Method Article
Isolation, Purification, and Identification of Bacitracin-producing Bacillus licheniformis from Fresh Feces of Healthy Pigs
In This Article
Summary
This protocol provides a complete and comprehensive description of the detailed process for the isolation, purification, and identification of bacitracin-producing Bacillus licheniformis from healthy pig feces.
Abstract
Bacillus licheniformis and bacitracin have a huge application market and value in the fields of medicine, chemistry, aquaculture, agricultural, and sideline products. Therefore, the selection of B. licheniformis with high production of bacitracin is of great importance. In this experimental protocol, Bacillus with a high yield of bacitracin was isolated, purified, and identified from the fresh feces of healthy pigs. The inhibitory effect of secondary metabolite bacitracin on Micrococcus luteus was also tested. Thin-layer chromatography and high-performance liquid chromatography were used for the qualitative and quantitative detection of bacitracin. The physiological and biochemical characteristics of B. licheniformis were determined by relevant kits. The phylogenetic relationships of B. licheniformis were determined and constructed using gene sequence detection. This protocol describes and introduces the standard isolation, purification, and identification process of B. licheniformis from animal fresh feces from multiple perspectives, providing a method for the large-scale utilization of B. licheniformis and bacitracin in factories.
Introduction
Bacillus licheniformis is a species of Bacillus in the family Firmicutes, which is widely distributed in various environments such as water, soil, and animal intestines1. B. licheniformis has a short and stout rod-like structure and moves individually2. The colony is nearly round and dull, with a central bulge and neat edges of grayish white3. It has strong growth and reproduction ability and can absorb and utilize nutrients from various carbon sources, such as monosaccharides, polysaccharides, ketose, and organic acids4. In the later stage of growth and development, B. licheniformis can exist in the form of dormant spores and produce antibacterial substances such as bacitracin, lichenysin, and surfactin. It can also resist nutritional deficiency and extreme external environment5. There is no obvious codon preference, and the efficient secretion system determines the heterologous protein secretion of B. licheniformis, which is twice that of Bacillus subtilis6. It is often used to produce enzyme preparations such as protease, amylase, and cellulase7. Because of its lack of endogenous toxins, it is certified as a food-safe strain and listed on the QPS by EFSA8. Therefore, there are several potential uses, including bioactive compound production, which have a wide range of applications in aquaculture, agriculture, food, biomedicine, and pharmaceutical industries. Also, B. licheniformis is an important component of animal intestinal flora, which can promote animals to improve production performance, improve intestinal flora balance, and prevent diseases. The entire genome of B. licheniformis ATCC14580 was analyzed in 2004, and the background information of transcription translation, protein folding, and secretion mechanism has been gradually understood9. This genetic information makes it conducive for genetic modification at the molecular level, contributing to facilitating the large-scale production of B. licheniformis.
Bacitracin is a dodecacyclic peptide antibiotic produced by non-ribosomal peptide synthetase by secondary metabolism in B. subtilis and B. licheniformis. Bacitracin is a mixture composed of various components such as bacitracin A, B, and C, where one or two amino acids differ between each component; among these, bacitracin A has the strongest biological activity10. Bacitracin can inhibit gram-positive bacteria such as Staphylococcus and Micrococcus luteus and some gram-negative bacteria by inhibiting cell wall formation and interacting with membrane-binding proteins11. Meanwhile, bacitracin is safe and stable, not easy to produce drug resistance, and can be compatible with other antibacterial drugs12. Therefore, bacitracin is used in medical and veterinary practice. In addition, because of its fast elimination rate and low absorption rate, it can also be used as an additive for animal feed13.
B. licheniformis can colonize the intestine and improve the gastrointestinal microenvironment. The adhesion and reproduction ability and related physiological functions of Bacillus from different sources in the gastrointestinal tract of different animals are different. Pig-derived B. licheniformis is more conducive to colonization in the intestines of pigs and other livestock. There is a close relationship between the relative abundance of intestinal probiotics and the health status of host14. Dietary supplementation with B. licheniformis mix in weaned piglets improves the intestinal ecosystem by changing microbiota composition and metabolic activity, and also effects the intestinal mucosa15. Animal feces can reflect the type and quantity of animal intestinal flora. This protocol describes the isolation and purification of bacitracin-producing Bacillus spp. from healthy pig feces. The feces are derived from Taihu sows that are not fed with compound feed and have excellent production performance in pig farms. The isolates were identified as B. licheniformis based on their morphological characteristics, physicochemical properties, and biochemical identification.
Protocol
All experimental procedures were documented and approved by the Ethics Committee of Nanjing Tech University. The feces were derived from Taihu sows about 2 years old (see Table of Materials), which were raised on professional and standard pig farms.
1. Preparation of media
- Luria-Bertani (LB) liquid medium: Add 10 g of NaCl, 5 g of yeast extract powder, and 10 g of tryptone into a conical bottle, add distilled water to 1 L, stir, and dissolve with a glass rod. Transfer 100 mL of medium to a 500 mL conical bottle and tie it tightly with breathable sealing film. Sterilize at 121 °C in an autoclave for 20 min(see Table of Materials).
