Method Article
Mouse Electroacupuncture Fixation Device Fabrication for Electroacupuncture Pretreatment in Diabetic Cardiomyopathy Mouse Model
* These authors contributed equally
In This Article
Summary
Here, we present a protocol to establish a mouse model of diabetic cardiomyopathy and a mouse electroacupuncture fixation device for the use of electroacupuncture in mice, which can fix the mice more gently during treatment.
Abstract
Diabetic cardiomyopathy (DCM) is a complication of diabetes that can progress to heart failure and is limited by the use of medications. Electroacupuncture is an effective way as a traditional Chinese medicine external treatment method to treat diabetic cardiomyopathy. A mouse electroacupuncture fixation device can easily and gently fix mice during electroacupuncture treatment and expose acupoints on the abdomen and limbs of mice. This article describes the methods used to fabricate the mouse electroacupuncture fixation device with all the steps and precautions, establishing a mouse model of diabetic cardiomyopathy with comprehensive measures to comprehensively record changes in blood biochemical indices such as blood glucose. The experiment was done with 3 groups: a control group, a model group, and a pretreatment group, with 10 mice in each group. The pretreatment group was treated by Biaoben acupoints electroacupuncture pretreatment once every alternate day for a total of 7 sessions. After electroacupuncture, the mice in the pretreatment group showed a decrease in blood glucose accompanied by a reduction in the symptoms of diabetes.
Introduction
Diabetic cardiomyopathy (DCM) is a common complication of diabetes mellitus characterized by changes in cardiac structure and ventricular diastolic and contractile function of a specific lesion, further developing into heart failure. It is one of the leading causes of death in diabetes mellitus patients1,2, and its increasing prevalence has attracted widespread attention in recent years3. It has been found that the pathogenesis of this disease mainly includes insulin resistance, inflammatory response, abnormal lipid metabolism, oxidative stress, mitochondrial damage4,5.
At present, clinical treatment of diabetic cardiomyopathy is mainly based on oral medication for controlling blood glucose and alleviating the symptoms of heart disease, but it has certain limitations, such as drug reactions, kidney function effects, and dependence on long-term medication6. Acupuncture, as a traditional Chinese medicine external treatment method, has a better-regulating effect on blood glucose, blood lipids, and inflammatory response in patients with diabetic cardiomyopathy, and the clinical efficacy is remarkable7. Acupuncture has no negative effects on gastrointestinal and kidney functions, and long-term treatment does not produce dependence. The theory of Biaoben acupoints is an important method of selecting and using acupuncture points in the traditional theory of acupuncture8. It defines the acupoints that can improve the symptoms of diseases as Biao acupoints, and the acupoints that can regulate the basic functions of the body related to the diseases and treat the root causes of the diseases are called Ben acupoints. The stimulation of Biao acupoints and Ben acupoints at the same time with acupuncture can achieve the purpose of treating the diseases as well as increasing the body's resistance to the diseases and preventing the exacerbation and recurrence of the diseases9. It can prevent the aggravation and recurrence of the disease. Modern research has proved that acupuncture with Biaoben acupoints is effective for diabetes and its many complications, including diabetic heart disease, diabetic nephropathy, diabetic retinopathy, diabetic foot, and so on10,11. An acupoint selection scheme in line with the theory of Biaoben acupoints is to select PC6 (Neiguan), which can regulate the functions of the heart and improve the inflammatory response as the Biao acupoint12, and ST36 (Zusanli), which can improve blood glucose and blood sugar situation, improve insulin resistance, and reduce oxidative stress, as the Ben acupoint13.
