotransduction signaling to simulate human-like scarring. This method involves increasing mechanical tension across a healing incision in a mouse and using a specialized device to create reproducible, excessive scar tissue for detailed histological and bioinformatic analyses.
Abstract
Hypertrophic scarring (HTS) is an abnormal process of wound healing that results in excessive scar tissue formation. Over the past decade, we have demonstrated that mechanotransduction—the conversion of mechanical stimuli into cellular responses—drives excessive fibrotic scar healing. A mouse model to assess human-like hypertrophic scarring would be an essential tool for examining various therapeutics and their ability to reduce scarring and improve healing. Specifically, our laboratory has developed a murine wound model that increases mechanical strain to promote human-like HTS. This protocol utilizes biomechanical loading devices, made from modified 13 mm palatal expanders, whose arms are placed on either side of the incision and distracted incrementally apart in order to apply continuous tension across the wound bed during healing. Over nearly two decades of use, this model has been significantly advanced to improve efficacy and reproducibility. Using the murine HTS model, significant dermal fibrotic scars can be induced to be histologically comparable to human hypertrophic scars. This murine model provides an environment to develop biologics involved in the treatment of HTS and mechanotransduction-related conditions such as foreign body response.
Introduzione
Wound healing, the process by which the body attempts to repair damaged tissue and rebuild the skin barrier, can result in atypical healing if its processes of hemostasis, inflammation, proliferation, and remodeling are irregular1. Hypertrophic scarring (HTS) is an example of irregular wound healing, characterized by excessive deposition of extracellular matrix and connective tissue at the site of injury resulting in the formation of an enlarged scar tissue area1,2,3. Areas on the body that undergo repeated mechanical stretch stimulations, such as around joints or on the face, are more prone to developing HTS and fibrosis4,5,6,7,8,9,10. We and others have shown that mechanical stretch across a wound bed promotes HTS formation through the activation of mechanotransduction pathways—the conversion of mechanical stimuli into cellular responses9,11.
HTS not only involves complex biological processes but also carries significant social, medical, and economic challenges for the people affected. Affected individuals can struggle with self-esteem and depression, especially when the scars are in visible areas like the face and hands1,9,10,12. Scientific review articles indicate that the prevalence of HTS varies between 32% and 72% in the United States10,13. The severity of these aesthetic concerns, especially in cases of serious burn injuries in the facial region, is underscored by the increasing number of full facial transplantation cases to improve appearance10. These scars can also cause functional impairments by restricting movement6,14, and surgical intervention is often required to excise scars and restore mobility10. The cost of HTS treatment can be substantial, including expenses for surgery, treatments, physical therapy, or even long-term care1,10. In the United States alone, the annual cost of treating HTS exceeds $4 billion10.
Considering the pervasiveness of HTS and the extreme measures taken to address its complications, conventional therapies (e.g., surgical excision, corticosteroid injections, and laser therapy) remain highly variable1,2,15,16,17. While these treatments can offer relief in some cases, they can be insufficient due to the complex nature of scar pathology. Factors such as genetic differences among individuals and an incomplete understanding of the mechanisms driving HTS cause therapeutic strategies to remain clinically unsatisfactory18,19,20. The future of HTS therapy seems to lie in new innovative approaches that target cell mechanistic drivers of HTS, such as mechanotransduction11,21, which we have extensively demonstrated to drive excessive fibrotic scar healing5,6,7,8,11,21,22,23,24,25. Specifically, we had previously developed a murine model that increases wound mechanical strain to promote human-like HTS9. However, after nearly two decades of use, the model has been significantly advanced to improve efficacy and reproducibility. This protocol will allow researchers to best utilize an updated and optimized HTS mouse model to explore the cell populations and drivers behind excessive scarring. The overall goal of this method is to provide researchers with a protocol designed to produce human-like hypertrophic scarring in mice.
Protocollo
Approval from the University of Arizona Institutional Animal Care and Use Committee (IACUC) was obtained for all experiments (control number: 2021-0828). This protocol uses 15-week-old C57BL/6J male mice although it could be applied to other ages and strains9,26.
1. Creating the HTS biomechanical loading device
NOTE: Modifying the palatal expanders into the HTS device can occur at any point before the experiment.
- Take the unmodified palatal expander and present to makerspace core or fabrication core with specifications according to Figure 1A,B.
NOTE: If no makerspace or fabrication core is available, continue to the following steps. - Use a Mini Universal Bender (MUB) wire bending tool to alter the palatal expander to conform to the specifications of Figure 1A,B.
- Place the device flat on a surface as shown in Figure 1A1.
- Bend the arms 90° up for the upper arms and 90° down for the lower arms when the device lays flat with the MUB as seen in Figure 1A2. See Figure 1A3 for the appearance of the device when resting flat on a surface, where the arms now are perfectly parallel with the body of the device.
- Bend each arm back into the page 90° along the axis of the dotted line as seen in Figure 1A4. See Figure 1A5 for the device after bending.
2. Hair removal and initial incision on postoperative day 0 (POD 0)
NOTE: Clean and autoclave several sets of surgical instruments before surgery (e.g., dissection scissors, scalpel, Adson forceps, Needle driver). Prepare sterilized 5-0 sutures for use and have a surgical marker on hand.
- Clean the operating space with 70% ethanol spray.
- Place a heating pad set at 35 °C on the presurgical prep table. Tape down an absorbent pad over the heating pad. Place another absorbent pad over the previous pad (to collect shaved hair). Tape the anesthesia cone (or cones) on the absorbent pad.
NOTE: [Optional] If a benchtop fume extractor is available, place the cone near the workspace and turn on the suction. - Place the mouse in the anesthesia induction chamber with 1-3% isoflurane and 2 L/min oxygen flow until the mice breathe calmly (2-3 min).
- Insert each mouse's nose into the nose cone opening, allowing for inhalation of anesthesia composed of 1-3% isoflurane with 2 L/min of oxygen. Confirm adequate anesthesia by the lack of reaction to a toe pinch. Apply ophthalmic lubricating ointment to the eyes of the mouse.
- Use an electric razor to shave the hair on the dorsum in the area as seen in Figure 1C.
- Apply the depilatory paste with a gloved finger or cotton swab onto the skin, covering the shaved area. After 45 s, wipe away the paste with gauze and then an alcohol swab. Wipe the dorsum with another alcohol swab to remove the depilatory paste, leaving behind a hairless patch (Figure 1C).
NOTE: It is critical to use a moderate amount of paste, remove the paste promptly, and wipe the mouse after removal of the paste to prevent dermal burns by the depilatory paste. Murine skin is thin, making it susceptible to rapid chemical burns that can have detrimental effects both on mouse health and on experimental quality. - Ear tag the mouse for identification.
- Upon removing the hair, place the mouse back in the cage to prevent excessive isoflurane exposure. Monitor the mouse.
- Remove the top absorbent pad covered with hair to have a clean presurgical pep work surface.
- Set up a separate surgical work area. Place a heating pad set at 35 °C on the surgical table. Tape down an absorbent pad over the heating pad. Tape the anesthesia cone (or cones) on the absorbent pad.
- Place a mouse in the anesthesia induction chamber with 2-4% isoflurane and 2 L/min oxygen flow until the mouse breathes calmly (2-3 min).
- Take the mouse out of the induction chamber and place it on the operating surface. Insert each mouse's nose into the nose cone opening, allowing for inhalation of anesthesia composed of 1-3% isoflurane with 2 L/min of oxygen. Confirm adequate anesthesia by the lack of reaction to a toe pinch. Apply ophthalmic lubricating ointment to the eyes of the mouse.
- Inject 0.05 mg/kg sustained-release buprenorphine into the shoulder subcutaneously using a 21 G needle for postsurgical pain treatment.
- Disinfect the mouse's dorsum with three alternating rounds of iodine/chlorhexidine based scrub and alcohol swab. With the surgical marking pen and a ruler, mark a 2 cm line on the dorsal midline where the full-thickness incision will be made, as shown in Figure 1D.
- Use a scalpel or dissection scissors (surgical preference) to make the full-thickness dorsal midline incision through the marked area.
NOTE: Be careful not to cut the underlying tissue (e.g., muscle), as seen in Figure 1D. - Using 5-0 sutures in a simple interrupted pattern, close the incision by bisecting the wound as shown in Figure 1D. Use at least 5 evenly spaced sutures.
- Cut a Telfa gauze into a 3 cm x 1 cm piece. Place it in the center of a foam adhesive dressing and place the adhesive dressing on the mouse dorsum such that the gauze covers the incision as shown in Figure 1D. Place a halved dressing on the abdomen and wrap circumferentially until it meets the dorsal dressing.
- After the dressing is complete, place the mouse in a separate sterile cage and monitor it until it has fully recovered from the anesthetic.
- Repeat the procedure with all mice, regardless of the experimental group. Let the incision heal over the next 4 days before the next step.
3. Placement of HTS biomechanical loading device (POD 4)
NOTE: Clean and autoclave the HTS devices and several sets of surgical instruments before surgery (e.g., dissection scissors, scalpel, Adson forceps, Needle driver, skin stapler, skin staples). Prepare sterilized 5-0 sutures for use. [Optional] If a benchtop fume extractor is available, place the cone near workspace and turn on the suction.
- Prepare the area of surgery as described in steps 2.1 and 2.2.
- Anesthetize the mouse and provide analgesia by following steps 2.11-2.13.
- Use the forceps or needle driver to separate the dressing from the mouse's abdomen by working the tool side to side against the ventral side. Leaving the tool between the wrap and skin to elevate the dressing off the skin, use scissors to cut the dressing off.
NOTE: Be careful not to cut the mouse skin, rip the dressing off, or disturb the healing incision. - Clean the dorsum with an alcohol swab. Examine the incision for wound dehiscence or signs of infection.
- Ensure the HTS device is not extended/expanded and is in its most reduced form, as shown in Figure 1B. Lightly coat the arms of the device with medical glue.
- With one hand, render the skin on the mouse dorsum slightly taut in the transverse direction. With the other hand, place the HTS device on the mouse dorsum such that the incision is equidistant from each arm of the HTS device, thereby centering the device over the incision. Ensure the skin between the arms of the HTS device is uniformly taut. Hold the device in place until the glue has dried (~30 s), as seen in Figure 2A.
NOTE: Keeping the skin uniformly taut is important to ensure equal amounts of tension are placed on the incision with the device. Leave the sutures intact during this process because the process of placing the device adds mechanical tension to the skin, which can reopen the healing incision. The sutures remain in place until the first day of stretching to ensure that the wound remains closed. - Secure four sutures around each arm and through the skin as shown in Figure 2B. While suturing, make sure that the needle exits the skin towards the incision.
NOTE: Inserting the needle from the incision side of the device can sometimes tear the incision open due to the force necessary to puncture the skin. - Now place three skin staples around the arms and through the skin, securing the HTS device to the skin as shown in Figure 2B.
- Bandage the mouse by following steps 2.17 and 2.18.
- Repeat the procedure with all mice, regardless of the experimental group.
NOTE: All mice receive the same preparation; however, they are randomly assigned in each experimental group (e.g., control, stretch) to ensure an unbiased distribution of mice in the experimental design. Specifically, both control and stretch mice will have the device attached to their dorsum. The control mice's devices will remain untouched, while the stretch mice will undergo the next steps.
4. Initial stretch of HTS biomechanical loading device (POD 5)
NOTE: Clean and autoclave several sets of surgical instruments (e.g., dissection scissors, scalpel, Adson forceps, Needle driver) prior to surgery. [Optional] If a benchtop fume extractor is available, place the cone near the workspace and turn on the suction.
- Randomly assign each mouse via ear tag to the desired group. For control mice, simply remove the sutures on the incision and change the dressings of the wounds (steps 1-6, and then 8-9). If desired, remove the device at the time of stretching and take a photo with a measuring implement for scale to track the scar size.
- Prepare the surgical area, anesthetize the mouse, and remove the dressing by performing steps 3.1-3.4.
NOTE: Be careful not to tear the dressing off or disturb the healing incision. If an arm of the HTS device has detached from the skin, lightly glue back into place and add a skin staple or suture depending on the size of the detachment. - Remove the sutures from the wound with dissection scissors or other method of choice.
- Insert the HTS device key into the device and turn to expand the device until the skin is taut but not at risk of tearing the skin, as seen in Figure 2C.
NOTE: This initial distraction may take around 4-8 full turns of the key since the skin may be loose between the arms of the device. - Bandage the mouse by performing steps 2.17 and 2.18.
5. Subsequent stretch of HTS biomechanical loading device (POD 7, 9, 11, 13, 15, 17)
NOTE: Clean and autoclave several sets of surgical instruments (e.g., dissection scissors, scalpel, Adson forceps, Needle driver) prior to surgery. [Optional] If benchtop fume extractor is available, place the cone near the workspace and turn on the suction.
- For control mice, simply change the dressings of the wounds (steps 1-5, and then steps 13-14). If desired, remove the device at the time of stretching and take a photo of the scar with a measuring implement for scale to track the scar size.
- Prepare the surgical area, anesthetize the mouse, and remove the dressing by following steps 3.1-3.4.
NOTE: If an arm of an HTS device has detached from the skin, glue it in place and add a skin staple or suture depending on the size of the detachment. - Insert the HTS device key into the device and turn to expand the device until the skin is taut but not at risk of tearing the skin.
NOTE: This may take approximately 4 turns or ~2 mm of total distraction. If the device has reached maximum extension and cannot be extended further, perform steps 5.4-5.7 to take off and re-attach a new device. Otherwise, bandage the mouse by performing steps 2.17 and 2.18. - If the device has reached maximum extension, remove the device from the mouse's dorsum by removing the staples with a staple remover or scissors by prying the prongs of the staples open. Then, remove the sutures and carefully remove the device.
- Clean the device with a scalpel, alcohol swabs, and paper towels. Soak the device in 70% ethanol for ~20 min to aid in cleaning. Then, use the key to contract the device to its thinnest form.
- Reattach the HTS device following steps 3.5-3.8.
- Bandage the mouse by following steps 2.17 and 2.18.
6. Harvesting the HTS tissue (POD 19)
NOTE: Harvesting tissue can take place at any point in the process. We have harvested tissue after only 4 days of stretch to examine early time points; however, tissue is most consistently harvested at POD 19 (2 weeks after strain was initiated). Clean and autoclave several sets of surgical instruments (e.g., dissection scissors, scalpel, Adson forceps) prior to surgery. To get photos of the scar over time, the device can be removed before each stretching step to take a photo of the scar before re-applying the device and re-initiating mechanical strain. [Optional] If a benchtop fume extractor is available, place the cone near the workspace and turn on the suction. The benchtop fume extractor may be turned off when the isoflurane gas is no longer being used.
- Prepare the surgical area, anesthetize the mouse, and remove the dressing by following steps 3.1-3.4.
- Sacrifice the mouse via cervical dislocation.
NOTE: Take care not to pull the skin and tear the mouse's dorsum. - Use dissecting scissors or a skin staple remover to remove the skin staples. Cut off the sutures. Gently remove the HTS device, taking care not to tear the skin.
- Using either a scalpel or scissors, cut the skin surrounding the HTS scar. Preserve the skin for histological analysis in the desired fashion.
NOTE: If the skin is to be used for transcriptomic or protein analysis (e.g., qPCR, western blot, single-cell analysis), be sure to excise just the HTS scar tissue to minimize the amount of surrounding healthy tissue. This will ensure that the analysis will only capture the scar tissue. - Scrape the HTS devices clean with a scalpel. Place devices in a beaker with 70% ethanol to soften any remaining adhesive or tissue.
NOTE: After soaking in 70% ethanol, the devices may require further wiping, scraping, and cleaning before autoclaving.
7. Measuring average scar width
NOTE: This was accomplished with image analysis software ImageJ, and the information was recorded on a spreadsheet.
- Open images in ImageJ. Trace the edges of the scar with the polygon tool. Click analyze | measure to measure this area.
NOTE: The scar can be identified by its discoloration and lack of hair follicles. - Measure the length of the scar from end to end using the segment tool. Click analyze | measure to measure this length.
- Measure the length of 1 cm or any other standardized unit of length in the picture, using the segment tool. Click analyze | measure to measure this length.
- Using the spreadsheet, take the Area of the scar (in pixels; px) and divide that by the Length of the scar (px length). That result gives the average scar width in pixels.
- Divide that result by the measured standard unit of length (px length). The result will be the average scar width in the unit of the standard unit of length (e.g., cm) used for the experiment.
Risultati
To clearly demonstrate the effective use of the HTS protocol and identify successful "positive" results, the model was established as shown in Figure 3A. In the representative study, there were two groups: No Stretch Control (n = 6) and Mechanical Stretch HTS group (n = 6) where human-like levels of mechanical strain were induced across the incision to generate an HTS, seen in Figure 3B,C. Within the experimental plan given in Figure 3A-C, gross photography was taken of the scar at PODs 5, 9, 15, and 19. The scars were traced with ImageJ and analyzed for the average width of the scars at the different time points (Figure 3D). The average scar widths were graphed against time and between the groups in Figure 3E. Following the application of mechanical strain at POD 5, where the scars were not significantly different between the Stretch and Control, the Stretch Group exhibited significantly wider scars than the Control, reaching a peak width of 0.99 mm at POD 9 (p = 0.045). This significantly greater scar width compared to the Control continued at POD 15 (p = 0.043) and 19 (p = 0.045). It is recommended that gross photography only be taken on days the HTS device needs to be removed (i.e., the HTS device has reached maximum expansion and needs to be reattached or during explantation) since the device blocks proper photography of the scar. In this protocol, images and scar width analyses across multiple time points give a clearer view of the model's wound progression for the reader.
Analysis of the HTS tissue can be done in a number of ways, such as IHC staining for fibrotic markers, H&E and Trichrome staining to examine the histological scar areas, picrosirius red staining to assess collagen architecture of the scars, and other methods for wound analysis -- all of which have been used by the lab historically6,8,21,24,25,27,28(Figure 4). Figure 4 shows histological features of the HTS tissue, such as raised scar tissue (Figure 4A), loss of adnexal structures and hair follicles (Figure 4B), alignment of collagen fibers in the direction of mechanical loading (Figure 4C), and collagen whorls (Figure 4F). Figure 4 also demonstrates immunohistochemical staining of mast cell density and vasculature of murine HTS and compares it to human HTS tissue (Figure 4D,E,G,H). Furthermore, analysis of the HTS scar can be done with single-cell analysis if the tissue is harvested, prepared, and taken to a bioinformatics core immediately post excision.

Figure 1: Preparation of the HTS device and of the mouse's dorsum. (A) Modification process of a 13 mm palatal expander to create the HTS device, (1) showing the initial expander structure, (2) bending of the arms 90° up for the upper arms and down for the lower arms when the device lays flat, (3) result of initial bending, where the arms now are perfectly parallel with the body of the device, (4) bending of arms to form final device with measurements; the arms should each be bent 90° into the page as seen in the (5) final device. (B) The fully assembled HTS device from various angles, both un-extended and extended. (C) Sequential steps for preparing the mouse's dorsum: (1) initial state with fur, (2) after shaving, and (3) post application of depilatory paste. (D) Procedure for creating the dorsal incision: (1) marking the incision site with a 2 cm guideline, (2) making the incision, (3) suturing the wound, (4) applying the Telfa gauze, and (5) securing with adhesive dressing. (E) Gross photography of the (1) incision, and (2) after suturing the incision closed. Abbreviation: HTS = hypertrophic scarring. Please click here to view a larger version of this figure.

Figure 2: Positioning and stretching the HTS device. (A) Illustration of the correct positioning of the HTS device on the mouse's dorsum, ensuring proper alignment with the incision site. The left image shows the device placement as viewed from a posterior position. The right image shows the device placement viewed laterally. (B) Illustration of the attachment of the HTS device: (1) initial placement, (2) location of the sutures, (3) location of the skin staples. (C) Demonstration of stretching the HTS device with the labeled HTS Device Key: (1) starting configuration, (2) application of tension, (3) the final expanded state designed to apply consistent mechanical strain across the healing incision to promote hypertrophic scar formation, 2 mm of expansion applied. (D) Gross photography of applying tension to the HTS device, with HTS Device Key labeled. (E) Gross photography of the fully bandaged mouse. Abbreviation: HTS = hypertrophic scarring. Please click here to view a larger version of this figure.
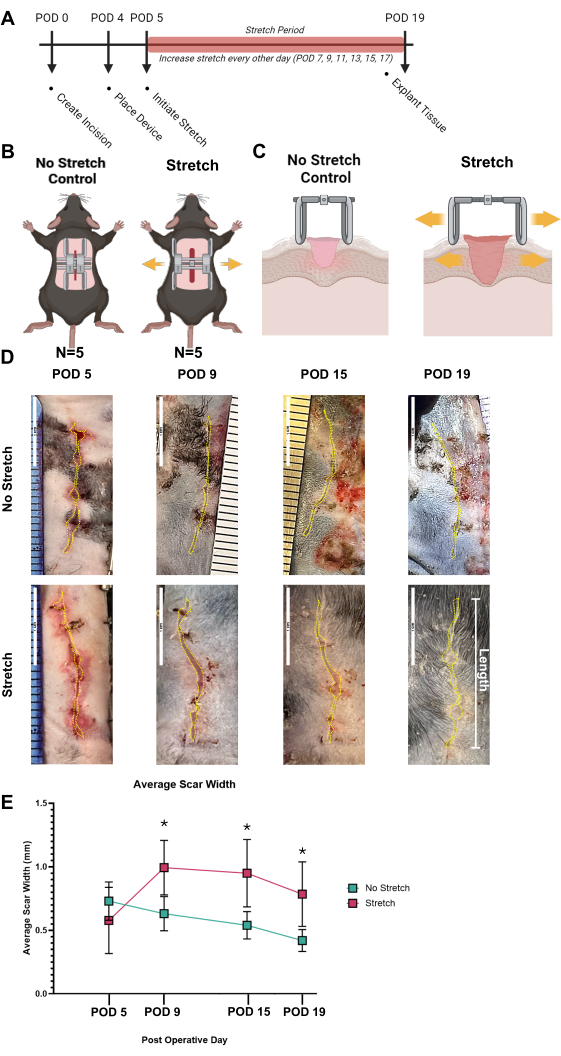
Figure 3: Application of mechanical strain to develop scars. (A) Timeline of the experimental procedure from the creation of the incision (POD 0) to the application of mechanical strain (POD 4) and through the stretching period (POD 5 to POD 19), culminating in tissue explantation (POD 19). (B) Illustration of the two groups: No Stretch Control and Mechanical Stretch HTS groups. (C) Cross-sectional schematic depicting the difference in tissue response between the No Stretch Control and Stretch HTS groups. (D) Representative gross images of scars of No Stretch Control and Stretch HTS groups at post-operative days (POD 5, 9, 15, and 19), with the hypertrophic scars traced in a yellow dotted line. (E) Quantitative analysis of average scar width over time, demonstrating significant differences in scar development between the No Stretch and Stretch groups, with error bars representing standard deviation. Significant differences are marked with asterisks (*). Statistical analysis was performed using two-way analysis of variance (ANOVA). Values of *p < 0.05 were considered statistically significant. Abbreviations: HTS = hypertrophic scarring; POD = postoperative day. Please click here to view a larger version of this figure.
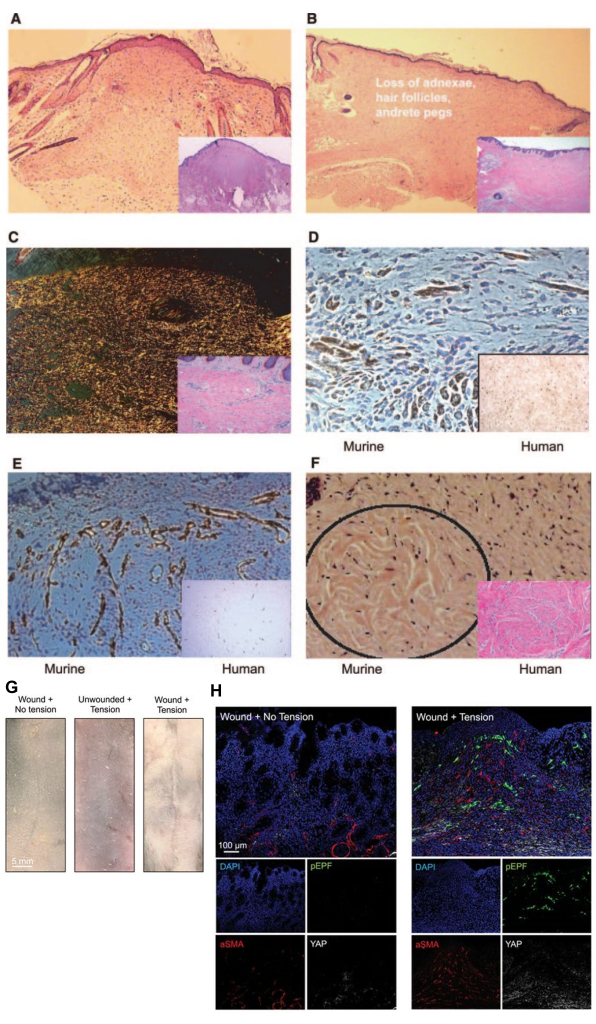
Figure 4: Histology of the scar. This murine scar histology has been harvested after 2 weeks of mechanical stress in our previously published work9,26. (A) The larger image shows H&E histological imaging of the scar cross section, while the bottom right is Trichrome histological imaging of the scar cross section, showing how the scar region is raised. (B) The larger image shows H&E histological imaging of the scar cross section, while the bottom right is Trichrome histological imaging of the scar cross section, showing how the scar region demonstrates a loss of adnexal structures and hair follicles. (C) The larger image shows picrosirius red imaging of the scar cross section, while the bottom right is trichrome histological imaging of the scar cross section, showing aligned collagen fibers in the direction of mechanical loading. (D) The larger image shows imaging of CD117 immunohistochemistry in murine HTS tissue, while the bottom right is imaging of CD117 immunohistochemistry in human tissue, demonstrating mast cell density comparable to human HTS tissue. (E) The larger image shows imaging of CD31 immunohistochemistry of vasculature in murine HTS tissue, while the bottom right is imaging of CD117 immunohistochemistry in human tissue, demonstrating mast cell density comparable to human HTS tissue. (F) Collagen whorls, seen in mature human hypertrophic scars in the image in the bottom right, are also seen in loaded murine scars after 24 weeks in the circled region in murine H&E imaging. (G) Representative gross images of scars of a Control group (Incision + No Stretch), a Stretch Control group (No Incision + Stretch), and a Stretch HTS group. (H) Shows immunohistochemistry comparing the Control group (Incision + No Stretch) and the Stretch HTS group. Using a co-stain for fibrotic markers pEPF, aSMA, and YAP with DAPI, there is an increase in the fibrotic markers in the HTS group. A-F have been adapted from Aarabi, S. et al.9. G,H have been adapted from Mascharak, S. et al. Reprinted with permission from FASEB26. Abbreviations: HTS = hypertrophic scarring; H&E = hematoxylin and eosin; pEPF = Engrailed-positive fibroblast; aSMA = alpha-smooth muscle actin; YAP = Yes-associated protein; DAPI = 4',6-diamidino-2-phenylindole. Please click here to view a larger version of this figure.
Discussione
The HTS mouse model is a cost-effective and highly reproducible method for inducing HTS via mechanotransduction and developing potential therapies. While there is an initial learning curve to effectively use the model, the protocol can, with practice, be performed by any researcher without surgical training. Using this model allows researchers to better understand HTS formation and the role of mechanotransduction in wound healing, which may lead to tangible improvements in patient wound care. The video demonstration accompanying this protocol will ideally reduce the learning curve for researchers and facilitate the smooth implementation of this model into future studies. However, there are a number of critical steps in the protocol that could benefit from further explanation to enhance clarity for users.
Progressing chronologically from the beginning of the protocol, the first key step is closing the surgical incision at Post Operative Day 0 (POD 0). It is imperative that the incision is closed completely with the skin layers in perfect apposition29. This is to ensure that the incision heals properly and will not open once the device is placed and tension is induced across the wound. If the wound opens (dehiscence), the animal should not be used for further experimentation.
When placing the HTS device on POD 4, it is crucial to ensure equal amounts of tension across the mouse's dorsum. With proper placement of the device, the tension exerted will be uniform along the entire incision, mitigating the risk of variable force application that could result in suboptimal experimental outcomes (non-uniform scarring). If the device is attached incorrectly, parts of the incision will not equally receive the average 2 mm of distraction every other day to induce HTS. Expansion of the device should eliminate slack in the skin and make it taut. Simultaneously, care must be taken to avoid overextension of the device, which could cause the wound to dehisce. If the devices are used for several rounds of experiments, the rotating gear in which the key is placed to expand and contract the device can become loose. If this happens, a new device should be made and the old device discarded.
When strain is initiated at POD 5, any therapy intended for scar prevention should begin to be administered. Therapies such as topical ointments are usually administered every other day when strain is initiated by being spread over the incision site. Hydrogels are similarly placed over the incision site. Systemic therapies can be injected into the blood stream either through the tail vein or retro-orbitally.
It is advisable to shave and/or lightly apply depilatory paste to the mouse's dorsum around the scar on POD 19 or prior to photographing the scar. This improves visibility and subsequent analysis of the scar size with ImageJ. Additionally, when using depilatory paste, err on the side of less paste and shorter amounts of time. Overexposure of the mouse's dorsum to the paste can cause chemical burns that may interfere with the wound healing process. Mouse skin is thinner than human skin and is more prone to chemical burns30.
The HTS mouse model is inherently limited since it does not utilize human skin and thus cannot fully recapitulate human HTS. However, it has a significant number of logistical advantages over other animal models; performed properly, this mouse HTS model is reliable, inexpensive, and easily performed. Biologically, the HTS is induced through physical increases in mechanical signaling. Other models of HTS in rabbits induce HTS on the ear or the dorsum using thermal and chemical injury30,31,32. The skin on the ventral side of the rabbit ear does not involve many cells other than skin cells during healing, such as chondrocytes1,30. Another method is a burn HTS model on porcine skin33, but it can be logistically challenging in terms of cost and handling. The porcine model is likely the most similar at recapitulating human HTS; however, it is an expensive method to gather initial results. A final HTS model is xenografting excised human HTS skin onto mice, but this could trigger an immune response and does not explore the formation of new scars34.
The above methods of inducing HTS in animal models do not adequately trigger the body's natural healing response in the same way that human HTS scars form. In contrast, the mechanotransduction-based mouse HTS model employs mechanical strain to initiate hypertrophic scarring, closely mirroring the human HTS1,9,21. This method relies on the physiologic process of mechanotransduction signaling to capture underlying tissue biomechanics and cell population drivers behind fibrosis. This model is optimal for studying biologic targets to inhibit or even induce mechanotransduction pathways, potentially leading to the discovery of novel translational therapies.
Divulgazioni
The authors have no competing interests or other conflicts associated with the contents of this article.
Riconoscimenti
This work was supported by the Center for Dental, Oral, and Craniofacial Tissue and Organ Regeneration Interdisciplinary Translational Project Awards supported by the National Institute of Dental and Craniofacial Research (U24 DE026914) (G.C.G) and the Plastic Surgery Foundation Translational Research Grant (837107) (K.C.).
Materiali
| Name | Company | Catalog Number | Comments |
| 100 mL PYREX Griffin beaker | Milipore Signma | CLS1000100 | |
| Aesculap Exacta mini trimmer | Aesculap | ||
| AutoClip System | Fine Surgical Instruments | 12020-00 | |
| BD brand isopropyl alcohol swabs | Fisher Scientific | 13-680-63 | |
| Buprenorphine SR (0.5 mg/mL) | Buprenex, Indivior Inc. | 12496-0757-1 | |
| C57/BL6 females (6–8 weeks old) | The Jackson Laboratory | 000664 | |
| Covidien sterile gauze | Fisher Scientific | 2187 | |
| Covidien TelfaTM non-adherent pads | Fisher Scientific, Covidien | 1961 | |
| Dental surgical ruler | DoWell Dental Products | S1070 | |
| Depilatory cream (Nair Hair Remover Lotion) | Church&Dwight, CVS | 339823 | |
| Ethanol 70% solution | Fisher Scientific | 64-17-5 | |
| Excel | Microsoft Cooperation | Microsoft.com | software program |
| ImageJ | ImageJ, Wayne Rasband | imagej.net | software program |
| Inhalation anesthesia system | VetEquip | 922130 | |
| Iris scissors 4½ in. stainless | McKesson | 43-2-104 | |
| Isoflurane, USP | Dechra Veterinary Products | 17033-094-25 | |
| Kaka industrial MUB-1 | Kaka Industrial | 173207 | Only necessary if there is no maker space or fabrication shop available |
| Leone Rapid Palatal Expander- 13 mm | Great Lakes Dental Technologies | 125-004 | The key necessary to expand and cotnract the device will come with this product in the box |
| Liquid repellent drape 75 x 90 cm with adhesive hole 6 x 9 cm | Omnia S.p.A. | 12.T4362 | |
| Medequip Depot Silk Black Braided Sutr 6-0 Rx | Medequip Depot D707N, Fisher Scientific | NCO835822 | |
| Needle holder 5 in. with serrated jaws | McKesson | 43-2-842 | |
| Prism 9 | GraphPad Holdings, LLC | graphpad.com | software program |
| Puralube ophthalmic ointment | Dechra, NDC | 17033-211-38 | |
| R studio Desktop | RStudio PBC | rstudio.com | software program |
| Surgical skin marker | McKesson | 19-1451_BX | |
| Tegaderm, 3 M | VWR | 56222-191 | foam adhesive dressing |
| Thermo-peep heating pad | K&H, Amazon | ||
| Tissue forceps 4¾ in. stainless 1 x 2 teeth | Mckesson | 43-2-775 | |
| Vetbond (3 M) | Saint Paul, MN | 1469SB |
Riferimenti
- Mony, M. P., Harmon, K. A., Hess, R., Dorafshar, A. H., Shafikhani, S. H. An updated review of hypertrophic scarring. Cells. 12 (5), 678 (2023).
- Limandjaja, G. C., Niessen, F. B., Scheper, R. J., Gibbs, S. Hypertrophic scars and keloids: Overview of the evidence and practical guide for differentiating between these abnormal scars. Exp Dermatol. 30 (1), 146-161 (2021).
- Cao, X., Sun, L., Luo, Z., Lin, X., Zhao, Y. Aquaculture derived hybrid skin patches for wound healing. Engineered Regeneration. 4 (1), 28-35 (2023).
- Ishise, H., et al. Hypertrophic scar contracture is mediated by the trpc3 mechanical force transducer via nfkb activation. Sci Rep. 5 (1), 11620 (2015).
- Padmanabhan, J., et al. Allometrically scaling tissue forces drive pathological foreign-body responses to implants via rac2-activated myeloid cells. Nat Biomed Eng. 7 (11), 1419-1436 (2023).
- Kussie, H. C., et al. Avenanthramide and β-glucan therapeutics accelerate wound healing via distinct and nonoverlapping mechanisms. Adv Wound Care (New Rochelle). 13 (4), 155-166 (2024).
- Chen, K., et al. Disrupting biological sensors of force promotes tissue regeneration in large organisms. Nat Commun. 12 (1), 5256 (2021).
- Chen, K., et al. Role of boundary conditions in determining cell alignment in response to stretch. Proc Natl Acad Sci USA. 115 (5), 986-991 (2018).
- Aarabi, S., et al. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J. 21 (12), 3250-3261 (2007).
- Aarabi, S., Longaker, M. T., Gurtner, G. C. Hypertrophic scar formation following burns and trauma: New approaches to treatment. PLoS Med. 4 (9), e234 (2007).
- He, J., et al. Mechanical stretch promotes hypertrophic scar formation through mechanically activated cation channel piezo1. Cell Death Dis. 12 (3), 226 (2021).
- Weng, W., et al. Ellipsoidal porous patch with anisotropic cell inducing ability for inhibiting skin scar formation. Engineered Regeneration. 3 (3), 262-269 (2022).
- Lawrence, J. W., Mason, S. T., Schomer, K., Klein, M. B. Epidemiology and impact of scarring after burn injury: A systematic review of the literature. J Burn Care Res. 33 (1), 136-146 (2012).
- Ziolkowski, N., et al. Psychosocial and quality of life impact of scars in the surgical, traumatic and burn populations: A scoping review protocol. BMJ Open. 9 (6), e021289 (2019).
- Gauglitz, G. G., Korting, H. C., Pavicic, T., Ruzicka, T., Jeschke, M. G. Hypertrophic scarring and keloids: Pathomechanisms and current and emerging treatment strategies. Mol Med. 17 (1-2), 113-125 (2011).
- Fu, X., et al. Oxygen atom-concentrating short fibrous sponge regulates cellular respiration for wound healing. Advanced Fiber Materials. 5 (5), (2023).
- Fu, X., et al. Living electrospun short fibrous sponge via engineered nanofat for wound healing. Advanced Fiber Materials. , (2022).
- Fomovsky, G. M., Holmes, J. W. Evolution of scar structure, mechanics, and ventricular function after myocardial infarction in the rat. Am J Physiol Heart Circ Physiol. 298 (1), H221-H228 (2010).
- Macintyre, L., Baird, M. Pressure garments for use in the treatment of hypertrophic scars--a review of the problems associated with their use. Burns. 32 (1), 10-15 (2006).
- Brissett, A. E., Sherris, D. A. Scar contractures, hypertrophic scars, and keloids. Facial Plast Surg. 17 (4), 263-272 (2001).
- Chen, K., et al. Disrupting mechanotransduction decreases fibrosis and contracture in split-thickness skin grafting. Sci Transl Med. 14 (645), eabj9152 (2022).
- Ogawa, R., et al. Clinical applications of basic research that shows reducing skin tension could prevent and treat abnormal scarring: The importance of fascial/subcutaneous tensile reduction sutures and flap surgery for keloid and hypertrophic scar reconstruction. J Nippon Med Sch. 78 (2), 68-76 (2011).
- Chen, K., Henn, D., Gurtner, G. C. Holy grail of tissue regeneration: Size. Bioessays. 44 (9), e2200047 (2022).
- Sivaraj, D., et al. Nitric oxide-releasing gel accelerates healing in a diabetic murine splinted excisional wound model. Front Med (Lausanne). 10, 1060758 (2023).
- Chen, K., et al. Pullulan-collagen hydrogel wound dressing promotes dermal remodelling and wound healing compared to commercially available collagen dressings. Wound Repair Regen. 30 (3), 397-408 (2022).
- Mascharak, S., et al. Preventing engrailed-1 activation in fibroblasts yields wound regeneration without scarring. Science. 372 (6540), eaba2374 (2021).
- Fischer, K. S., et al. Protocol for the splinted, human-like excisional wound model in mice. Bio Protoc. 13 (3), e4606 (2023).
- Wang, P. H., Huang, B. S., Horng, H. C., Yeh, C. C., Chen, Y. J. Wound healing. J Chin Med Assoc. 81 (2), 94-101 (2018).
- Azmat, C. E. Wound closure techniques. Statpearls. , (2024).
- Tunca, M., et al. Cryosurgery to remove perichondrium for the rabbit ear hypertrophic scar model: A simplified method. Acta Dermatovenerol Alp Pannonica Adriat. 28 (2), 57-59 (2019).
- Sun, Q., et al. The effects of timing of postoperative radiotherapy on hypertrophic scar in a rabbit model. Med Sci Monit. 26, e921263 (2020).
- Zu, W., Jiang, B., Liu, H. Establishment of a long-term hypertrophic scar model by injection of anhydrous alcohol: A rabbit model. Int J Exp Pathol. 102 (2), 105-112 (2021).
- Molina, E. A., et al. Angiogenic gene characterization and vessel permeability of dermal microvascular endothelial cells isolated from burn hypertrophic scar. Sci Rep. 12 (1), 12222 (2022).
- Li, Z., et al. A highly simulated scar model developed by grafting human thin split-thickness skin on back of nude mouse: The remodeling process, histological characteristics of scars. Biochem Biophys Res Commun. 526 (3), 744-750 (2020).
Ristampe e Autorizzazioni
Richiedi autorizzazione per utilizzare il testo o le figure di questo articolo JoVE
Richiedi AutorizzazioneThis article has been published
Video Coming Soon