- LB solid medium: Add 10 g NaCl, 5 g yeast extract powder, 10 g tryptone, 20 g agar powder to a conical bottle, add distilled water to 1 L, dissolve with a glass rod, and tie tightly with a breathable sealing film. Sterilize at 121 °C in an autoclave for 20 min. When the medium is cooled to 50 °C, pour 20 mL of the medium into Petri dishes, allow them to cool, and then store them upside down.
- Bacitracin fermentation medium: Add 8 g soybean meal, 4 g soluble starch, 0.1 g (NH4)2SO4, 0.6 g CaCO3, and 100 mL of distilled water to a 500 mL conical bottle, stir with a glass rod to dissolve thoroughly, and tie tightly with a breathable sealing film. Sterilize at 121 °C in an autoclave for 20 min(see Table of Materials).
- M. luteus suspension: Select a ring of M. luteus and inoculate on an LB culture plate, prepared in step 1.2 (see Table of Materials). Culture in a constant temperature incubator at 37 °C for 16 h (see Table of Materials). Add 5 mL of normal saline to the plate and shake gently to elute the colonies. Measure the OD600 and dilute the solution in a test tube to get a suspension with OD600 of 0.3-0.7. Store the suspension of M. luteus at 4 °C for later use.
- Antibacterial activity assay medium: Add 1 mL of M. luteus suspension, prepared in step 1.4, to 100 mL of liquid LB solid medium prepared in step 1.2 and shake slightly. Immediately take 5 mL of this solution containing M. luteus and spread it evenly on the LB culture plate.
2. Isolation and purification of Bacillus from fresh feces of healthy pigs
- Add 9 mL of normal saline and 1.0 g fresh pig feces into a 150 mL erlenmeyer flask. Stir with a magnetic stirrer (see Table of Materials) at room temperature at 60 rpm/min for 15 min.
- Put the fecal extract in an 80 °C thermostat water bath (see Table of Materials) for 20 min to kill non-spore-producing bacteria.
- Add the suspension to 100 mL of LB liquid medium in a 250 mL Erlenmeyer flask and culture in a shaker (see Table of Materials) at 180 rpm/min at 37 °C for 8 h.
- Perform serial dilution of the cultured bacterial solution with normal saline from 10-1 to 10-8. Apply 0.1 mL of 10-6, 10-7, and 10-8 bacterial diluent to individual LB culture plates and let dry. After 10 min, place plates upside down in a constant temperature incubator at 37 °C for 48 h.
- Use the inoculation ring to pick the colonies that look similar to B. licheniformis (creamy white color, soft consistency, mucoid appearance, convex, and flat elevation, and irregular shape)16and streak them using a zigzag pattern on antibacterial activity assay medium prepared in step 1.5 (see Figure 1).
- Put the plates into the incubator at 37 °C for 48 h and observe whether there is an inhibition zone around the colony.
3. Screening for inhibition of M. luteus activity
- Select a single colony that generated an inhibitory zone and add to 100 mL of LB liquid medium, culture in the shaker at 37 °C and 180 rpm/min for 12 h.
- Inoculate 5 mL of the culture medium into the bacitracin fermentation medium prepared in step 1.3 and culture at 37 °C for 48 h.
- Collect the fermentation broth in a centrifuge tube and centrifuge at 13,400 x g at 4 °C for 15 min, and then filter using a 0.22 µm microporous filter membrane (see Table of Materials).
- Add an Oxford cup to a fresh antibacterial activity assay medium plate and then add 50 µL of filtrate (fermentation supernatant) to the Oxford cup (see Table of Materials). Culture in an incubator at 37 °C for 12 h. After incubation, measure the diameter of the inhibitory zone with a vernier caliper (see Table of Materials).
- Select the strains and the corresponding fermentation supernatant with an inhibitory zone diameter greater than 15 mm for subsequent experiments.
4. Identification of bacitracin by thin-layer chromatography
- Add 60 mg of bacitracin standard and dissolve in 1% EDTA-2Na (see Table of Materials) in a 10 mL volumetric bottle to make a 6.0 mg/mL bacitracin solution.
- Dilute 1 mL of the fermentation supernatant to 10 mL with 1% EDTA-2Na. Spot 5 µL of sample and 5 µL of bacitracin standard solution on the activated silica gel thin layer plate (see Table of Materials).
- Add 100 mL of n-butanol-acetic acid-water-pyridine-ethanol (60:15:10:6:5) to the chromatographic tank (see Table of Materials). Place the thin layer plate of silica gel into the chromatographic tank. Cover and let it sit at room temperature for 30 min.
- Take out the silica gel thin layer plate, spray butanol-pyridine solution (99:1) containing 1% ninhydrin (see Table of Materials), and heat at 105 °C for 5 min until brownish red spots appear. Calculate Rf (Retention Factor Value) for the standard and the sample according to the following formula:
Rf = Spot travel distance/Solvent travel distance.
5. Detection of bacitracin by HPLC
- Add 0.3 mL of fermentation supernatant and 1.2 mL of 50% ethanol into a 2 mL microcentrifuge tube, shake for 5 min by hand, and let stand at 4 °C for 12 h.
- Centrifuge at 13,400 x g for 15 min and filter into a sample bottle labeled as S1 with a 0.22 µm microporous filter membrane (see Table of Materials).