Animal models are an important tool in the study of diabetic cardiomyopathy because it has been experimentally demonstrated that rat or mouse models well mimic the structural abnormalities and functional changes of the heart caused by diabetic cardiomyopathy and provide the samples required for biochemical detection indices of diabetic cardiomyopathy14,15. The use of high-sugar and high-fat feed combined with Streptozotocin solution to induce mice can lead to the formation of diabetic models in 4-5 weeks, effectively shortening the modeling time and improving the success rate, and there are no special requirements for the rearing environment16,17. Diabetic cardiomyopathy is a kind of cardiomyopathy caused by the damage to the cardiac structure and function caused by continuous hyperglycemia. With the prolongation of the course of the disease, the diabetic heart disease model can be naturally developed by the diabetic model under long-term hyperglycemia18,19,20. Acupuncture pretreatment refers to the pretreatment of mice after the formation of diabetes and before the formation of obvious myocardial injury. However, after repeated experimental stimulation, mice often become mobile and difficult to immobilize, and violent experimental manipulation techniques may cause mice injury or make them manic or depressed21. Therefore, a suitable method to immobilize mice is needed. Commonly used methods of mouse immobilization include anesthesia and strapping22. Although the anesthesia method can better expose the acupoints, it may affect nerve conduction and ultimately affect the experimental results. The strapping method is a physical immobilization method and does not produce the same physiological effects as anesthesia, but the bound mice will experience significant tension, increased muscle tone, and enhanced defecation22,23. In addition, the needle cannot easily reach the desired position. Here, we introduce a mouse electroacupuncture fixation device that can be used to ensure that mice do not move and also expose acupoints during electroacupuncture treatment so that electroacupuncture treatment can be performed conveniently, safely, nonviolently, and without sequelae.
This article describes in detail the fabrication of the mouse electroacupuncture fixation device and the establishment of a mouse model of diabetic cardiomyopathy. We treated diabetic cardiomyopathy by Biaoben acupoints electroacupuncture pretreatment, demonstrated how its operation was realized on the mice fixation device, and evaluated the therapeutic effect of Biaoben acupoints electroacupuncture pretreatment on diabetic cardiomyopathy by measuring indices related to glycolipid metabolism.
Protocol
All animal experiments comply with the 3Rs principle of animal ethics and have been reviewed and approved by the Committee on the Ethics of Animal Experiments of Hubei University of Traditional Chinese Medicine (Ethical clearance number HUCMS00309300). Here, 30 SPF-grade mice were obtained from the Hubei Provincial Center for Disease Control and Prevention (Quality certificate NO.42010200009690).
1. Fabrication of the electroacupuncture fixation device
- For the assembly of an electroacupuncture fixation device obtain a transparent acrylic hollow plastic tube and an electroacupuncture therapy instrument as the main body (Figure 1A). In the tube maintain a tube body and prepare closure tabs located in the openings at each end. Make grooves in the hollow tube in the center of each end and secure the closure tabs in the grooves at each end using manually rotatable screws.
- In the middle of the hollow tube, make three oval holes about 2 cm long and 1 cm wide distributed adjacent to each other. The holes on both sides are used to pull out the forelimbs of the mouse for fixation for electroacupuncture treatment of the forelimbs, and the hole in the middle can be used to expose the acupuncture points of the thoracic and abdominal regions of the mouse for electroacupuncture treatment of the thoracic and abdominal regions.
- At the end of the hollow tube, make an oval hole symmetrically distributed on both sides of the center groove, which can be used to pull out the hind limbs of the mouse for electroacupuncture treatment of the hind limbs (Figure 1B).
- Check the integrity of the hollow plastic tubing and install the closure tabs. Move the closure tabs in the tube body as the screws slide in the grooves, ensuring that it can adjust the fixation length to suit the length of the mouse.
- Turn on the electroacupuncture therapy instrument and make sure it is operational. Each button can be adjusted by pressing it once (Figure 1C).
- Prepare the electroacupuncture therapy instrument to be placed next to the hollow plastic tubes for conducting electroacupuncture treatments on mice. The electroacupuncture therapy instrument consists of an electroacupuncture therapy machine and several pairs of energizing clips; each pair of energizing clips can provide two poles of energizing.
- When using the clips to clip the needle handle to energize the needle, use a clip to fix the needle.
- The electroacupuncture therapy machine has 11 buttons. Use the upper 5 to set the frequency, time, and waveform of electroacupuncture and are used from left to right for frequency, time, continuous wave, sparse and dense wave, and intermittent wave. Use the lower 6 to set the current strength of electroacupuncture. Each of these buttons has a notch underneath it for attaching energizing clips; the number of energizing clips can increase or decrease with the number of mice, up to a maximum of 6.
2. Establishing a mouse model of diabetic cardiomyopathy
- Divide the 30 mice into 3 groups by the random number table method: a control group, a model group, and a pretreatment group, with 10 mice in each group. Subject a total of 20 mice in the model group and pretreatment group to establish the model of diabetic cardiomyopathy. Treat the pretreatment group with Biaoben acupoints electroacupuncture.
