Method Article
Mobile Device-assisted Dietary Ecological Momentary Assessments for the Evaluation of the Adherence to the Mediterranean Diet in a Continuous Manner
In questo articolo
Riepilogo
Presented here is a study protocol aimed at monitoring continuous adherence to the Mediterranean diet (MedDiet) by means of ecological momentary assessments. The method evaluates the intake of key food groups of the MedDiet and calculates an index of adherence.
Abstract
Mobile device-assisted dietary ecological momentary assessments (EMAs) have emerged as a new tool allowing the evaluation of dietary intake in real time, in a real-world setting and in a continuous manner. They have the potential to minimize recall bias, participant, and investigator burden, and decrease economic and time investment while maximizing ecological validity.
We developed a set of EMAs aimed at evaluating continuous adherence to the MedDiet. Four multiple-choice EMAs are sent daily in a randomized manner from a total of eight questions. The EMAs enquire about the consumption of 11 key food groups of the Mediterranean diet in the last 24-48 h in a semi-quantitative way. EMAs capture the daily frequency of consumption of fruits, vegetables, and extra virgin olive oil on different days of the week. Additionally, EMAs capture the weekly frequency of consumption of whole grain products, sugary drinks, nuts, legumes, sweets, fish and seafood, and red and processed meats. A designed scoring system behind the EMAs extracts the percentage of adherence to the MedDiet recommendations and calculates a quality index of the diet every week. Individualized reports are sent periodically to the volunteers highlighting the strengths and weaknesses of their diet. EMAs are also expected to have a behavioral effect, reinforcing the choice of Mediterranean foods.
Introduzione
The Mediterranean diet (MedDiet) is a dietary pattern associated with longevity and multiple health benefits. High adherence to the MedDiet has been related to a decreased risk of overall mortality, cardiovascular disease, overall cancer incidence, neurodegenerative disease and diabetes1. In particular, the MedDiet, based on seasonal and local products, is characterized by a high intake of plant-based food (fruit, vegetables, legumes, nuts, and unrefined cereals) and a moderate intake of fish, eggs, dairy, and poultry. Olive oil represents the main source of fat. The consumption of saturated fatty acids is low, with very low consumption of red and processed meats, sweets, and processed foods. The MedDiet is also characterized by a moderate intake of ethanol, mostly in the form of wine consumed during meals2.
The evaluation of diet is complex and challenging. The correct evaluation of dietary practices and MedDiet adherence in the study of nutrition is the key in attempting to find associations between this dietary pattern and its health outcomes. The traditional methods used to assess diet are food frequency questionnaires, food diaries and 24-h dietary recall. They have been broadly used in nutrition epidemiology and in clinical nutrition, however, they are highly subjected to misreporting, recall bias and depend on the participant's capacity to estimate food content and portion size. These traditional dietary assessment methods are time-consuming, expensive and represent an important burden for both participant and researcher3,4. To overcome these limitations, there is a need to reformulate traditional dietary assessment. The goal of the dietary assessment is to achieve a balance between the collection of accurate and reliable data with the resources consumed and the burden for the participant3. Several researchers have developed complementary approaches to evaluate adherence to the MedDiet. These approaches calculate composite dietary scores that result from the combination of different dietary characteristics associated with the MedDiet2. The first MedDiet score was created in 1995 by Trichoupoulou et al. (1995)5 and includes a total of 8 components. The score assessed the frequency of consumption of 7 food groups: vegetables, fruits and nuts, legumes, cereals, meat and meat products, milk and dairy products and alcohol intake. The eighth component was a fat quality measurement; the ratio between monounsaturated and saturated fatty acid (MUFA/SFA)5. One of the most used MedDiet assessment questionnaires, the MEDAS (Mediterranean Diet Adherence Screener) was developed during the PREDIMED study (Prevención con Dieta Mediterránea) with the aim of controlling dietary intervention compliance6. The MEDAS is a validated 14 item dietary screener which considers additional items compared to the first MedDiet score such as the type of oil used in cooking, consumption of sugar in drinks and sweets and the consumption of the typical Spanish spiced tomato sauce known as "sofrito". The questionnaire was found to be useful in evaluating the adherence to the MedDiet especially in time-limited settings such as large epidemiological studies and general clinical practice6.
The widespread availability of new technologies and the changes in how people use them have created the opportunity to incorporate these innovations into dietary assessment. They offer the chance to capture the complexity of food intake while overcoming the aforementioned limitations of traditional methods. In this context, ecological momentary assessments (EMAs) have been developed as a tool to use new technologies in performing repeated sampling of an individual's current behavior and experience7. The introduction of EMAs into dietary assessment can improve accuracy, ecological validity, and data robustness while minimizing reporting and recall bias and decreasing participant and research burdens. Additionally, the use of EMAs allows for the continuous assessment of diet; through the observation of fluctuations across time, observation of within-person changes, and the modeling of these variations. EMAs have the potential to minimize the reactivity bias, yield higher rates of compliance and lower the occurrence of missing data4,7,8. In summary, the major advantages associated with the use of EMAs are: (1) the collection of data as it occurs in the natural environment, (2) real time or near real time data collection rather than retrospective survey, thereby avoiding recall bias, and (3) repeated sampling, which allows for the study of behaviors and experiences over a given time period4,9. The use of EMAs to assess dietary intake is increasing and several clinical trials have used them to collect dietary information. The type of data collected in these studies include: the frequency of meals and snacks, the consumption of predefined food groups and beverages, and the recording of food images4,7.
To the best of our knowledge, the EMA approach has never been used to study the adherence to the MedDiet. The aim of the present study was to develop a set of mobile device assisted EMAs to continuously assess the adherence to the MedDiet. To do so, we developed a set of 8 mobile device-assisted EMAs to measure the consumption of 11 food groups, including the assessment of those typical of the MedDiet (olive oil, fruit, vegetables, etc.) along with the intake of food groups that represent a typically low consumption in the MedDiet (processed and red meat, sugary drinks, etc.).
Protocollo
This protocol demonstrates how to continuously assess adherence to the MedDiet by means of tailored EMAs. This protocol has been reviewed and approved by the local ethics committee of the Hospital del Mar: CEIm-PSMAR (reference number: 2019/8972).
1. Study design: Sampling protocol
- Determine the number of days to assess the dietary intake; a minimum of 1 week is required to obtain the first score and, therefore, ensure that the total number of days is adapted on a 7-days basis (e.g., 4 weeks of assessment to obtain the MedDiet adherence score over a month).
NOTE: The determination of the number of days is closely related to the aim of the study. In studies monitoring the adherence to the MedDiet exclusively, short periods of time such as 2 weeks is recommended. In the case of intervention studies aimed at improving MedDiet adherence, longer periods of evaluation are recommended. - Determine the number of waves for the study (e.g., two monitoring periods of two weeks separated by a month). In the case of longitudinal studies, the evaluation of MedDiet adherence can be integrated in the repeated sampling of volunteers.
NOTE: We leave the decision of the number of waves to the researcher's criteria. This decision is likely going to be subject to a set of appraisal criteria such as the aim of the survey in the context of the study, the type of volunteers among others. Based on the literature, most of the studies monitor during only one period, while some other included up to 8 waves of data collection. Studies with more than one monitoring period had smaller duration than those with only one monitoring period8. - Determine the schedule of the delivery of EMAs (e.g., every evening at 21:00h).
- Determine the latency of EMAs; as in the amount of time given to the volunteers to answer the questions (e.g., 2 h).
2. Selection of participants
- Define the inclusion criteria for the participants' selection.
- Ensure that participants possess a smartphone.
NOTE: Alternatively, the study can provide smartphones in cases where participants do not have one. - Ensure participants have good internet connection on their smartphone.
NOTE: Alternatively, the study can provide internet connection to the participants.
- Ensure that participants possess a smartphone.
- Define the exclusion criteria for the participant's selection.
- Do not include participants who are illiterate and/or do not have digital skills.
- Do not include participants residing in environments without internet connection.
- Select study participants based on the inclusion exclusion criteria.
3. Meeting with the participants before the assessment
- Schedule an introduction session with the participants.
- Explain in detail how to answer daily questions, emphasizing how they will receive notifications, how to respond to EMAs and the amount of time required to respond.
- Inform them that they will receive the questions in their phone via SMS. The SMS has attached a specific link that leads to the questionnaire. Each link is specific for the volunteer and the day.
- Introduce participants to the 8 EMA questions, explain which foods belong and do not belong to each category and describe the serving sizes when appropriate (Table 1).
- To ensure the correct comprehension of EMAs, prepare a pilot test with participants, providing them with an example of a diet and enabling them to respond to EMAs based on the presented example.
- Prepare an example of a diet of an individual describing specifically the types of food consumed and portion sizes. An example has been included as supplementary material.
- Provide the example to the participants via email and ask them to read it carefully.
- Give the volunteers the 8 questions included in the EMA survey on an online form and ask them to answer them based on the diet example given. The online form consists of 8 multiple-choice questions and volunteers must be identified with their id study number.
- Correct the questions and return a personalized feedback via email to each volunteer, with an explanation of the mistakes done.
| Question Nº | Food categories enquired | Food included | Food excluded of the group | Servings (s) |
| Q1 | Extra virgin olive oil | Extra virgin olive oil | Olive oil | Not applicable |
| (EVOO) | ||||
| Q2 | Vegetables | Salads, cooked vegetables, “sofrito”, frozen vegetables... | Potato, sweet potatoes, peas... | 1 s = 200g grams |
| ½ s = Side dish | ||||
| Q3 | Fruit | All fruit including raw, cooked… | Juices, yogurt with fruits, jam… | 1 s = 1 medium piece, 1 slice of melon/ watermelon, 2-3 small pieces… |
| Q4 | Whole-grain food | All whole grain products | Refined cereals and non-whole grain multigrain products | Not applicable |
| Q5 | Sugary drinks (including juices) | Soft drinks with and without sugar, natural and packaged juice | Water and alcoholic drinks | 1 s = 1 glass |
| Q6 | Legumes | Dried and cooked legumes, peas, tofu… | Corn | 1 s = 150 g |
| Q6 | Nuts | All nuts | Dried fruit | 1 s = 25 g |
| Q7 | Sweets | Home-made and industrial baking | Not applicable | |
| Q8 | Fish and seafood | All types: raw, frozen, canned, smoked… | Surimi and derivates | 1 s fish = 100-150 g |
| 1 s seafood = 200 g | ||||
| Q8 | Red meat | Bovine, game, viscera, duck… | Chicken, turkey, lean pork cuts | 1 s = 100 – 150 g |
| Q8 | Processed meat | Sausages, ham, mince, or cured meat… | - | 1 s = 50 g |
| Q: Question; s: Serving | ||||
Table 1: List of food categories enquired, detailing examples of food products included and excluded, and the size of the serving reported.
4. Measurement of the adherence to the MedDiet using EMAs.
- Send daily four randomized EMAs via a link attached in a SMS (Figure 1) following the weekly frequency outlined in Table 2 for the period of time established in the study. In this experiment the platform LimeSurvey (https://www.limesurvey.org) was used for the EMA delivery and for data storage.
| Nº | Food group | Type of question | Interval of enquiry | Weekly frequency of the question | |
| Question | |||||
| Q1 | EVOO | Qualitative | Last 24 hours | 4 times | |
| (Yes/No and usage) | |||||
| Q2 | Vegetables | Semi-Quantitative (Nº of servings) | Last 24 hours | 4 times | |
| Q3 | Fruit | Semi-Quantitative (Nº of servings) | Last 24 hours | 4 times | |
| Q4 | Whole-grain food | Qualitative | Last 24 hours | 3 times | |
| (Yes/No) | |||||
| Q5 | Sugary drinks (including juice) | Semi-Quantitative (Nº of servings) | Last 24 hours | 3 times | |
| Q6 | Legumes | Semi-Quantitative (Nº of servings) | Last 48 hours | 3 times | |
| Q6 | Nuts | Semi-Quantitative (Nº of servings) | Last 48 hours | 3 times | |
| Q7 | Sweets | Qualitative | Last 48 hours | 3 times | |
| (Yes/No) | |||||
| Q8 | Fish and seafood | Semi-Quantitative (Nº of servings) | Last 48 hours | 4 times | |
| Q8 | Red meat | Semi-Quantitative (Nº of servings) | Last 48 hours | 4 times | |
| Q8 | Processed mead | Semi-Quantitative (Nº of servings) | Last 48 hours | 4 times | |
Table 2: Description of the EMAs, type of question, interval of enquiry and frequency.
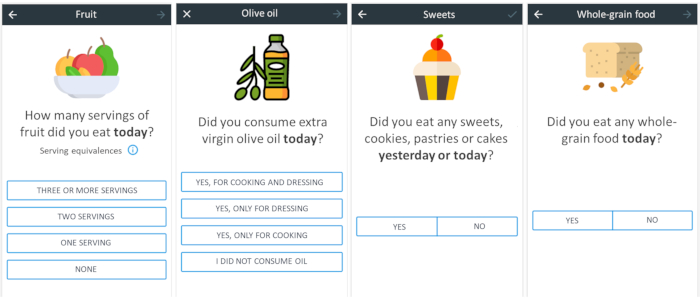
Figure 1: Example of four daily EMAs received by the participants. Please click here to view a larger version of this figure.
- During the first days of the study check that participants are replying to EMAs:
- Log in to the questionnaire administrator interface.
- Click on the answer space and search with the number of volunteer whether they have answered and whether the answer is complete (indicated with a green tick) or incomplete (indicated with a red cross).
- Contact participants who are not replying to detect technical problems, if any.
5. Assess the weekly adherence to the MedDiet
- After 7 days of receiving EMAs, calculate a score of adherence to the MedDiet.
- Download all completed answer from the survey website.
- Log in into the questionnaire administrator interface.
- Click on the menu to answers and then click to export answers.
- Select the data format to export and click on "only completed answers".
- Click to export to download the file.
- Open the excel file and sort columns by order of day and number of volunteers.
- Check for duplicated answers and eliminate, if any.
- Using a spreadsheet, sort all the answers given by each volunteer and translate the answer obtained into frequencies of consumption of the enquired food groups as detailed in Table 3.
- Download all completed answer from the survey website.
- Compare the frequencies of consumption obtained for each individual with the ones described in the MedDiet awarding the score for each food category as detailed in Table 3.
- Add up the scores of all enquired food groups to obtain the final score. The score has a maximum of 11 points. Additionally, translate the score into a percentage and classify into the following MedDiet adherence levels:
Weekly score ≤ 5 points (≤ 45%): Low adherence to the MedDiet
Weekly score > 5 and < 8 points (< 73%): Moderate adherence to the MedDiet
Weekly score ≥ 8 points (≥ 73%): High adherence to the MedDiet - Assess the compliance rate of the participants by means of the calculation of the accuracy of data of the week using the following formula:

Number of EMAs answered: total number of EMA prompts completely answered
Number of EMAs delivered: total number of EMA prompts send to the participants: 4 prompts x 7 days of the week =28
NOTE: Accuracies ≥80% are considered acceptable. Low accuracy percentages indicate low compliance of the participants and hence the quality of the data obtained is poor and probably not representative. - Send personalized feedback to the participant by email; including the weekly score and the score achieved in each food category. Extend the assessment for a total of 4 consecutive weeks to obtain the monthly score by adding the scores achieved in the 4 weeks period (maximum 44 points). The monthly calculated score can be classified in the following MedDiet adherence levels:
Monthly score ≤20 points (≤45%): Low adherence to the MedDiet
Monthly score >20 and <32 points (<73%): Moderate adherence to the MedDiet
Monthly score ≥32 points (≥73%): High adherence to the MedDiet - Send personalized feedback to the participant including the monthly score.
| Nº Question | Food enquired | Punctuation | Fulfillment with the recommendations | Recommendations * | |
| Q1 | EVOO | 1 | ≥ 75 % 1 | Use of EVOO as the main source of fat in each meal | |
| 0.5 | ≥ 50% | ||||
| 0 | < 50% | ||||
| Q2 | Vegetables | 1 | ≥ 75 % 1 | 2-3 servings/day | |
| 0.5 | ≥ 50 | ||||
| 0 | < 50% | ||||
| Q3 | Fruit | 1 | 75 % 1 | 3-4 servings/day | |
| 0.5 | ≥ 50% | ||||
| 0 | < 50% | ||||
| Q4 | Whole-grain food | 1 | ≥ 66 % 2 | Preference for whole-grain foods | |
| 0 | < 66% | ||||
| Q5 | Sugary drinks | 1 | ≥ 66 % 3 | Occasional and moderate consumption | |
| 0 | < 66% | ||||
| Q6 | Legumes | 1 | ≥ 2 times/week | 2-4 servings/week | |
| 0 | < 2 times/week | ||||
| Q6 | Nuts | 1 | ≥ 3 times/week | 3-7 servings/week | |
| 0.5 | ≥ 2 times/week | ||||
| 0 | < 2 times/week | ||||
| Q7 | Sweets | 1 | ≥ 66 % 3 | Occasional and moderate consumption | |
| 0 | < 66% | ||||
| Q8 | Fish and seafood | 1 | ≥ 2 times/week | 2-3 servings/week | |
| 0 | < 2 times/week | ||||
| Q8 | Red meat | 1 | ≤ 1 time/ week 4 | Occasional and moderate consumption | |
| 0 | > 2 times/week | ||||
| Q8 | Processed meat | 1 | ≤ 1 time/ week 4 | Occasional and moderate consumption | |
| 0 | > 1 time/ week | ||||
| * Recommendations are based on Dietary guidelines for the Spanish population (Spanish Society on Community Nutrition, December 2016) | |||||
| 1. We considered a good adherence to the recommendation when subjects complied with more than 75% with the recommendations. | |||||
| 2. We considered a good adherence to the recommendations when whole-grain products where consumed in two or more occasions of the 3 times asked per week. | |||||
| 3. We considered that the consumption was occasional when the intake of sugary drinks and sweets reported was less than once out of the 3 times asked per week. | |||||
| 4. We considered that the consumption was occasional and moderate when the total number of servings reported in the week was ≤1. | |||||
Table 3: Items and punctuation criteria to calculate the weekly MedDiet adherence score.
Risultati
The present protocol was used in a proof-of-concept study which included a total of 63 subjects with an age range of 22 to 76 years. The aim of the proof-of-concept study was to compare the adherence to the Mediterranean diet obtained with the proposed EMAs approach with the validated MEDAS test. The present study did not intend to validate the EMAs but to compare both instruments as tools to measure the adherence to the MedDiet, to test its feasibility and the adherence of study participants to a two-week EMAs evaluation.
Study design and study population
The present study is an observational clinical trial including a total of 63 healthy volunteers. Table 4 summarizes the baseline characteristics of the study participants. Participants in this protocol were medical/human biology students from the University Pompeu Fabra and subjects already enrolled in the PENSA study. They were included from July 2020 to November 2020. Eligibility criteria were participants aged over 18 years old, Spanish speaking and in possession of a smartphone with internet connection. Exclusion criteria were illiteracy and participant with unstable health chronic conditions that could potentially alter their habitual diet.
| n | % | ||
| Age (years) | |||
| 20-29 | 21 | 33.3 | |
| 30-49 | 16 | 25.4 | |
| 50-72 | 26 | 41.3 | |
| Gender | |||
| Men | 19 | 30.2 | |
| Women | 44 | 60.8 | |
| Highest educational degree received | |||
| Primary education | 3 | 4.8 | |
| Secondary education | 6 | 15.8 | |
| Medium grade education | 10 | 19.1 | |
| Bachelor or superior education | 34 | 60.3 | |
| Occupational status | |||
| Student | 8 | 12.7 | |
| Worker | 31 | 47.6 | |
| Homemaker | 3 | 4.8 | |
| Retired | 22 | 34.9 | |
| Type of diet | |||
| Diet without restrictions | 44 | 79.4 | |
| Flexitarian diet | 6 | 11.1 | |
| Gluten-free diet | 1 | 1.6 | |
| Lactose-free diet | 3 | 4.8 | |
| Diet restricted by other intolerances / allergies | 2 | 3.2 | |
Table 4: Characteristics of study participants (n= 63)
Informed consent was obtained from all screened participants before any study procedure. Initially, all study participants completed the MEDAS test. Prior to the beginning of the two-week EMAs evaluation period an individual induction session was carried out by a trained nutritionist and included the EMAs principles. The session included the detailed explanation of each individual question, including what type of food was included and excluded in each category and a description of serving sizes in grams and portion sizes (Table 1). All the information was given in paper to the participants to be consulted throughout the study. After the initial training, individuals were given an example of EMAs and answered under the supervision of the nutritionist. Doubts and misunderstandings detected at this moment were clarified before the beginning of the EMAs evaluation period. At the end of the study, participants received a feedback of their adherence to the MedDiet during the two weeks and the MEDAS results. The feedback was not provided at the end of the first week in order of avoid changes in their habitual diet. Volunteers were not paid for the study participation. The trial was performed following good clinical practices and in accordance with the Helsinki declaration.
Evaluation of MedDiet adherence using EMAs
Volunteers received 4 randomized EMAs daily at 20:00 h starting on a Monday. First and second week of EMAs evaluation differed on the pattern of randomization of the questions and is detailed in Table 5. Overall, the EMAs followed the frequencies detailed in Table 2 and the time window included in each question did not overlap within the same week.
| Day | Questions delivered during | Questions delivered during |
| Week 1 | Week 2 | |
| Monday | Q1, Q3, Q4, Q7 | Q3, Q5, Q7, Q8.1 |
| Tuesday | Q2, Q5, Q6, Q8.2 | Q1, Q3, Q4, Q6 |
| Wednesday | Q1, Q2, Q3, Q7 | Q2, Q5, Q4, Q8.2 |
| Thursday | Q3, Q5, Q6, Q8.2 | Q1, Q2, Q3, Q7 |
| Friday | Q1, Q2, Q3, Q4 | Q2, Q6, Q5, Q8.2 |
| Saturday | Q2, Q5, Q6, Q8.2 | Q1, Q2, Q3, Q7 |
| Sunday | Q1, Q4, Q7, Q8.1 | Q1, Q4, Q6, Q8.2 |
Table 5: Pattern of EMAs survey followed in the proof-of-concept study: questions delivered each day of the week of the study.
MedDiet Adherence based on EMAs
The observed mean (SD) scores obtained using EMAs were 6.3 (2.2) during the first week and 6.4 (2.1) during the second week of a maximal score of 11 points. The scores between weeks did not differ significantly (p>0.05). The coefficient of variation (CV) for within-individual variation was calculated between the score obtain the first and the second week obtaining a mean value of 25.5 % (SD = 39.5). The average weekly score ranged from 1.5 to 10.3 with a mean (SD) of 6.4 (2.0). Figure 2 outlines a histogram of the scores obtained.
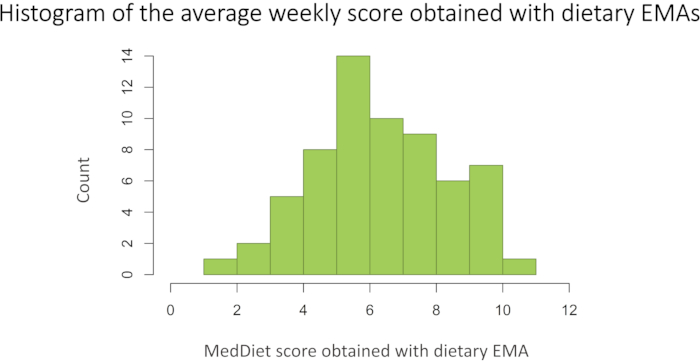
Figure 2: Histogram of the mean score obtained with EMAs for two weeks (n=63). Please click here to view a larger version of this figure.
Compliance rate
In terms of compliance, 43 volunteers (68%) answered 100% of the prompts sent, meaning an accuracy of 100% of the data obtained. Out of the remaining volunteers, 15 (24%) had an accuracy over 80%, 3 (5%) volunteers responded between 70-80% of the EMAS and only 2 volunteers (3%) exhibited accuracies lower than 70 %.
Comparison with MEDAS
Finally, as mentioned before, all subjects answered the MEDAS test for comparative purposes. The obtained scores ranged from 3 to 13 points, corresponding to an adherence ranging from 21.1% to 92.8% to the MedDiet. 26 (42.6%) volunteers presented a low adherence to the MedDiet, 19 (31.2%) volunteers a moderate adherence and 66 (26.2%) volunteers presented a high adherence to the MedDiet. The percentages of adherence to MedDiet obtained with MEDAS and with EMA did not show any statistical difference (p > 0.05). Figure 3 represents a correlation between the percentages of adherence to MedDiet obtained with both tests. A significant positive correlation was observed between both with a correlation coefficient of 0.672 (p<0.001). The correlation was calculated with volunteers answering more than 70% of the prompts sent (n=61).
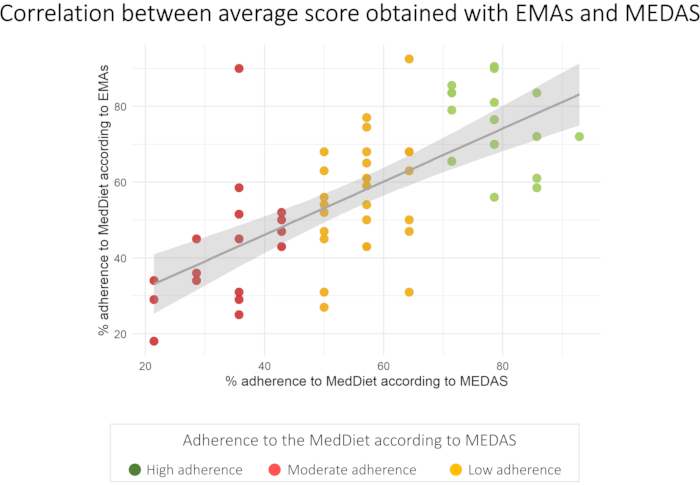
Figure 3: Correlation between percentage of adherence to the MedDiet measured with MEDAS and percentage of adherence measured with EMAs (n=61). Please click here to view a larger version of this figure.
MedDiet adherence assessment
Based on the answers given in EMAs, punctuation was given based on whether the volunteers achieved MedDiet recommendations or not. Participants were categorized in two groups: those who achieved recommendations (score assigned = +1) and those who did not achieve MedDiet recommendations (score assigned = 0). For certain food groups a half point was given when 50 % of recommendations were achieved. Figure 4 outlines the food categories in which the study population exhibits the higher adherence to the MedDiet (EVOO and low consumption of sugary drinks) and the food categories in which the adherence is lower (Vegetables, Nuts, and Legumes). This information can be important in the development of public health policies as it would help to identify and prioritize the food groups in which general population has a lower adherence to the MedDiet recommendations.
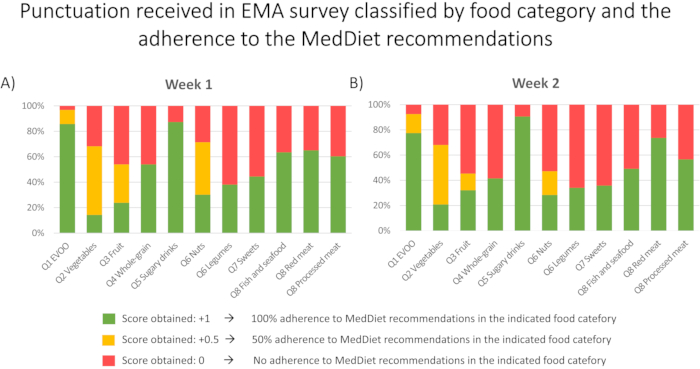
Figure 4: Punctuation received in each enquired food category in week 1 (A) and in week 2 (B) (n=63). Based on EMAs answers, the adherence to the MedDiet recommendation of each food category was obtained. For each specific food group evaluated, a score was given in terms of the degree of fulfillment of the MedDiet recommendations: 0, +0.5 or +1. The figure outlines the distribution of the scores obtained in each week for each single food category. Please click here to view a larger version of this figure.
Supplementary information:
Statistical analysis
Analyses were performed with R, version 3.0.2. Differences in MedDiet adherence scores and between-week changes in EMAs results were assessed by Student's t-test for paired samples. Pearson correlation coefficient was calculated to evaluate the existence of linear association between MedDiet measured using MEDAS and using our EMA approach. Significance was defined as p < 0.05. All statistical analyses were performed in participants with a rate of compliance to EMAs survey higher than 70%.
Discussione
Here we describe a protocol to assess an individual's adherence to the MedDiet via a mobile-based application. This method uses daily EMAs to capture the dietary pattern and by means of an algorithm, calculates a weekly score representing the degree of adherence to the MedDiet. A positive score is given to the high intake of healthy food items which are characteristic of the MedDiet. Conversely, a negative score is given to the intake of unhealthy food groups, in accordance with the recommendations given for the Spanish population10.
The use of EMAs to evaluate dietary intake represents an innovative approach capable of capturing the complexity of food sustained consumption and overcomes some of the limitations of traditional dietary assessment methods. A key strength of the present method is its capacity to monitor dietary intake continuously over a time period. The evaluation of the diet is performed in real time or near real time and in the real environment, maximizing the ecological validity of the data obtained. The present method is distinctive in its briefness and simplicity, representing a low burden for the participant. Once participants are habituated and properly trained to respond to EMAs, they can respond to the questions in less than 3 minutes. The evaluation of the dietary information obtained is performed automatically, thus reducing investigator burden and time investment. The presented score differs significantly from and does not substitute other nutritional composite scores such as Healthy Eating Index (HEI) and Alternative Healthy Eating Index (AHEI). The calculation of the MedDiet score by means of EMAs represents a semi-quantitative method and does not require software to further calculate the nutritional composition of individual's diet.
Despite the advantages of using EMAs in dietary assessment, the present method presents some limitations and challenges. The information on dietary consumption is limited to a predefined group of food, and does not consider other important food groups in the diet such as dairy, eggs or alcohol consumption. The consumption of eggs and dairy is not monitored in MEDAS either. Alcohol consumption and its recommendation has been the subject of a major debate. Therefore, we decided not to include alcohol consumption in the EMAs to avoid the promotion of its consumption and the consequences associated with alcohol misuse and abuse. Different patterns in the consumption of the non-assessed groups could significantly impact on the overall quality of the diet and would not be reflected in the final score given by the present method. Another limitation of EMAs shared with traditional dietary assessment methods is that they rely on self-reported data and participant's interpretation of EMAs, they are thus prone to measurement errors, reactivity bias and social desirability. Although one of the main objectives of using EMAs is reducing participant burden, the desire to obtain accurate data may lead to an excess number of prompts that are too repetitive for participants and lead to a decrease in the response rate and an increased risk of study dropout. Indeed, it has been described that, within the studies using EMAs to assess dietary intake, the highest frequency of prompting was associated with the lowest compliance rates8. In this case, as the score is accumulated based on punctuations and percentages over an entire week, a sporadic absence of information would not compromise the quality of the data obtained. Nonetheless, higher amounts of missing data could trigger unreliable and insufficient information and hinder the calculation of adherence to the MedDiet.
Ultimately, part of the challenge of the present method is linked to its technological component. While young individuals may feel comfortable with mobile-based technology and easily adopt it as a routine, older participants can present lower acceptability to technology, which, in turn, could trigger difficulties when responding to daily EMAs and ultimately, lower response rates. Additionally, potential technical issues can arise with the platform and the devices used as well as connectivity problems4.
Special care should always be taken when performing research in humans. Therefore, all steps should be performed cautiously and follow good clinical practices. To obtain reliable information using the mentioned method, one of the most critical steps is the training of the participants. Researchers had to ensure the correct comprehension of all questions included in the EMAs, which food groups are included, and which are not. As the present method includes a report on the number of servings, participants must also be instructed on how to report them accurately. A poor understanding of the EMA will lead to misreporting and hence, to the collection of biased data. Finally, participants must be aware of the importance of answering all the EMAs received to avoid low compliance rates. Researchers could design reward program strategies to promote compliance. An additional critical step common in all studies using EMAs is the correct reporting of the data obtained in their publications. To ensure data comparability between studies, Liao et al (2016) have designed a set of guidelines and a checklist specifically tailored for studies using EMAs and their unique characteristics8. These guidelines emphasize the need to report information such as detailed study design and compliance rates in order to assess the quality of the data obtained8. Our protocol and the form of reporting the results of the proof-of-concept study followed the guidelines designed by Liao et al. (2016)8.
In addition, the tailored dietary EMAs proposed in the present work can be part of a behavioral intervention to trigger a change in food habits. As previously described, the mere act of measuring a behavior can trigger a change in that behavior11. Therefore, repetitive exposure to EMAs could lead to a positive change in food habits. The extended use of the application could have the aim of encouraging participants to follow the MedDiet. The application outlines the positive and negative food items present in the MedDiet. The delivery of personalized feedback has the purpose of increasing dietary self-awareness and dietary self-management. Feedbacks could be combined with automatic personalized dietary recommendations in response to low or high consumption of food items, providing recipes, healthier alternatives, and a list of seasonal foods to improve diet quality. Overall, the use of the application can encourage the choice of food based on MedDiet recommendations.
The development of new technologies together with the generalization of mobile use offers an opportunity for dietary assessment. The use of dietary EMAs, characterized by the repeated assessment of individuals in real time, represents a novel tool to evaluate dietary intake. The use of dietary EMAs could be integrated as a useful instrument in nutritional epidemiology as well as in clinical interventions. Moving forward, the use of EMAs could be combined with objective measurements such as dietary-associated biomarkers, to strengthen the overall method and overcome its challenges. Additionally, the described method could be integrated into a more complex platform able to simultaneously collect dietary habits and other types of data such as environmental (e.g. location), lifestyle (e.g. physical activity, sleep, etc.) and health outcomes (e.g. heart rate, blood pressure, glycemia, etc.). Finally, it is important to mention that the present method falls into the new approach of future medicine (P4) being predictive, preventive, participatory, and personalized12.
Divulgazioni
The authors have nothing to disclose.
Riconoscimenti
This work was supported by grants from Alzheimer Association (18PTC-R-592192; The PART THE CLOUD to RESCUE (REverse, reStore, Cease and UndErstand) Brain Cell Degeneration in Alzheimer's disease Program), Instituto de Salud Carlos III (FEDERPI17/00223), CIBER de Fisiopatología de la Obesidad y Nutrición (CIBEROBN) and DIUE de la Generalitat de Catalunya (2017 SGR 138) from Agència de Gestió d'Ajuts Universitaris i de Recerca (AGAUR).
Materiali
| Name | Company | Catalog Number | Comments |
| Data processing software (excel) | MS Office | - | Others suitable options like R studio |
| Google forms | - | Free online software that allows the creation of surveys and questionnaires to be delivered. It's part of Google's web-based apps sui | |
| Limesurvey platofrm (https://www.limesurvey.org/) | Limesurvey | - | A free software application for conducting online surveys |
Riferimenti
- Dinu, M., Pagliai, G., Casini, A., Sofi, F. Mediterranean diet and multiple health outcomes: an umbrella review of meta-analyses of observational studies and randomised trials. European Journal of Clinical Nutrition. 72 (1), 30-43 (2018).
- Zaragoza-Martí, A., Cabañero-Martínez, M. J., Hurtado-Sánchez, J. A., Laguna-Pérez, A., Ferrer-Cascales, R. Evaluation of Mediterranean diet adherence scores: a systematic review. BMJ Open. 8 (2), 019033 (2018).
- Rollo, M. E., Williams, R. L., Burrows, T., Kirkpatrick, S., Bucher, T., Collins, C. E. What Are They Really Eating? A Review on New Approaches to Dietary Intake Assessment and Validation. Current Nutrition Reports. 5, 307-314 (2016).
- Maugeri, A., Barchitta, M. A Systematic Review of Ecological Momentary Assessment of Diet: Implications and Perspectives for Nutritional Epidemiology. Nutrients. 11 (11), 2696 (2019).
- Trichopoulou, A., et al. Diet and overall survival in elderly people. BMJ. 311 (7018), 1457-1460 (1995).
- Schröder, H., et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. Journal of Nutrition. 141 (6), 1140-1145 (2011).
- Schembre, S. M., et al. Mobile Ecological Momentary Diet Assessment Methods for Behavioral Research: Systematic Review. JMIR mHealth and uHealth. 6 (11), 11170 (2018).
- Liao, Y., Skelton, K., Dunton, G., Bruening, M. A Systematic Review of Methods and Procedures Used in Ecological Momentary Assessments of Diet and Physical Activity Research in Youth: An Adapted STROBE Checklist for Reporting EMA Studies (CREMAS). Journal of Medical Internet Research. 18 (6), 151 (2016).
- Bruening, M., van Woerden, I., Todd, M., Brennhofer, S., Laska, M. N., Dunton, G. A Mobile Ecological Momentary Assessment Tool (devilSPARC) for Nutrition and Physical Activity Behaviors in College Students: A Validation Study. Journal of Medical Internet Research. 18 (7), 209 (2016).
- Spanish Society on Community Nutrition (SENC, Aranceta Bartrina J. et al. Guias alimentarias para la poblacion espanola (SENC, diciembre 2016): la nueva piramide de la alimentacion saludable [Dietary guidelines for the Spanish population (SENC, December 2016); the new graphic icon of healthy nutrition]. Nutricion Hospitalaria. 33, 1-48 (2016).
- Levav, J., Fitzsimons, G. J. When questions change behavior: the role of ease of representation. Psychological Science. 17 (3), 207-213 (2006).
- Flores, M., Glusman, G., Brogaard, K., Price, N. D., Hood, L. P4 medicine: how systems medicine will transform the healthcare sector and society. Personalized Medicine. 10 (6), 565-576 (2013).
Ristampe e Autorizzazioni
Richiedi autorizzazione per utilizzare il testo o le figure di questo articolo JoVE
Richiedi AutorizzazioneEsplora altri articoli
This article has been published
Video Coming Soon