- Preparation of bacitracin mother solution: Add 0.4 g bacitracin standard (60 U/mg; see Table of Materials), 100 mL of 40 g/L EDTA-2Na (see Table of Materials) into a 100 mL volumetric flask to prepare 240 U/mL bacitracin mother solution.
- Add 3 mL of bacitracin mother solution and 21, 9, 3, 1, and 0 mL of ultrapure water to test tubes labeled A1, B1, C1, D1, and E1, respectively. After filtration with 0.22 µm microporous filter membrane, put the filtrate into sample bottles labeled A2, B2, C2, D2, and E2.
NOTE: The concentrations of bacitracin standard solution are 30, 60, 120, 180, and 240 U/mL. - Place A2, B2, C2, D2, E2 and S1 into the test tray of the HPLC instrument. Place 1000 mL of 50 mM/L ammonium formate and 1000 mL of acetonitrile in the mobile phase tray and set them as mobile phases A and B, respectively.
NOTE: The pH of ammonium formate should be adjusted to 4.0 with formic acid. - Install C18 (5 µm, 4.6 mm x 250 mm) HPLC column in the direction indicated on the column (see Table of Materials).
- Set the mobile phase parameters as 0 min, 77% A and 23% B; 30 min, 70% A and 30% B; 40 min, 60% A and 40% B; 50 min, 60% A and 40% B; 51 min, 77% A and 23% B; 58 min, 77% A and 23% B. Set sample size as 100 µL; Flow rate, 1.0 mL/min; Column temperature, 30 °C.
- Click the Run button to execute the program.
6. Morphological identification
- Dilute and coat the strain with the highest bacitracin titer on an LB culture plate and culture in an incubator at 37 °C for 48 h. Stain and observe colony morphology as described below.
- Add a drop of sterile purified water to the slide, pick up a small amount of colonies, and add to the water droplet. Smear evenly, let the slide dry naturally, and then fix over a flame.
- Add crystal violet for 1 min to stain the sample and then rinse with tap water. Add iodine for 1 min to stain the sample and then rinse with distilled water.
- Add 95% alcohol, shake the slide to decolorize for 1 min, and then rinse with distilled water.
- Add saffron for 1 min to stain the sample and then rinse with distilled water. Let the slide dry naturally and observe the colony morphology under a microscope with an oil immersion (see Table of Materials).
7. Physiological and biochemical identification
- Add 2 mL of sterile saline into the test tube and inoculate a single colony from the plate into the sterile saline solution using an inoculation ring to obtain bacterial suspension.
- Add 100 µL of the bacterial suspension into each hole for measuring V-P, citrate, gelatin, 7% sodium chloride, pH 5.7, nitrate reduction, and starch hydrolysis on the Bacillus biochemical identification strip (see Table of Materials).
- Use the inoculation ring to pick another single colony and streak them in the zigzag pattern on the anaerobic growth biochemical tube of the Bacillus biochemical identification strip.
- Use the inoculation ring to pick the other colonies and puncture them vertically into the pores for propionate, D-xylose, L-arabinose, and D-mannitol identification on the Bacillus biochemical identification strip.
8. Determination of strain gene sequence
- Extract the DNA of the strain using a bacteria DNA extraction kit (see Table of Materials): Add 2 mL of seed liquid into a 5 mL microcentrifuge tube and centrifuge at 11,500 x g for 1 min. Discard the supernatant, and add 110 µL of buffer (containing 20 mM Tris, pH8.0; 2 mM Na2-EDTA, and 1.2% Triton) and 70 µL of 50 mg/mL lysozyme solution to the pellet and incubate at 37 °C for 30 min.
- Add 4 µL of 100 mg/mL RNase A for 15 s and leave for 5 min at room temperature. Add 20 µL of proteinase K solution and mix well.
- Add 220 µL of buffer GB, shake for 15 s until the pellet is suspended, place at 70 °C for 10 min to make the solution clear, and perform a short spin to remove the water beads on the tube wall.
- Add 220 µL of anhydrous ethanol, shock fully for 15 s, short separation. Transfer the solution and flocculation precipitation to the adsorption column CB3 of the collection tube, centrifuge at 13,400 x g for 30 s, and discard the supernatant.
- Add 500 µL of buffer GD into the adsorption column, centrifuge for 30 s at 13,400 x g, and discard the supernatant. Add 600 µL of PW to the column, centrifuge for 30 s at 13,400 x g, and discard the supernatant.
- Put the adsorption column back into the collection tube, centrifuge for 2 min at 13,400 x g, and discard the supernatant.
- Place the adsorption column at room temperature for 10 min to dry. Transfer the column to the centrifuge tube and add 100 µL of eluting buffer TE into the middle of the adsorption membrane.
- Leave at room temperature for 5 min to collect the flow through into the centrifuge tube and centrifuge for 2 min at 13,400 x g.
- Add 25 µL of 2x master mix (see Table of Materials), 2.5 µL of DNA sample obtained in step 8.8, 2 µL of 27F forward primer, 2 µL of 1492R reverse primer, and 18.5 µL of double distilled H2O to a 0.1 mL PCR tube.
NOTE: The gene sequences of the primers are 27F: 5'-AGAGTTTGATCCTGGCTCAG-3' and 1492R: 5'-GGTTACCTTGTTACGACTT-3'. - Place the PCR tube into the PCR machine (see Table of Materials) under the following conditions: pre-denaturation at 95 °C for 10 min, denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, extension at 72 °C for 90 s, extension at 72 °C for 7 min. Run the denaturation, annealing, and extension three steps cycle for 32x.
- Take 5 µL of the product and perform electrophoresis with 1% agarose gel at 120 V for 30 min.
- Dissolve 500 mg agarose in 50 mL of 1x TAE buffer. Heat the solution in the microwave for 1-2 min until the agarose is completely dissolved. Add 5 µL of DNA staining dye, then pour into a gel tray and insert the orifice plate. Let stand at room temperature for 30 min until it solidifies; put the gel into the electrophoresis tank and add 1x TAE buffer to submerge the gel. Add 2 µL of 2 kb DNA ladder (10 mg/µL) into the first well and 5 µL PCR product mixed with 1 µL of loading dye to the remaining wells in the gel.
- Place the gel in the gel imaging system and take a picture. Cut the DNA strips quickly under ultraviolet light and weigh.
- Add strips to a microcentrifuge tube and add an equal volume of PN solution. Keep the tube in water bath at 50 °C until the gel is fully dissolved.
- Add 500 µL of equilibrium liquid BL to the adsorption column CA2, centrifuge at 13,400 x g for 1 min, and discard the supernatant.
- Add the dissolved gel solution to the adsorption column CA2 and place it at room temperature for 2 min, centrifuge it at 13,400 x g for 30 s, and discard the supernatant.
- Add 600 µL of PW to the adsorption column CA2, centrifuge at 13,400 x g for 1 min, discard the supernatant, and repeat 1x.
- Place the adsorption column CA2 into a collection tube, centrifuge at 13,400 x g for 2 min, and discard the supernatant.
- Place the adsorption column CA2 at room temperature for 10 min to dry thoroughly and then put it into a clean centrifuge tube.
- Add 50 µL of eluting buffer EB to the adsorption film suspended at room temperature for 2 min, and collect the DNA solution by centrifugation at 13,400 x g for 2 min.
NOTE: The above-mentioned solutions or reagents are included in the Purification Kit (see Table of Materials).- Send the collected DNA solution to a professional sequencing company (see Table of Materials) for sequencing. Compare the sequencing results by Blast in the GenBank database of NCBI. According to sequence homology, select different strains and construct phylogenetic trees by the Maximum Likelihood method in MEGA-X to determine the species relationships of the strains.
תוצאות
In this experiment, 48 strains of Bacillus were isolated from fresh feces of healthy pigs, numbered from 1001 to 1048. Among them, 15 strains had antibacterial activity against M. luteus. From the 15 strains, the titers of bacitracin were measured by high-performance liquid chromatography, as shown in Table 1. Among them, B. licheniformis No. 1026 had the highest bacitracin titer, 456.35 ± 21.75 U/mL, so No. 1026 was selected for subsequent experiments.
The identification results by TLC are shown in Figure 2A. The position of the main spots in the fermentation broth of strain No. 1026 and the bacitracin standard solution was the same, and the Rf value was 0.61. For HPLC analysis, the standard curve equation of bacitracin is y = 50.287x - 250.55, and the correlation coefficient R2 = 0.9968 (Figure 2B). The peak time of fermentation supernatant was consistent with the bacitracin standard (Figure 2C), so the bacteriostatic substance was identified as bacitracin, and the titer of bacitracin was 456.35 ± 21.75 U/mL.
The colony of strain No. 1026 was cultured on LB medium for 12 h, as shown in Figure 3A. The colony was round and transparent in the shape of water droplets, with full protrusions and neat edges. From 12 h to 24 h, the bacteria entered the later stages of growth. As shown in Figure 3B, the colonies spread around in a plum shape, the center formed folded, and the color gradually deepened to milky white. It is consistent with the description of the colony morphology of B. licheniformis16. There was a transparent mucus at the edge. After Gram staining, strain No. 1026 was observed under the microscope, as shown in Figure 3C. The cells were purple rods, indicating Gram-positive bacteria.
As shown in Table 2, strain No. 1026 could grow in an anaerobic environment. The strain tested positive for V-P test, citrate utilization, nitrate reduction, and starch hydrolysis. The strain was able to decompose and utilize most of the carbon sources. It had the same physiological and biochemical characteristics as B. licheniformis.
The 16S rDNA fragment size of strain No. 1026 was about 1400 bp in size (Figure 4A). The sequencing results of strain No. 1026 showed 99.58% similarity to that of B. licheniformis DSM 13 in GenBank. The phylogenetic tree was then constructed, as shown in Figure 4B. The evolutionary branch length of B. licheniformis DSM 13 is 0.000, indicating that it is B. licheniformis.
Based on the morphological, physiological, and biochemical characteristics of strain No. 1026 and the homology analysis of the 16S rDNA gene sequence, strain No. 1026 was identified as B. licheniformis.
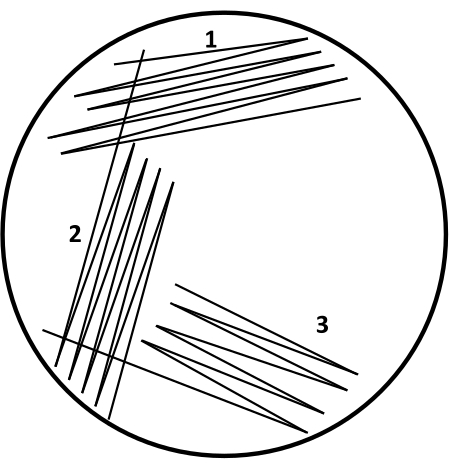
Figure 1: Schematic diagram of inoculation of strain. The inoculation ring was used to pick the colonies and to mark them in a zigzag pattern on the antibacterial activity assay medium. Please click here to view a larger version of this figure.
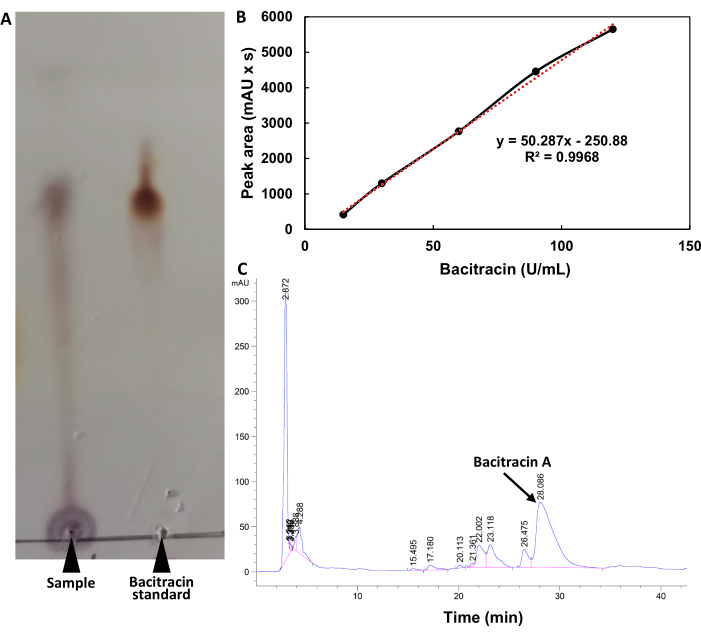
Figure 2: Identification of bacteriostatic substances. (A) Thin-layer chromatography. (B) HPLC standard curve of bacitracin. (C) HPLC chromatogram. Please click here to view a larger version of this figure.
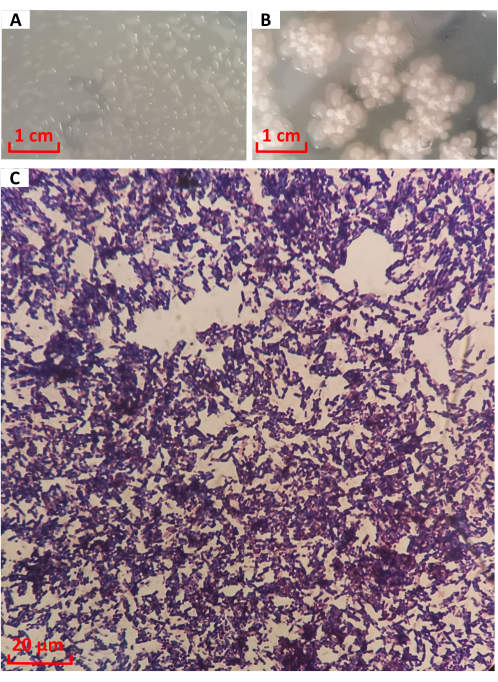
Figure 3: Morphological characteristics and Gram staining of strain No. 1026. Colony morphology at (A) 12 h and (B) 24 h. (C) Gram staining. Please click here to view a larger version of this figure.
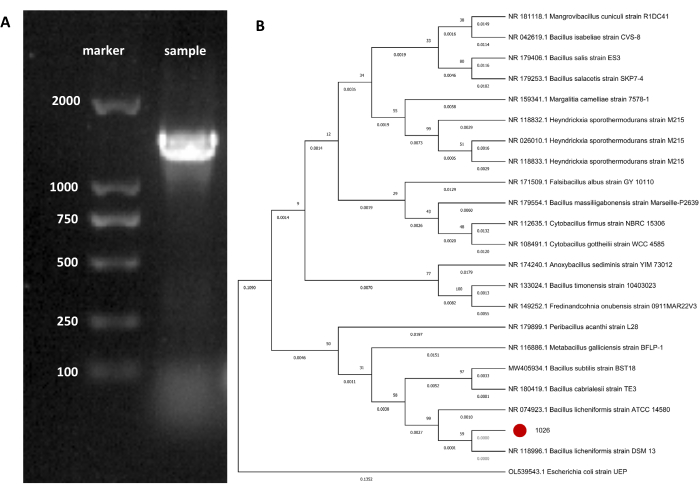
Figure 4: Molecular biological identification of strain No. 1026. (A) Gene amplification of strain NO. 1026 16S rDNA. (B) Phylogenetic tree of strain No. 1026. Please click here to view a larger version of this figure.
| NO. | Bacitracin titer (U/mL) | NO. | Bacitracin titer (U/mL) |
| 1003 | 423.57±18.62 | 1021 | 317.46±13.46 |
| 1004 | 325.82±13.23 | 1026 | 456.35±21.75 |
| 1009 | 326.26±14.52 | 1027 | 435.57±19.18 |
| 1011 | 376.65±16.11 | 1030 | 382.48±17.64 |
| 1015 | 325.27±12.37 | 1031 | 215.37±11.73 |
| 1016 | 256.56±15.37 | 1039 | 353.67±16.16 |
| 1017 | 352.47±16.47 | 1041 | 342.36±14.36 |
| 1018 | 328.73±16.12 |
Table 1: The bacitracin titer of antibacterial activity strains.
| Items | Results | Items | Results |
| Anaerobic growth test | + | D-mannitol test | + |
| V.P assay | + | Gelatin test | + |
| Citrate utilization test | + | 7%NaCl test | + |
| Propionate test | + | pH5.7 test | + |
| D-xylose test | + | Nitrate reduction test | + |
| L-arabinose | + | Starch hydrolysis test | + |
Table 2: Physiological and biochemical identification of strain no. 1026.
Discussion
B. licheniformis grows rapidly with simple culture conditions and fast sugar consumption, and the mature fermentation technology is helpful in saving industrial production costs13. The wide application of B. licheniformis and its secretions, bacitracin, has determined its promising market value. In agriculture, B. licheniformis is employed as a biofertilizer to improve plant growth and nutrient uptake by enhancing soil fertility, promoting root development, and aiding in the degradation of organic matter15. For industrial enzyme production, B. licheniformis produces a range of enzymes such as proteases, amylases, cellulases, and lipases, which determines its irreplaceable market value in food processing, detergent manufacturing, leather processing, and textile industry16. In view of the superior antibacterial pharmacological activity of B. licheniformis and bacitracin, it has been used in the medical field to treat a variety of infectious diseases induced by bacteria and fungi1. Meanwhile, considering the growth of herbal plants as well as the accumulation of pharmacologically active ingredients in plants, B. licheniformis is a potential enhancer of plant growth as well as useful for the production of compounds such as Rhodiola-derived salidroside17,18. The above advantages of B. licheniformis and bacitracin make them widely useful in industry, agriculture, aquaculture, biotechnology and medical industries. Therefore, the separation and purification of B. licheniformis with a high yield of bacitracin is a decisive factor in ensuring subsequent large-scale production and application.
Although bacitracin can be prepared by chemical synthesis, the steps are cumbersome, and there are many by-products. In industry, bacitracin is mainly produced by the secondary metabolic process of corn flour and soybean meal fermented by B. licheniformis. In the modern industrial production of bacitracin, high cost and low yield hinder its further application10. Therefore, screening high-yield bacitracin strains and improving the utilization rate of raw materials are the key to improving the production of bacitracin.
At present, the breeding of high-yield bacitracin strains is mainly through natural breeding from soil and other environments, mutation breeding using existing bacitracin-producing strains, or genetic engineering-based breeding. The natural breeding operation is simple, and the screened strains have stable production capacity, but the process takes a long time, and the workload is large. Mutation breeding has a high mutation rate and shortens the breeding time, but the mutation may not be able to be stably inherited in subsequent generations16. Genetic engineering-based breeding is more targeted for obtaining high-yield strains10, but there are safety concerns caused by the introduction of exogenous genes. Healthy adult animal feces contain abundant probiotic resources that can maintain the balance of flora.
In this paper, based on the characteristics of B. licheniformis to produce dormant spores in extreme environments19, healthy pig feces dilution was placed in a high-temperature environment to kill the heat-resistant non-spore-producing bacteria and fungi. Since M. luteus is sensitive to the production of bacteriostatic peptides by B. licheniformis at the late stage of growth and produces a yellow pigment for easy observation, it is used to screen the antibacterial activity based on the inhibition zone assessment. According to the colony morphology similar to the morphology of B. licheniformis, the cell morphology is short rod-shaped, Gram-positive, physiological and biochemical characteristics of B. licheniformis, 16 s RNA is closely related to B. licheniformis, so as to determine that the strain no. 1026 is B. licheniformis. The bacteriostatic substance was identified by TLC and HPLC as bacitracin. Thus, bacitracin-producing B. licheniformis was obtained. This method also has shortcomings, such as the screening process taking a long time and the operation requiring some experience.
In summary, this experimental protocol comprehensively and in detail introduced the acquisition and routine laboratory identification process of bacitracin-producing B. licheniformis, which has a wide application prospect and inestimable market value20. These methods are simple, feasible, and easy to implement, and undoubtedly will be an effective reference for large-scale production of B. licheniformis. This paper also provides a screening idea for the production of strains for other antimicrobial peptides.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This study was supported by the National Key Research and Development Program of China (No. 2022YFC2104800), and the Six Talent Peaks Project in Jiangsu Province (No. 2019-NY-058).
Materials
| Name | Company | Catalog Number | Comments |
| 2 × Phanta Flash Master Mix | Nanjing Vazyme Biotechnology Co., Ltd., Nanjing,China | P252-01 | |
| 2kb DNA Marker | Beijing Trans Biotechnology Co., Ltd., Beijing, China | BM121-01 | |
| Acetonitrile | Shanghai Aladdin Biochemical Technology Co.,Ltd., Shanghai, China | A104443 | |
| Agar powder | Shanghai Macklin Biochemical Technology Co., Ltd., Shanghai, China | A800730 | |
| Agarose | Shanghai Aladdin Biochemical Technology Co.,Ltd., Shanghai, China | A104062 | |
| Ammonium sulfate ((NH4)2SO4) | Sinopharm Chemical Reagent Co., Ltd., Shanghai, China | 10002917 | |
| Autoclave sterilizer | Zealway Instrument Inc., Xiamen, China | GI36DWS | |
| Bacillus biochemical identification strip | Qingdao Haibo Biotechnology Co., Ltd., Qingdao, China | HBIG14 | |
| Bacitracin | Shanghai Yuanye Bio-Technology Co., Ltd., Shanghai, China | B65740 | |
| Bacteria DNA Extraction Kit | Tiangen Biochemical Technology Co., Ltd., Beijing, China | DP209 | |
| Breathable sealing film | Beijing Leiborun Biotechnology Co., Ltd. | BS-QM-01A | |
| Butanol | Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China | B433378 | |
| C18 (5 μm, 4.6 × 250 mm) HPLC column | Rizhao Kepuno New Material Co., Ltd., Rizhao, China | C1805-462510 | |
| Calcium carbonate (CaCO3) | Sinopharm Chemical Reagent Co., Ltd., Shanghai, China | 10005717 | |
| Centrifuge | New Brunswick Scientific Co., Inc., UK | 5452 | |
| Chromatographic tank | Nanjing Tenghui Experimental Technology Co., Ltd., Nanjing, China | P-1 | |
| Conical bottle | Sichuan Shubo Co., Ltd., Chengdu, China | 18012 | |
| Constant temperature incubator | Taist Instrument Co., Ltd., Tianjin, China | GH4500 | |
| Dipotassium phosphate (K2HPO4) | Xilong Chemical Co., Ltd., Guangdong, China | XL0015 | |
| EDTA-2Na | Shanghai Aladdin Biochemical Technology Co.,Ltd., Shanghai, China | E397526 | |
| Electronic balance | Mettler Toledo International Co., Ltd. | FA2104 | |
| Ethyl alcohol | Shanghai Aladdin Biochemical Technology Co.,Ltd., Shanghai, China | E130059 | |
| Gel Midi Purification Kit | Tiangen Biochemical Technology Co., Ltd., Beijing, China | DP302 | |
| Glass rod | Chengdu Yibang Kexi Instrument Co., Ltd. | 1294 | |
| Glucose | Shanghai Macklin Biochemical Technology Co., Ltd., Shanghai, China | D823520 | |
| Gram 's staining solution kit | Qingdao Haibo Biotechnology Co., Ltd., Qingdao, China | HB8278 | |
| High performance liquid chromatograph | Agilent Technologies, Inc., California, America | 1260 | |
| Horizontal electrophoresis apparatus | Beijing Liuyi Biotechnology Co., Ltd., Beijing, China | DYCP-31DN BIOMATE | |
| Inoculation ring | Shanghai Muchen Biotechnology Co., Ltd., Shanghai, China | 3171026 | |
| Magnetic stirrer | Wiggens GmbH Co., Ltd., Germany | WH220 PLUS | |
| Methyl alcohol | Shanghai Aladdin Biochemical Technology Co.,Ltd., Shanghai, China | M116115 | |
| Microcentrifuge tube | Shanghai Muchen Biotechnology Co., Ltd., Shanghai, China | 1351171 | |
| Micrococcus luteus | Bena Culture Collection, Suzhou, China | BNCC102589 | |
| Microporous filter membrane | Nantong Suri Experimental Equipment Co., Ltd. | PES0.22 | |
| Ninhydrin | Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China | N105629 | |
| Optical microscope | Optical Instrument Factory, Shanghai, China | DYS-108 | |
| Pig feces | Nanjing Quanfu Pig Farm, Nanjing, China | ||
| Polymerase chain reaction (PCR) Amplifier | Suzhou Dongsheng Xingye Scientific Instrument Co., Ltd., Suzhou, China | ETC811 | |
| Professional sequencing company | General Biology (Anhui) Co., Ltd., Anhui, China | ||
| Pyridine | Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China | P111516 | |
| Shaker | Taicang Qiangle Experimental Equipment Co., Ltd.,Taicang, China | HYL-C | |
| Silica gel GF254 thin layer plate | Yantai Huayang New Material Co., Ltd., Yantai, China | HPT-HSGF5025023 | |
| Sodium chloride (NaCl) | Shanghai Macklin Biochemical Technology Co., Ltd., Shanghai, China | S805275 | |
| Sodium citrate | Sinopharm Chemical Reagent Co., Ltd., Shanghai, China | C39197100001 | |
| Soluble starch | Shanghai Macklin Biochemical Technology Co., Ltd., Shanghai, China | S817547 | |
| Thermostat water bath | Shanghai Heheng Instrument Equipment Co., Ltd., Shanghai, China | DK-8D | |
| Tryptone | Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China | T139519 | |
| Ultra GelRed | Nanjing Vazyme Biotechnology Co., Ltd., Nanjing,China | GR501-01 | |
| Ultra pure water instrument | Merck KGaA Co., Ltd., Germany | Milli Direct-Q8 | |
| Ultrasonic cleaner | Jiangsu Huaguan Electric Appliance Group Co., Ltd., Jiangsu, China | SB-100DT | |
| Vernier caliper | Sanfeng Company, Japan | N20P | |
| Yeast extract powder | Vicbio Biotechnology Co., Ltd., Beijing, China | LP0021 |
References
- Shleeva, M. O., Kondratieva, D. A., Kaprelyants, A. S. Bacillus licheniformis: A producer of antimicrobial substances, including antimycobacterials, which are feasible for medical applications. Pharmaceutics. 15 (7), 1893 (2023).
- Muras, A., Romero, M., Mayer, C., Otero, A. Biotechnological applications of Bacillus licheniformis. Crit. Rev. Biotechnol. 41 (4), 609-627 (2021).
- He, H., et al. Biotechnological and food synthetic biology potential of platform strain: Bacillus licheniformis. Syn Syst Biotechno. 8 (2), 281-291 (2023).
- Sun, Y., et al. Enhanced β-mannanase production by Bacillus licheniformis by optimizing carbon source and feeding regimes. Prep Biochem Biotech. 52 (7), 845-853 (2022).
- Trunet, C., et al. Suboptimal Bacillus licheniformis and Bacillus weihenstephanensis spore incubation conditions increase heterogeneity of spore outgrowth time. Appl Environ Microb. 86 (6), e02061-e02119 (2020).
- Lee, N. K., Kim, W. S., Paik, H. D. Bacillus strains as human probiotics: characterization, safety, microbiome, and probiotic carrier. Food Sci Biotechnol. 28 (5), 1297-1305 (2019).
- Yi, W., et al. Dietary novel alkaline protease from Bacillus licheniformis improves broiler meat nutritional value and modulates intestinal microbiota and metabolites. Anim Microbiome. 6 (1), 1-16 (2024).
- Vasileios, B., et al. Safety and efficacy of Bacillus licheniformis DSM 32457 as a silage additive for all animal species. EFSA Journal. 17 (8), e05787 (2019).
- Birgit, V., et al. The complete genome sequence of Bacillus licheniformis DSM13, an organism with great industrial potential. J Mol Microb Biotech. 7 (4), 204-211 (2004).
- Zhu, J., et al. Microbial synthesis of bacitracin: Recent progress, challenges, and prospects. Synth Syst Biotechnol. 8 (2), 314-322 (2023).
- Shleeva, M. O., Kondratieva, D. A., Kaprelyants, A. S. Bacillus licheniformis: A producer of antimicrobial substances, including antimycobacterials, which are feasible for medical applications. Pharmaceutics. 15 (7), 1893 (2023).
- Willdigg, J. R., et al. The Bacillus subtilis cell envelope stress-inducible ytpAB operon modulates membrane properties and contributes to bacitracin resistance. J Bacteriol. 206 (3), e0001524 (2024).
- Silva, K. G. S., et al. Effects of bacterial direct-fed microbial mixtures offered to beef cattle consuming finishing diets on intake, nutrient digestibility, feeding behavior, and ruminal kinetics/fermentation profile. J Anim Sci. 102, (2024).
- Jia, D., et al. Probiotic Bacillus licheniformis ZW3 alleviates DSS-induced colitis and enhances gut homeostasis. Int J Mol Sci. 25 (1), 561 (2024).
- Wang, X., et al. Dietary supplementation with Bacillus mixture modifies the intestinal ecosystem of weaned piglets in an overall beneficial way. J Appl Microbiol. 130 (1), 233-246 (2021).
- James, N., et al. Unravelling the potential plant growth activity of halotolerant Bacillus licheniformis NJ04 isolated from soil and its possible use as a green bioinoculant on Solanum lycopersicum L. Environ. Res. 216 (2), 114620 (2024).
- Shen, P., et al. Exploitation of ammonia-inducible promoters for enzyme overexpression in Bacillus licheniformis. J Ind Microbiol Biot. 48 (5-6), (2021).
- Hou, Y., et al. Rhodiola crenulata alleviates hypobaric hypoxia-induced brain injury by maintaining BBB integrity and balancing energy metabolism dysfunction. Phytomedicine. 128, 155529 (2024).
- Fan, B., Chen, T. Y., Zhang, S., Wu, B., He, B. F. Mining of efficient microbial UDP-glycosyltransferases by motif evolution cross plant kingdom for application in biosynthesis of salidroside. Sci Rep. 7 (1), 463 (2017).
- Hugo, R. O., Bernardo, R. B., Alejandra, C. S. R. Potential application of the probiotic Bacillus licheniformis as an adjuvant in the treatment of diseases in humans and animals: A systematic review. Front Microbiol. 13, 993451 (2022).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved