Method Article
Intravascular Lithotripsy-Assisted Transfemoral Transcatheter Aortic Valve Implantation
In questo articolo
Riepilogo
Transcatheter aortic valve implantation (TAVI) has been shown to generate the best clinical outcomes when performed by the percutaneous transfemoral approach. Intravascular lithotripsy (IVL) can facilitate a transfemoral process in patients with calcified iliofemoral vascular disease and borderline intraluminal diameters. The present protocol describes IVL-assisted transfemoral TAVI.
Abstract
During the last decade, transcatheter aortic valve implantation (TAVI) has evolved as a well-established therapy for aging patients suffering from symptomatic severe aortic valve stenosis. This is also reflected in the recently updated international guidelines on managing patients with valvular heart disease. A transfemoral (TF) TAVI approach has proven superior to alternative access strategies. With the introduction of intravascular lithotripsy (IVL), patients with calcified iliofemoral vascular disease and borderline intraluminal diameters have also become candidates for percutaneous TF-TAVI. Moreover, IVL reduces the risk of major vascular complications by modifying the superficial and deep vascular calcium, thereby changing the vessel compliance and controlling luminal expansion. In this way, IVL has shown to safely facilitate TF delivery of TAVI devices in patients with calcified peripheral artery disease. The present article aims to provide a detailed step-by-step description on how to perform IVL-assisted TF-TAVI safely and efficiently. Furthermore, a literature review on the outcomes obtained with this technology is included, along with a concise discussion on this unique TAVI approach.
Introduzione
Transcatheter aortic valve implantation (TAVI) has proven to be a valuable therapy for elderly patients suffering from symptomatic severe aortic valve stenosis (AS) across all surgical risk categories1,2. The data and outcomes are most convincing for those patients in whom the TAVI procedure can be performed by transfemoral (TF) approach. TAVI by alternative access, such as transsubclavian, transaxillary, transcarotid, transcaval, and transapical access, can also be considered. However, the complication rates reported for TAVI by alternative access are higher than TF-TAVI3,4. This is also reflected in the most recent EU and US guidelines on managing patients with valvular heart disease5, in which there is a prominent role for TF-TAVI as a treatment option for patients with symptomatic severe AS.
Although there is consensus that TF-TAVI should be the default strategy for patients with proper iliofemoral access5, peripheral arterial disease (PAD) is not uncommon in patients planned for TAVI, given their advanced age and often multiple co-morbidities6. There has been a rapid technological evolution in the TAVI field over the past few years, resulting in TAVI devices with lower insertion profiles and more flexible delivery systems. Also, increased operator experience has increased the use of a fully percutaneous TF-TAVI approach. Nowadays, more than 90% of TAVI cases are performed in this way in most advanced TAVI centres7.
Still, a group of patients (5%-10%) remain good candidates for TAVI but suffer from severely calcified PAD. For many of these patients, the introduction of peripheral intravascular lithotripsy (IVL) has opened up the possibility to be treated by TF-TAVI. When using IVL, one generates sonic pressure waves using miniaturized lithotripters emitters integrated within a balloon. A steam bubble is created inside the balloon that rapidly expands and collapses by delivering electrical energy. This generates sonic pressure waves, similar in their waveform to those used during extracorporeal lithotripsy of nephrolithiasis. These waves travel through the vessel with a positive peak pressure of around 50 atm, thereby cracking and modifying both the superficial and deep vascular calcium, ultimately changing the vessel compliance and allowing for controlled luminal expansion8,9,10 (Figure 1). In this way, IVL has been shown to facilitate TF delivery of TAVI devices in patients with calcified PAD in a safe manner11,10,13. These IVL balloons are available in different diameters ranging between 3.5 mm-7 mm with a length of 60 mm.
The present article aims to provide a detailed description of performing IVL-assisted TF-TAVI safely and efficiently. Furthermore, a literature review on the outcomes obtained with this technology is included, along with a concise discussion on this new TAVI approach.
Patients (male/female) with a diseased iliofemoral anatomy corresponding to the following criteria could be suitable for IVL-assisted TF-TAVI (Figure 2): (1) Iliofemoral vascular disease with a lesion length of <20 mm and a calcium arc of ±270°, having a minimal lumen diameter of >3.0 mm, (2) iliofemoral vascular disease with a lesion length of <20 mm and calcium of arc ±360°, having a minimal lumen diameter of >4.0 mm, (3) iliofemoral vascular disease with a lesion length of >20 mm and a calcium arc of ±270°, having a minimal lumen diameter of >3.5 mm, and (4) iliofemoral vascular disease with a lesion length of >20 mm and calcium of arc ±360°, possessing a minimal lumen diameter of >4.5 mm. These recommendations are based upon expert opinion and local practice.
Protocollo
The protocol is approved by the human research ethics committee of Copenhagen University Hospital, and the studies are conducted following the guidelines of the said ethics committee. Following local policies, all patients gave informed consent for the TAVI procedure, cardiac CT scanning, and anonymous data for research.
1. Preprocedural planning
- Inspect the aorta-iliofemoral vessels ranging from the aortic valve, over the aortic arch, down to the common femoral artery (CFA) and the femoral bifurcation. For an accurate evaluation, perform a dedicated computed tomography (CT) angiography14 and make an angiography-based three-dimensional (3D) reconstruction using dedicated software15 (see Table of Materials).
- Visually assess the degree of vessel tortuosity.
- Visually assess the degree and amount of vessel calcification (arc, morphology, etc.)16. Pay specific attention to calcified spots with a calcified circumference of >270°.
- Measure the minimal luminal diameter (MLD), maximal luminal diameter, and mean luminal diameter at the site of the most critical calcifications and stenoses16.
- Decide upon the feasibility of a TF approach for TAVI. Assess the need for and benefit of an IVL-assisted TF approach. Take into account the recommendations provided in Figure 2.
- Determine the region of interest for possible IVL-treatment: iliac bifurcation, common iliac artery and/or external iliac artery.
- Determine the preferred TAVI access/puncture site referring to the preprocedural CT angiography; this concerns the femoral bifurcation and femur head.
- Decide upon the diameter and length of (un)covered stents if this is needed in vascular bailout situations.
2. Obtaining vascular access
- Apply local anesthesia by injecting ~10-20 mL of Xylocaine (10 mg/mL) solution in the skin and subcutaneous tissue at the preferred puncture site for TAVI. Confirm the effect of anesthesia by testing skin sensitivity with a needle.
- Perform an echo-guided puncture of the CFA and insert a 0.035" guidewire (see Table of Materials).
NOTE: Avoid a CFA puncture too close to the femoral bifurcation as this may complicate and compromise the option for bailout stenting of the CFA in case of vascular closure device failure. Verify this by fluoroscopy. - Make a skin incision of 2-3 cm and insert a 7 F-8 F dilator (see Table of Materials) over the wire.
- Keep the 0.035" guidewire into the artery and remove the 7 F-8 F dilator. Decide on vascular closure strategy (e.g., suture-based, plug-based closure, etc.) and perform pre-closure maneuvers if necessary.
- Insert the 7 F-8 F sheath (see Table of Materials) over the wire.
- Administer intravenous heparin according to the local protocol (e.g., 100 IU/kg).
3. Usage and positioning of a safety wire
- In a percutaneous intervention at an IVL-treated site, position a safety wire across the main access site and a wire in the abdominal aorta. Strictly keep this wire even after retraction of the TAVI system and the large bore insertion sheath.
NOTE: If the main TAVI access site is not diseased, there is no absolute need to position a safety wire across the main puncture site. - In case of vascular disease at the TAVI access site, consider a safety wire crossing the main puncture site, either introduced by a contralateral (e.g., through a 6 F-8 F long sheath), lower ipsilateral, or transradial secondary arterial access. Use this safety wire to treat vascular complications at the puncture and/or IVL-treated sites.
- Choose a 0.018" safety wire (which is stiff enough, see Table of Materials) to deliver vascular balloons and/or stents, if needed, but with a floppy, non-traumatic tip. Keep this safety wire in place during the entire TAVI procedure until confirmation of good vascular closure.
4. IVL system set-up
- Introduce a 0.014" guidewire, preferably with extra supportive characteristics, into the 7F-8F sheath at the main TAVI access site. Do not push this wire across the aortic arch.
- Turn on the IVL generator and get the connector cable (see Table of Materials) connected to it.
- Choose a 110 cm long IVL catheter with a balloon length of 60 mm and diameters ranging from 3.5-8 mm on an over-the-wire (OTW) system (see Table of Materials).
NOTE: There are three ports on the distal end of the catheter: one to connect the IVL connector, one for inflating and deflating the balloon, and one that fits the 0.014" guidewire. - Prepare the IVL catheter/balloon following the steps below.
- Aspirate 5 mL of a 50% contrast (see Table of Materials) and 50% saline mixture into a 20 mL syringe. Connect to the inflation/deflation port of the balloon catheter.
- Pull-on the syringe to aspirate air and replace this air inside the catheter with the fluid mixture in the syringe. Repeat at least three times.
- Fill an indeflator device (see Table of Materials) with 50% saline/50% contrast medium. Disconnect the 20 mL syringe and connect the indeflator to the inflation port of the IVL catheter with a three-way stopcock in an intermediate position, ensuring no air is introduced into the system.
- Flush the guidewire exit port at the distal end of the IVL catheter with a saline solution.
- Wrap the connector cable in a sterile cover.
- Connect the IVL catheter in a sterile fashion to the connector cable. Wrap some adhesive tape or a rubber band around the connection between the sterile catheter and the sterile cover to prevent it from sliding.
- Push the therapy button on the IVL generator. The light will switch from orange to green.
NOTE: Do not press the therapy button unless the balloon is filled with 50% saline/50% contrast medium (otherwise, it risks damaging the lithotripsy emitters). The IVL system is now ready for use (Figure 1).
5. IVL treatment
- Make the balloon and shaft of the IVL catheter wet before insertion to activate the hydrophilic coating.
- Insert the IVL catheter over the wire (OTW) into the 7 F-8 F sheath (main access site).
- Use fluoroscopy to position the marker bands at the region of interest.
- Inflate the IVL balloon to 4 atm. Document correct positioning and inflation using fluoroscopy. Ensure no air visible in the inflated balloon.
- Press and hold the activation button on the connector handle. Hold for 10 s to apply one cycle of 30 IVL pulses. Audible clicks and light flashes will confirm that the therapy is delivered.
- At the end of the 30 IVL pulses, increase the inflation of the IVL balloon to 6 atm. Hold this pressure for 4 s.
- Deflate the balloon and keep the negative pressure for 30 s to ensure it is empty. Repeat this action two more times.
- Repeat steps 5.3-5.7 for a maximum amount of 10 cycles with 30 IVL pulses (300 pulses in total).
- Confirm that the balloon is fully deflated before removing the IVL catheter.
- Exchange the 0.014" guidewire for a 0.018"-0.035" guidewire, depending on further planning (steps 5.11-5.12).
- If needed, perform additional percutaneous transluminal angioplasty (PTA) with a non-compliant balloon (e.g., 6-8 mm).
- Ensure that a 0.035" stiff guidewire is in place before inserting the large bore TAVI introducer sheath into the main access site.
- Continue the TAVI procedure, as usual.
6. Vascular closure after TAVI
- Before removing the insertion sheath, check the position of the safety wire. Always have a bailout option for percutaneous intervention with balloons and/or (un)covered stents in mind and ready in the cath lab.
- Perform vascular closure employing a suture-or plug-based vascular closure device (see Table of Materials).
- Asses the vascular closure with contrast injection, either from the secondary access site or through a 6 F sheath which replaced the large bore insertion sheath.
NOTE: A digital subtraction angiography (DSA) can be helpful in better identifying possible vascular complications17. Select and use the DSA mode on your module and ask the patient to hold their breath while performing the recording. - If a vascular complication occurs, treat accordingly. For example, place a covered stent in case of significant extravasation and an uncovered stent in a spiral dissection, etc.
Risultati
IVL treatment (Figure 1) of calcified PAD was first investigated in the DISRUPT-PAD European pre-market study18. The study showed an acute increase in vessel diameter in 35 patients following peripheral IVL treatment at the cost of only minimal vessel injury. The multi-center DISRUPT-PAD II trial19 confirmed these findings in 60 patients. DISRUPT PAD III20 was designed as a real-world, prospective, multi-center study in which 306 patients were randomized 1:1 to IVL or PTA treatment of severely calcified femoropopliteal arterial disease. Procedural success, defined as residual stenosis <30% without flow-limiting dissection after balloon dilatation, was greater in the IVL group than the classical PTA group (65.8% vs. 50.4%; p = 0.01). Furthermore, in the PTA group (1.4% vs. 6.8%; p = 0.03), flow-limiting dissections occurred more frequently.
Considering these promising results, IVL is increasingly used to enable TF delivery of large-bore devices, as needed in TAVI and (thoracic) endovascular aortic repair ((T)EVAR). Table 1 gives an overview of registries reporting on the use of IVL to facilitate TF-TAVI11,13,21,22. The delivery of the TAVI device was successful in all cases, with low complication rates. The Copenhagen registry11 reported the largest single-center series so far, in which 50 patients were included and treated. Patient selection was based on the CT recommendations described in Figure 2. All 50 TAVI cases were performed successfully with no VARC-2-defined major vascular complications (Table 1). No vascular perforations or ruptures were documented, and only one dissection occurred, which was treated with a non-covered stent. There was a relatively higher rate of vascular closure device failure (14%), which necessitated the use of a covered stent in 5 patients (10%)11. This underlines once more the degree of PAD in these patients and the need for diligent preprocedural planning and use of safety measurements during TAVI in this patient population.
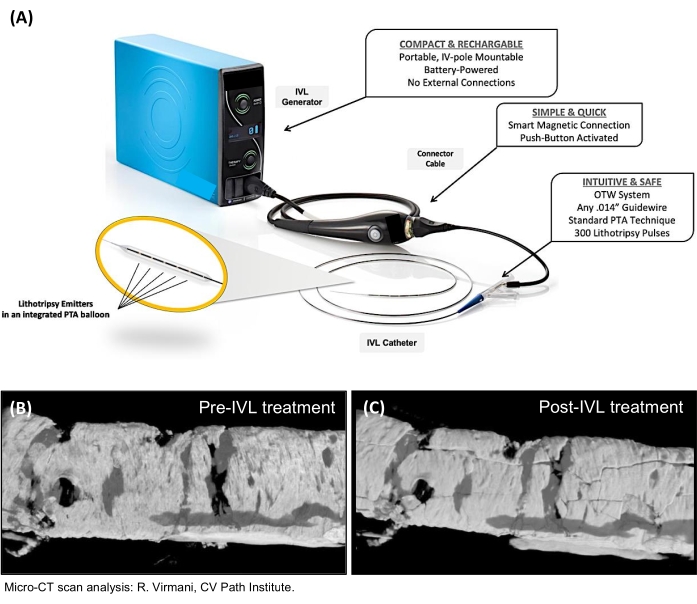
Figure 1: IVL system. (A) The IVL system components. (B,C) Micro-CT scan analysis showing 'micro-cracks' in the calcified vascular wall after IVL treatment. IVL, intravascular lithotripsy; OTW, over-the-wire; PTA, percutaneous transluminal angioplasty. Please click here to view a larger version of this figure.
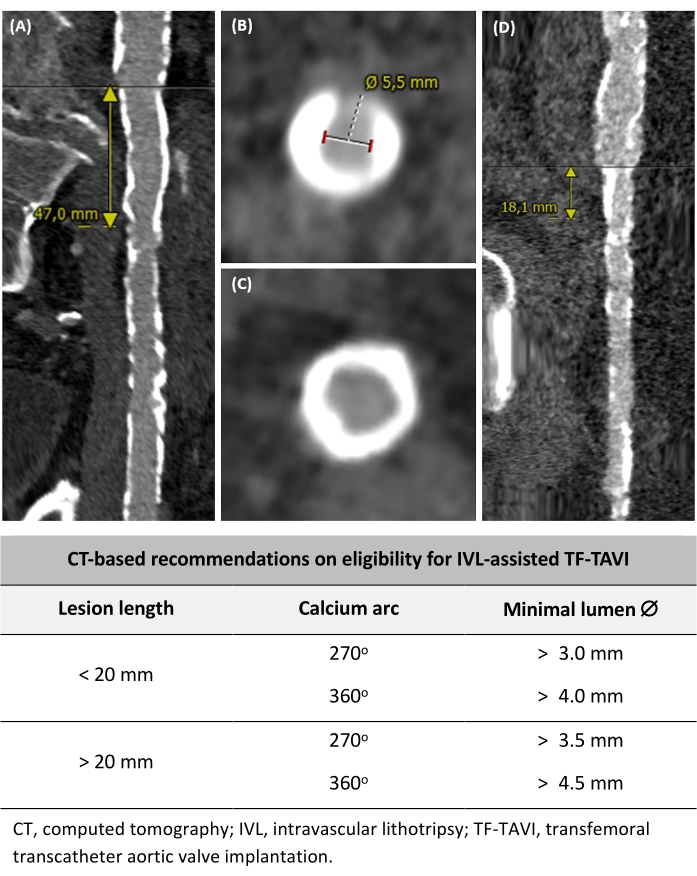
Figure 2: Determining the eligibility for IVL-assisted TF-TAVI. (A) Long calcified vessel segment >20 mm length. (B) 270° calcium arc and measurement of the minimal lumen diameter. (C) Circumferential 360° calcium arc. (D) Severely calcified but focal lesion measuring <20 mm. Based on preprocedural CT criteria, recommendations on eligibility for IVL-assisted TF-TAVI are also included. Please click here to view a larger version of this figure.
| Sawaya et al.11 | Nardi et al.22 | Armstrong et al.21 | Price et al.13 | |
| N = 50 | N = 108 | N = 17 | N = 9 | |
| Age | 78.3 ± 6.7 | 80.5 ± 6.2 | 72.5 ± 8.3 | 79.3 ± 9.8 |
| Procedure type | ||||
| TAVI | 50 (100%) | 108 (100%) | 4 (24%) | 4 (44%) |
| TEVAR | - | - | - | 1 (11%) |
| EVAR | - | - | 13 (76%) | 1 (11%) |
| Fenestrated EVAR | - | - | - | 3 (33%) |
| Reference vessel diameter, mm | 8.7 ± 2.2 | 9.1 (8.3-10.0) | 8.4 ± 2.5 | N/A |
| Vessel diameter stenosis, % | 55 ± 13 | 50 ± 11 | 79 ± 19 | N/A |
| Target lesion length, mm | 37 ± 16 | 20 (12-30) | 43 ± 22 | 42 ± 31 |
| IVL catheter size | ||||
| 5.0 x 60 mm | - | 2 (1.8%) | N/A | 0 (0) |
| 6.0 x 60 mm | - | 8 (7.1%) | N/A | 4 (40%) |
| 6.5 x 60 mm | - | 6 (5.4%) | N/A | 0 (0) |
| 7.0 x 60 mm | 50 (100%) | 96 (85.7%) | N/A | 6 (60%) |
| # pulses per lesion | N/A | 300 (270-300) | 234 ± 144 | N/A |
| Successful TF delivery | 50 (100%) | 108 (100%) | 17 (100%) | 9 (100%) |
| Complications | ||||
| Perforation | 0 (0) | 0 (0) | 0 (0) | 1 (11%) |
| Rupture | 0 (0) | 1 (0.9%) | 0 (0) | 0 (0) |
| Dissection – stenting | 1 (2%) | 2 (1.8%) | 0 (0) | 2 (22%) |
Table 1: Studies on IVL-assisted large-bore transfemoral access. Values are mean ± SD or N (%). N/A, not available; IVL, intravascular lithotripsy; EVAR, endovascular aortic repair; TAVI, transcatheter aortic valve implantation; TF, transfemoral; TEVAR, thoracic endovascular aortic repair.
Supplementary File 1: Why aim to maximize TAVI by percutaneous TF approach? Possible advantages of TAVI procedures by percutaneous transfemoral approach compared to alternative approaches. TAVI, transcatheter aortic valve implantation; TF, transfemoral. Please click here to download this File.
Discussione
Since the introduction of TAVI as a treatment option for patients with severe symptomatic AS, studies and registries have demonstrated that TAVI by TF approach generates better procedural success and lower complication rates3,4,23. As a result, most centers nowadays seek to perform most of their TAVI procedures by percutaneous TF approach23.
The introduction of IVL as a new tool in the TAVI armamentarium has resulted in an increased number of patients suitable for TF-TAVI and offers operators the possibility to perform TF-TAVI more safely and efficiently in case of calcified PAD. In the example of Copenhagen, the use of IVL has increased the percentage of TAVI cases that could be treated by the TF approach from 90%-95%, without increasing the risk of vascular complications7.
When considering IVL-assisted TF-TAVI, proper patient selection is of utter importance to perform these cases successfully and without complications. A CT-based algorithm has been proposed by the Copenhagen group11 and has also been successfully implemented in other Nordic and Western EU countries. It is also essential to understand that wall apposition of the balloon is important in transmitting the energy to the vessel wall10. In real-world practice mostly 6 mm–7 mm IVL balloons have been used in the context of 22, as the outer diameter of current TAVR delivery systems/introducer sheaths are 16 F–22 F (i.e., 5.3–7.3 mm). Based on the available data in the literature, IVL-assisted TF-TAVI has already been shown to be reliable, resulting in 100% successful TF delivery of the TAVI systems22. This is encouraging, especially considering the more difficult transcatheter heart valve (THV) deliveries with alternative TAVI access.
Finally, it should be emphasized that diligent safety measures need to be taken when engaging in challenging TF-TAVI cases with severely calcified iliofemoral vascular disease. Even recent state-of-the-art TAVI trials still show an incidence of 6%-8% major vascular complications24. This risk only increases when treating patients with a more hostile access25,26. However, with good preprocedural planning and proper safety measures during TAVI, significant vascular complication rates can be kept close to zero. Many bailout strategies are available7, primarily relying on a secondary access site. A classical approach is to perform a cross-over from a contralateral femoral access site and advance a safety wire in the main access vessel, passing the main puncture site. Also, a distal ipsilateral or a transradial access can be considered to introduce a safety wire. If a vascular complication occurs, either at the IVL-treated or the main puncture site, an occlusion balloon and/or (un)covered stent can be deployed quickly and efficiently to salvage vascular issues. Using such bailout strategies provides safety when treating these challenging TF-TAVI cases, which is a must in current practice as early ambulation and next-day discharge have become more routine in many TAVI centers27,28.
The current approach suffers certain limitations. Presently, there are no well-defined guidelines or instructions to indicate which patients are suitable for IVL-assisted TF-TAVI. Based on experience in large TAVI centers, a CT-based algorithm has been proposed11. However, these cut-off values may be different depending on the experience of the interventional team. It is essential to verify that the interventional team has the skills and expertise to perform percutaneous peripheral vascular interventions and that all tools/materials are present in the cath lab when engaging in these cases. IVL treatment also adds a specific economical cost to the TAVI procedure; however, the avoidance of general anesthesia, need for intensive care monitoring, a surgical wound, and the possibility of early ambulation29 and discharge27,28 is expected to (largely) compensate this additional cost for IVL treatment. Furthermore, transfemoral access is associated with less radiation exposure to the operator30 (Supplementary File 1).
If a patient is deemed unsuitable for TF-TAVI after careful preprocedural CT-angio analysis, TAVI by alternative access is typically considered. However, these strategies are more invasive, require specific experience, are often missing in lower-volume TAVI centers, and are associated with higher peri-procedural complication rates3,4. Classical balloon PTA could also be considered in patients with borderline-acceptable iliofemoral access for TF-TAVI. This strategy is less expensive and more time-efficient, but could also be associated with less luminal gain and increased risk of major vascular complications16 as reported in the DISRUPT PAD III trail20 for below-the-knee interventions. Further research on this topic is needed.
Another alternative strategy is to use a so-called 'pave-and-crack'-technique, in which multiple covered stents are implanted in the iliofemoral axis as sort of 'endo-conduits' causing controlled rupture of the access vessels and resulting in dilation to larger diameters31. The advantage of this technique is that it protects against extensive vascular perforation, rupture, and dissection. On the other hand, this technique also has multiple disadvantages: high cost, risk of restenosis, and more invasive character.
Divulgazioni
Prof. Dr. De Backer received speaker fees from Shockwave Medical Inc. All other authors do not report relevant conflicts of interest.
Riconoscimenti
The authors have none to acknowledge.
Materiali
| Name | Company | Catalog Number | Comments |
| 0.014” guidewire | Floppy II Extra Support Guide Wire, Abbott, USA | 22299M | |
| 0.035’’ stiff guidewire | Amplatz superstiff j-tip 7 cm floppy, Boston Scientific, USA | M001465020 | |
| 20 mL syringe | |||
| 6 F or 8 F femoral sheat | Radifocus Introducer II, Terumo | RS*B70N10MRD and RS*B80N10MRD | |
| 6-8 F Arrow sheat 35 cm- if contralateral access | Teleflex | CL07635 and CL07835 | |
| Arterial puncture needle | Percutaneous entry thinwall needle, Cook Medical | SDN18-18-7.0 | |
| Contrast solution | Visipaque 350, GE Healthcare | ||
| CT angiography-based 3D reconstruction dedicated software | 3mensio, Pie Medical, The Netherlands | ||
| Diagnostic catheter | 6F IMA diagnostic catheter, Cordis | 534-6605 | |
| Echo probe sterile cover | CIV-flex transducer cover, CIVCO | 610-1212 | |
| Indeflator device (20 mL) | Everest 30, Medtronic | AC3200 | |
| IVL Connector Cable | Shockwave medical | IVLCC | |
| IVL generator | Shockwave medical | IVLGCC | |
| Local anesthetic | Xylocain 10 mg/mL, Aspen | ||
| Non-compliant balloon | Z-MED II balloon 6 to 8 mm, Numed Canada inc. | PDZ622 | |
| Safety wire | 0.018’’ Platinum Plus guidewire, Boston Scientific, USA | M0014666050 | |
| Shockwave M5/M5+ catheter (7 mm-8 mm diameter) | Shockwave medical | M5IVL7060 - M5PIVL7060 - M5PIVL8060 | |
| Standard J-wire | angiodyn guide wire j-tip, B. Braun | 5050200 | |
| Sterile cover for shockwave connector cable | camera drape, Mönlycke health care | ||
| Three-way stopcock | |||
| Unfractionated heparin | 10 mL vials of 1000 IE/mL, Amgros I/S | ||
| Vascular closure device | Perclose Prostyle device, Abbott, USA | 12773-02 | |
| Vascular echo probe | |||
| Manta VCD, Essential Medical, USA | 2156NE, 2115NE |
Riferimenti
- Mack, M. J., et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. The New England Journal of Medicine. 380 (18), 1695-1705 (2019).
- Popma, J. J., et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. New England Journal of Medicine. 380 (18), 1706-1715 (2019).
- Blackstone, E. H., et al. Propensity-matched comparisons of clinical outcomes after transapical or transfemoral transcatheter aortic valve replacement. A placement of aortic transcatheter valves (PARTNER)-I trial substudy. Circulation. 131 (22), 1989-1999 (2015).
- Siontis, G. C. M., et al. Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of severe aortic stenosis: a meta-analysis of randomized trials. European Heart Journal. 37 (47), 3503-3512 (2016).
- Vahanian, A., et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. European Heart Journal. 43 (7), 561-632 (2021).
- Ueshima, D., et al. The impact of pre-existing peripheral artery disease on transcatheter aortic valve implantation outcomes: A systematic review and meta-analysis. Catheterization and Cardiovascular Interventions. 95 (5), 993-1000 (2020).
- Costa, G., Bieliauskas, G., Fukutomi, M., Ihlemann, N., Søndergaard, L., De Backer, O. Feasibility and safety of a fully percutaneous transcatheter aortic valve replacement program. Catheterization and Cardiovascular Interventions. 97 (3), 418-424 (2021).
- Cleveland, R. O., McAteer, J. A. Chapter 38, The physics of shock wave lithotripsy. Smith's Textbook on Endourology. 1, 529-558 (2007).
- Dini, C. S., et al. Intravascular lithotripsy for calcific coronary and peripheral artery stenoses. EuroIntervention. 15 (8), 714-721 (2019).
- Kereiakes, D. J., et al. Principles of intravascular lithotripsy for calcific plaque modification. JACC: Cardiovascular Interventions. 14 (12), 1275-1292 (2021).
- Sawaya, F. J., et al. Intravascular lithotripsy-assisted transfemoral TAVI: The Copenhagen experience and literature review. Frontiers in Cardiovascular Medicine. 8, 1-7 (2021).
- Nardi, G., et al. Peripheral intravascular lithotripsy to facilitate transfemoral TAVR: a multicentric prospective registry. European Heart Journal. 42, 1-11 (2021).
- Price, L. Z., Safir, S. R., Faries, P. L., McKinsey, J. F., Tang, G. H. L., Tadros, R. O. Shockwave lithotripsy facilitates large-bore vascular access through calcified arteries. Journal of Vascular Surgery Cases and Innovative Techniques. 7 (1), 164-170 (2021).
- Blanke, P., et al. Computed Tomography Imaging in the context of Transcatheter Aortic Valve Implantation (TAVI)/Transcatheter Aortic Valve Replacement (TAVR): An expert consensus document of the Society of Cardiovascular Computed Tomography. JACC: Cardiovascular Imaging. 12 (1), 1-24 (2019).
- Okuyama, K., et al. Transfemoral access assessment for transcatheter aortic valve replacement: evidence-based application of computed tomography over invasive angiography. Circulation Cardiovascular Imaging. 8 (1), 001995 (2015).
- Staniloae, C. S., et al. Systematic transfemoral transarterial transcatheter aortic valve replacement in hostile vascular access. Structural Heart. 3 (1), 34-40 (2019).
- El-Mawardy, M., et al. Impact of femoral artery puncture using digital subtraction angiography and road mapping on vascular and bleeding complications after transfemoral transcatheter aortic valve implantation. EuroIntervention. 12 (13), 1667-1673 (2017).
- Marianne, B., et al. Safety and performance of lithoplasty for treatment of calcified peripheral artery lesions. Journal of the American College of Cardiology. 70 (7), 908-910 (2017).
- Brodmann, M., et al. Primary outcomes and mechanism of action of intravascular lithotripsy in calcified, femoropopliteal lesions: Results of Disrupt PAD II. Catheterization and cardiovascular interventions Official journal of the Society for Cardiac Angiography & Interventions. 93 (2), 335-342 (2019).
- Tepe, G., et al. Intravascular lithotripsy for peripheral artery calcification: 30-day outcomes from the randomized Disrupt PAD III Trial. JACC: Cardiovascular Interventions. 14 (12), 1352-1361 (2021).
- Armstrong, E. J., et al. Intravascular lithotripsy for treatment of calcified, stenotic iliac arteries: a cohort analysis from the Disrupt PAD III Study. Cardiovascular revascularization medicine including molecular interventions. 21 (10), 1262-1268 (2020).
- Nardi, G., et al. Peripheral intravascular lithotripsy of iliofemoral arteries to facilitate transfemoral TAVI: a multicentre prospective registry. EuroIntervention. , (2021).
- Carroll, J. D., et al. STS-ACC TVT Registry of transcatheter aortic valve replacement. Journal of the American College of Cardiology. 76 (21), 2492-2516 (2020).
- Scarsini, R., et al. Impact of complications during transfemoral transcatheter aortic valve replacement: How can they be avoided and managed. Journal of the American Heart Association. 8 (18), 013801 (2019).
- Hayashida, K., et al. Transfemoral aortic valve implantation new criteria to predict vascular complications. JACC. Cardiovascular interventions. 4 (8), 851-858 (2011).
- Stefan, T., et al. Percutaneous aortic valve replacement. Journal of the American College of Cardiology. 59 (2), 113-118 (2012).
- Barbanti, M., et al. Optimising patient discharge management after transfemoral transcatheter aortic valve implantation: the multicentre European FAST-TAVI trial. EuroIntervention. 15 (2), 147-154 (2019).
- Wood, D. A., et al. The vancouver 3M (multidisciplinary, multimodality, but minimalist) clinical pathway facilitates safe next-day discharge home at low-, medium-, and high-volume transfemoral transcatheter aortic valve replacement centers. JACC: Cardiovascular Interventions. 12 (5), 459-469 (2019).
- Vendrik, J., et al. Early mobilisation after transfemoral transcatheter aortic valve implantation: results of the MobiTAVI trial. Netherlands Heart Journal: Monthly journal of the Netherlands Society of Cardiology and the Netherlands Heart Foundation. 28 (5), 240-248 (2020).
- Aquino, A., et al. Radiation exposure during transcatheter valve replacement: what cardiac surgeons need to know. The Annals of Thoracic Surgery. 109 (1), 118-122 (2020).
- Asciutto, G., Aronici, M., Resch, T., Sonesson, B., Kristmundsson, T., Dias, N. V. Endoconduits with "Pave and Crack" technique avoid open ilio-femoral conduits with sustainable mid-term results. European Journal of Vascular and Endovascular Surgery. 54 (4), 472-479 (2017).
Ristampe e Autorizzazioni
Richiedi autorizzazione per utilizzare il testo o le figure di questo articolo JoVE
Richiedi AutorizzazioneEsplora altri articoli
This article has been published
Video Coming Soon