NOTE: The model was built over a long period of time, and significant cardiomyopathy develops after 16 weeks. Our treatment time is from day 36 to day 70 (week 6 to week 10). This treatment was selected after the establishment of diabetes but before significant myocardial damage. At the end of 6 weeks, the mouse heart was removed and found to have significant diabetic cardiomyopathy. Therefore, the selection of treatment at this time point belongs to the category of pretreatment. - Feed mice in the control group with normal chow for 16 weeks. Feed mice in the model group and pretreatment group with a high-fat and high-sugar diet for 16 weeks to induce a metabolic state closely resembling the diabetic condition. The high-fat and high-sugar feed composition is 67.0% standard chow, 10.0% lard, 2.5% cholesterol, 20.0% sucrose, and 0.5% bile salts.
- Inject intraperitoneally Streptozotocin solution to establish a mouse diabetic cardiomyopathy model.
- Prepare the 1% solution of Streptozotocin on day 29. Dissolve Streptozotocin in a buffer prepared by mixing citric acid with trisodium citrate (pH = 4.4).
- Provide just water and no food for 12 h on day 29. Then, give each mouse a single intraperitoneal injection of 150 mg/kg of Streptozotocin solution as described below.
- Grab the mouse by the tail with the right hand and move it out of the cage. Place the mouse on the cage's surface and gently pull its tail out. Immobilize the mouse with the left hand and flip it over to expose the abdomen.
- Inject the mouse intraperitoneally using the right hand with a 1 mL syringe prepared with the appropriate amount of the Streptozotocin solution already withdrawn.
- Perform acupuncture treatment by Biaoben acupoints.
- Select the acupoints according to the theory of Biaoben acupoints13 including bilateral PC6 and ST36. For the pretreatment group, perform acupuncture on day 36, once every other day for a total of 18 treatments, with the treatment lasting a total of 5 weeks (from day 36 to day 70; Figure 2).
- Biao acupoint: Select bilateral PC6 as the Biao acupoint; the PC6 is located on the medial side of the forelimb, between the ulnar-radial sutures about 3 mm from the wrist joints. Ben acupoint: Select bilateral ST36 as Ben acupoints; the ST36 is located at the posterior-lateral aspect of the knee joint, approximately 2 mm below the fibular tuberosity.
- Grasp the mouse, pinch its tail, and place it on the cage's surface. Keep the mouth of the acrylic hollow plastic tube at an angle of 30° to the horizontal plane, with the small hole in the center facing the ground and the mouth of the tube aligned with the mouse's head.
- Accommodate the mouse's head to the mouth of the tube. Slowly rotate the mouse's head into the tube using voluntary movement and a slight push. Gently pat the mouse's buttocks so that the mouse is autonomously and completely pushed into the tube.
- Adjust the closure tabs, lift the mouse's tail out of the groove, tighten the nut, and use the closure tabs at the upper and lower ends to restrict the mouse's movement. At this point, the mouse can no longer move freely in the tube body.
- Use tweezers to gently pull out the four limbs of the mouse from each of the four small holes on both sides. After the four limbs are pulled out of the small holes, gently secure them to the outside of the tube body. At this point, the mouse's four limbs could no longer be displaced.
- Hold the disposable stainless-steel needle handle (0.3 mm x 13 mm) with the thumb and index finger of the right hand and hold the acrylic tube and the immobile mice in it with the left hand.
- Touch and locate where PC6 and ST36 are situated with the middle finger of the right hand, then gently prick the skin of the mouse with the needle tip. Quickly insert the needle injected obliquely around 3 mm and rapidly remove the right hand.
- Select the acupoints according to the theory of Biaoben acupoints13 including bilateral PC6 and ST36. For the pretreatment group, perform acupuncture on day 36, once every other day for a total of 18 treatments, with the treatment lasting a total of 5 weeks (from day 36 to day 70; Figure 2).
- Perform electroacupuncture pretreatment
- Turn on the electroacupuncture therapy machine and adjust to intermittent wave, 1 Hz. For each mouse, carry out electroacupuncture treatment for 15 min.
- Use the matching energized clip of the electroacupuncture therapy machine to hold the needle handle, one clip for one needle handle.
- Adjust the current of the button with the clip attached to 1 mA (Figure 3).
- Wait for 15 min and observe the state of the mice during the electroacupuncture pretreatment.
- At the end of the treatment, release the clip and remove the acupuncture needle. Adjust the closure tabs and release the mice from the electroacupuncture fixation device.
- Examine the whole body of the mice and observe if the mice are injured or have any abnormalities.
- Perform acupuncture treatments every other day after 7 days of injection of streptozotocin solution for a total of 18 treatments on a mouse electroacupuncture fixture from day 36 to day 70.
- End experiment on day 112 (end of week 16).
- Anesthetize by intraperitoneal injection of 2% pentobarbital sodium and confirm anesthesia by toe pinch response. Measure the cardiac function of the mice using an echocardiography.
- Record left ventricular end-diastolic diameter (LVEDD), left ventricular end-systolic diameter (LVESD), left ventricular ejection fraction (LVEF), and left ventricular fractional shortening (LVFS).
- Euthanasia the mice by cervical dislocation. Remove the heart, wash in physiological saline, and immerse in 4% paraformaldehyde.
- Anesthetize by intraperitoneal injection of 2% pentobarbital sodium and confirm anesthesia by toe pinch response. Measure the cardiac function of the mice using an echocardiography.
3. Hematoxylin-eosin (HE) staining
- Dehydrate, embed, slice, dewax the heart, and perform HE staining as per standard procedure.
- Observe the pathological changes of mouse heart tissue under a light microscope.
4. Observations on mice
- Measure blood sugar using a blood glucose meter on days 27, 36, and 49.
- Observe how much the mice eat, quantify their water intake and urination volume every day, and record their weight changes.
5. Statistical analysis
- Collect blood glucose data and use one-way ANOVA to identify statistical significance. Establish statistical significance as p < 0.05.
- Quantify the data by assistants who are blind to the experimental conditions. Express the data as mean ± standard deviation.
תוצאות
We followed the procedure described above and established a mouse model of diabetic cardiomyopathy. As can be observed in the HE-stained pictures (Figure 4), the mice in the control group had intact myocardial tissue structure, regular arrangement of cardiomyocytes, and no obvious lesions. In the model group, the myocardial cells were irregularly arranged, some myocardial cells were damaged and disintegrated (black arrow), and obvious inflammatory cell infiltration was observed in the interstitium (yellow arrow). It is suggested that the mice in the model group have myocardial injury. In the pretreatment group, the myocardial tissue structure of the mice was clear, the myocardial cells were arranged irregularly, a small number of myocardial cells were damaged and disintegrated (black arrows), and no other obvious lesions were observed. In comparison, there was no obvious lesion in the myocardial tissue of the control group, and the myocardial tissue injury in the model group was more serious. The degree of myocardial tissue injury in the pretreatment group was significantly lower than that in the model group. This suggests that the pretreatment treatment can reduce myocardial injury.
The cardiac function indexes of echocardiography (Figure 5) showed that compared with the control group, the left ventricular end-diastolic volume and left ventricular end-systolic volume in the model group were significantly increased, and the left ventricular ejection fraction and left ventricular fractional shortening in the model group were significantly decreased. The difference was statistically significant (P < 0.05). It is suggested that the mice in the model group have decreased myocardial systolic and diastolic function, and cardiac morphology and structure were abnormal24,25. Compared with the model group, the left ventricular end-diastolic diameter and left ventricular end-systolic diameter in the pretreatment group were significantly reduced, and the left ventricular ejection fraction and left ventricular fractional shortening in the pretreatment group were significantly increased. The difference was statistically significant (P < 0.05). It is suggested that the cardiac function of mice in the pretreatment group was improved. This showed that the diabetic cardiomyopathy model was successfully established in the model group and the pretreatment treatment can improve cardiac function26.
Assemble the mouse electroacupuncture fixation device and connect the electroacupuncture therapy instrument to perform the electroacupuncture, as shown in Figure 3. The mice were confined in acrylic plastic tubes during the needling of bilateral PC6 and ST36, and the transparent tubes allowed the mice to be observed at all times.
During the treatment, mice could be observed quietly receiving electroacupuncture in the fixation device. After being released, the mice had no stress reaction, did not tear, and did not run quickly in the cage. This suggests the safety and repeatability of the mouse electroacupuncture fixation device.
After 7 days of injection of streptozotocin solution, mice in the model group and pretreatment group showed symptoms associated with diabetes, such as excessive drinking water, eating, urinating, and weight loss. Compared with the control group, the blood glucose of the model and pretreatment groups was significantly higher on day 36, and the difference was significant (p < 0.05), which indicated that the modeling of diabetic heart disease mice was successful. After completing the electroacupuncture regime, compared with the model group, the blood glucose of mice in the pretreated group decreased significantly on day 49. The difference was significant (p < 0.05), which indicated that the blood glucose was significantly reduced after Biaoben acupoints electroacupuncture pretreatment (Figure 6). This showed that pretreatment therapy can effectively control blood glucose levels, improve hyperglycemia to alleviate myocardial cell damage and improve diabetic cardiomyopathy. It also proved the feasibility of mouse electroacupuncture fixation device assisted electroacupuncture treatment.
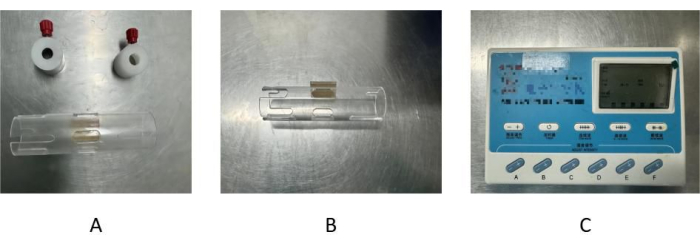
Figure 1: Composition of the electroacupuncture fixation device. (A) The acrylic hollow plastic tube consists of a tube body and closure tabs located in the openings at each end. The closure tabs are secured in the grooves at each end by manually rotatable screws. The tube is 11 cm long and 3 cm in diameter. (B) There are four oval holes symmetrically distributed on both sides of the center groove. (C) The electroacupuncture machine has 11 buttons. The upper 5 are used to set the frequency, time, and waveform of electroacupuncture, and from left to right: frequency, time, continuous wave, sparse and dense wave, and intermittent wave. The lower 6 are used to set the current strength of electroacupuncture. Please click here to view a larger version of this figure.
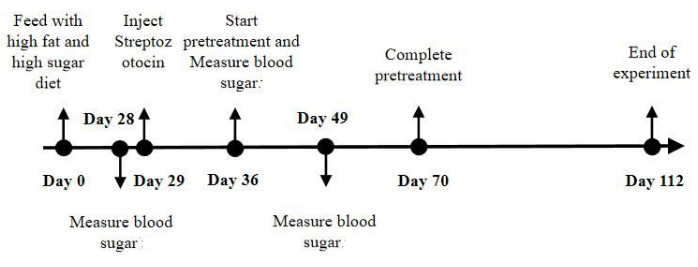
Figure 2: Flow and time points of the experiment. The mice in the control group were fed normal chow for 16 weeks. The mice in the model group and the pretreatment group were fed a high-fat and high-sugar diet for 16 weeks and injected with Streptozotocin solution on day 29. Followed by acupuncture treatments every other day, for a total of 18 treatments on the mouse electroacupuncture fixation device from day 36 to day 70. And we ended the experiment on day 112. In order to verify the feasibility of the electroacupuncture fixation device in mice, we detected the blood glucose of mice in each group on day 29, day 36, and day 49. Please click here to view a larger version of this figure.
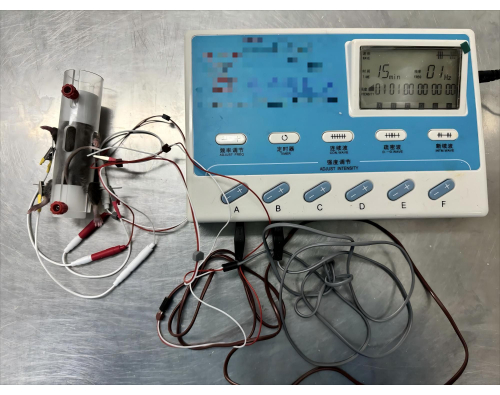
Figure 3: Electroacupuncture of mice using an electroacupuncture fixation device. Start the electroacupuncture therapy machine, adjust the current to 1 mA, intermittent wave, 1 Hz, and 15 min. Please click here to view a larger version of this figure.
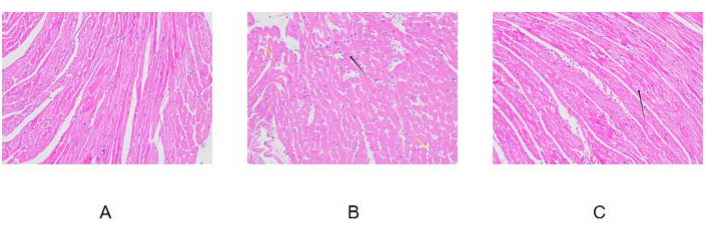
Figure 4: HE staining results. (A) Control group; (B) Model group; (C) Pretreatment group. Images were taken at 200x. Please click here to view a larger version of this figure.

Figure 5: Cardiac function indexes of mice in each group. (A) Left ventricular end-diastolic diameter (LVEDD); (B) left ventricular end-systolic diameter (LVESD); (C) left ventricular ejection fraction (LVEF); (D) left ventricular fractional shortening (LVFS). The graph shows mean ± standard deviation (n = 10). Statistical significance was calculated using one-way ANOVA; compared with the control group, ##P < 0. 01; compared with the model group, **P < 0.01. Please click here to view a larger version of this figure.

Figure 6: Changes in blood glucose in each group of mice on days 27, 36, and 49. The graph shows mean ± standard deviation (n = 10). Statistical significance was calculated using one-way ANOVA; compared with the control group, ##P < 0. 01; compared with the model group, **P < 0.01. Please click here to view a larger version of this figure.
Discussion
In this study, mice that had high blood glucose and showed signs of diabetes were obtained by intraperitoneal injection of the Streptozotocin solution, which is an effective and recognized method of modeling27. PC6 was selected as the Biao point, and ST36 was selected as the Ben point according to the theory of Biaoben acupoints28. All of these points are on the limbs of the mouse, so the acrylic tubes that can expose the abdomen and immobilize the limbs are well suited for needling these points. The mice were introduced into the tube body and fixed, and the electroacupuncture device was turned on for electroacupuncture treatment after needling. It can be observed that the local muscles treated with electroacupuncture appear to be slightly shaking, which is normal and has no side effects.
Acupuncture, as an important tool in traditional Chinese medicine, treats diabetic cardiomyopathy by regulating glycolipid metabolism and improving inflammatory response29,30. Electroacupuncture is one of the commonly used and quantifiably implemented methods of acupuncture therapy and is widely used in the clinical treatment of diabetic cardiomyopathy31. Electroacupuncture delivers electrical signals in the form of intermittent waves to the acupuncture site to continuously provide stimulation to the acupuncture points and improve the therapeutic effect of acupuncture. In this study, PC6 was selected as the Biao point, and ST36 was selected as the Ben point. Among them, PC6 can improve myocardial remodeling and systolic and diastolic function in mice with diabetic cardiomyopathy, and ST36 can reduce oxidative stress, improve insulin resistance, and increase the level of glucose and lipid metabolism. Combined with electroacupuncture therapy, it achieves the purpose of treating diabetic cardiomyopathy32. In this experiment, mice in the electroacupuncture pretreatment group showed lower blood glucose and reduced diabetic symptoms such as excessive drinking, eating, and urination compared to mice in the control group from day 36 to day 49. It shows that pretreatment can control blood glucose level and reduce blood glucose to alleviate myocardial injury. During the 2 week treatment, it was observed that the mouse electroacupuncture device was more convenient and safer than the traditional mouse coat, which greatly improved the treatment efficiency. It shows the feasibility of a mouse electroacupuncture fixation device in assisting electroacupuncture therapy.
Currently, mice are immobilized during electroacupuncture treatment by using homemade mouse suits to restrain the mice, which are inconvenient to make and use33. The electroacupuncture fixation device can quickly and gently immobilize the limbs of mice for electroacupuncture treatment. This device is faster, safer, and less likely to agitate the mice, avoiding irritation, biting, and death, thus protecting the welfare of the animals and the safety of the experimenters. If a mouse develops an abnormal condition, such as intense scratching of the inner wall of the tube or body twitching, unfasten the immobilizer and remove the mouse, place the mouse on a flat tabletop or mouse cage frame, and calm the mouse, then the abnormality will be quickly resolved. In addition, if acupuncture points in other parts of the body, such as the back, are needed for needling, this can be accomplished by simply changing the position of the acrylic tubes at the time of entry of the mice. The limitation of this device is that it does not work well for needling acupoints located on the head, as the mice are confined in the tube body, exposing only the acupoints at the abdomen, back, and limbs and the head is encased in an acrylic tube and a closure sheet. In addition, this device is suitable for mice rather than for large animals such as rabbits and dogs. In the future, this device can be implemented for widespread use in order to make it easier to treat mice with electroacupuncture, improve the efficiency of experiments, and protect the safety of both the mice and the experimenter.
In summary, in this experiment, we provide a detailed description of a methodology to fabricate a mouse electroacupuncture fixation device. The device exposes and immobilizes acupuncture points on the abdomen and limbs of mice in order to deliver electroacupuncture therapy. The use of a mouse electroacupuncture fixation device helps to better use and analyze electroacupuncture treatment for diabetic cardiomyopathy mice and provides more possibilities for exploration of acupuncture treatment for diabetic cardiomyopathy.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work is supported by a grant from Hubei Natural Science Foundation joint project 2022CFD024; National Natural Science Foundation of China,No. 81704142; Hubei University of Traditional Chinese Medicine 2023 double first-class construction key special scientific research project,2023ZZXT005;To study the mechanism of Biaoben acupoints electroacupuncture preconditioning on regulating ferroptosis of myocardial cells in rats with myocardial ischemia-reperfusion injury (MIRI) under the key project of Hubei Provincial Administration of Traditional Chinese Medicine,ZY2025D001; Knowledge Innovation Project of Wuhan Science and Technology Bureau, No. 2023020201010172; Hubei Shi Zhen Talent Engineering Project, E Wei Han [2024]256.
Materials
| Name | Company | Catalog Number | Comments |
| acrylic hollow plastic | Henan Zhike | ZK-GDQ | |
| acupuncture needle | Hwato | 0.3 mm x 13 mm | |
| C57BL/6 mice | Hubei Provincial Center for Disease Control and Prevention | ||
| citric acid monohydrate | Sigma | C1909 | |
| electroacupuncture therapy instrument | Hwato | SDZ-V | |
| electronic balance | Henan Zhike | ZK-DST | |
| glucose meters | LifeScan | OneTouch VerioVue | |
| glucose test strip | LifeScan | OneTouch Verio | |
| streptozotocin | Sigma | S0130 | |
| trisodium citrate dihydrate | Sigma | W302600 | |
| tweezers | Labshark | 130302001 |
References
- Dillmann, W. H. Diabetic cardiomyopathy. Circ Res. 124 (8), 1160-1162 (2019).
- Murtaza, G., et al. Diabetic cardiomyopathy - a comprehensive updated review. Prog Cardiovasc Dis. 62 (4), 315-326 (2019).
- Peng, M. L., et al. Signaling pathways related to oxidative stress in diabetic cardiomyopathy. Front Endocrinol. 13, 907757 (2022).
- Tong, M., et al. Mitophagy is essential for maintaining cardiac function during high fat diet-induced diabetic cardiomyopathy. Circ Res. 124 (9), 1360-1371 (2019).
- Jia, G., Demarco, V. G., Sowers, J. R. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol. 12 (3), 144-153 (2016).
- Nakamura, K., et al. Pathophysiology and treatment of diabetic cardiomyopathy and heart failure in patients with diabetes mellitus. Int J Mol Sci. 23 (7), 3587 (2022).
- Ye, Y., et al. Acupuncture reduces hypertrophy and cardiac fibrosis, and improves heart function in mice with diabetic cardiomyopathy. Cardiovasc Drugs Ther. 34 (6), 835-848 (2020).
- Zhou, X. L., et al. Effect of acupuncture-moxibustion stimulation of combined "biao-ben" acupoints on autonomic nervous activity and related factors in rats with irritable bowel syndrome diarrhea. Zhen Ci Yan Jiu. 48 (7), 635-642 (2023).
- Chen, Q., Liang, F., Wu, S., Lu, W., Wang, H. Theoretic exploration and clinical application of acupoint combination based on biaoben theory. Zhongguo Zhen Jiu. 38 (5), 5053-5059 (2018).
- Chen, J., et al. Protective effect and mechanism of electroacupuncture of "biao-ben" acupoints combination for mitochondrial dysfunction in diabetic nephropathy rats. Zhen Ci Yan Jiu. 47 (9), 759-768 (2022).
- Wang, K. X., et al. Effect of electroacupuncture of "biao-ben" acupoints on renal function and hemorheology and enos level in patients with early diabetic nephropathy. Zhen Ci Yan Jiu. 47 (1), 46-52 (2022).
- Zhang, M., et al. Acupuncture at pc6 prevents cardiac hypertrophy in isoproterenol-treated mice. Acupunct Med. 37 (1), 55-63 (2019).
- Chen, Q., et al. Protective effect and metabonomics research of "biao-ben acupoint combination" electroacupuncture in chronic myocardial ischemia model rats. Zhen Ci Yan Jiu. 43 (11), 698-704 (2018).
- Pulinilkunnil, T., et al. Cardiac-specific adipose triglyceride lipase overexpression protects from cardiac steatosis and dilated cardiomyopathy following diet-induced obesity. Int J Obes (Lond). 38 (2), 205-215 (2014).
- Sverdlov, A. L., et al. High fat, high sucrose diet causes cardiac mitochondrial dysfunction due in part to oxidative post-translational modification of mitochondrial complex ii. J Mol Cell Cardiol. 78, 165-173 (2015).
- Marino, F., et al. Streptozotocin-induced type 1 and 2 diabetes mellitus mouse models show different functional, cellular and molecular patterns of diabetic cardiomyopathy. Int J Mol Sci. 24 (2), 1132 (2023).
- Ren, B. C., et al. Curcumin alleviates oxidative stress and inhibits apoptosis in diabetic cardiomyopathy via sirt1-foxo1 and pi3k-akt signalling pathways. J Cell Mol Med. 24 (21), 12355-12367 (2020).
- Wang, H., et al. Inhibition of fatty acid uptake by tgr5 prevents diabetic cardiomyopathy. Nat Metab. 6 (6), 1161-1177 (2024).
- Wang, R., et al. Fibroblast growth factor 21 improves diabetic cardiomyopathy by inhibiting ferroptosis via ferritin pathway. Cardiovasc Diabetol. 23 (1), 394 (2024).
- Xu, H., et al. Types of cell death in diabetic cardiomyopathy: Insights from animal models. Acta Biochim Biophys Sin (Shanghai). , (2024).
- Miyamoto, T., et al. Repeated cold stress enhances the acute restraint stress-induced hyperthermia in mice. Biol Pharm Bull. 40 (1), 11-16 (2017).
- Zhang, H. X., Wang, D. J., Yu, J. C., Han, J. X. Effect of the restriction on the efficacy of acupuncture in mice. Zhen Ci Yan Jiu. 34 (6), 429 (2009).
- Chen, H. C., et al. Sub-acute restraint stress progressively increases oxidative/nitrosative stress and inflammatory markers while transiently upregulating antioxidant gene expression in the rat hippocampus. Free Radic Biol Med. 130, 446-457 (2019).
- Marwick, T. H., et al. Echocardiographic phenotypes of diabetic myocardial disorder: Evolution over 15 months follow-up in the arise-hf trial. Cardiovasc Diabetol. 24 (1), 16 (2025).
- Fiordelisi, A., et al. L-arginine supplementation as mitochondrial therapy in diabetic cardiomyopathy. Cardiovasc Diabetol. 23 (1), 450 (2024).
- Li, J. P., et al. Nlrp3 inflammasome-modulated angiogenic function of epc via pi3k/ akt/mtor pathway in diabetic myocardial infarction. Cardiovasc Diabetol. 24 (1), 6 (2025).
- Racine, K. C., et al. The high-fat diet and low-dose streptozotocin type-2 diabetes model induces hyperinsulinemia and insulin resistance in male but not female c57bl/6j mice. Nutr Res. 131, 135-146 (2024).
- Wu, F., et al. Effects of moxibustion of "biaoben acupoint combination" on heart rate variability, atrial natriuretic peptide in the model rats of ibs-d complicated with anxiety. Zhongguo Zhen Jiu. 43 (10), 1139-1147 (2023).
- Zhang, S., et al. Research progress on the mechanism of acupuncture on type II diabetes mellitus. Zhen Ci Yan Jiu. 49 (6), 641-649 (2024).
- Zhang, Z., et al. Acupuncture-assisted lifestyle intervention improve the metabolic status and spontaneous brain activity of type 2 diabetes mellitus patients: A randomized, clinical trial. Diabetol Metab Syndr. 16 (1), 255 (2024).
- Jia, X., et al. "Adjusting internal organs and dredging channelon" electroacupuncture glycolipid metabolism disorders in nafld mice by mediating the ampk/acc signaling pathway. Diabetol Metab Syndr. 16 (1), 173 (2024).
- Li, S., Wang, S., Feng, X., Chang, Y., Yan, D. Biao and ben acupoints regulate mitochondrial function through p2x receptor to improve myocardial ischemia. Altern Ther Health Med. 29 (4), 43-51 (2023).
- Guo, J., et al. A new mouse-fixation device for iop measurement in awake mice. Vision Res. 219, 108397 (2024).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved