Method Article
Establishment of A Mouse Model of Aqueous Deficiency Dry Eye
* Ces auteurs ont contribué à parts égales
Dans cet article
Résumé
Here, we present a method to establish a mouse model of aqueous-deficient dry eye by excising the extraorbital and infraorbital lacrimal glands and evaluate the changes in the ocular surface in aqueous deficiency dry eye.
Résumé
Dry eye disease is a prevalent condition affecting 5%-50% of the global population. Animal model investigations play a crucial role in understanding its underlying mechanisms. Therefore, we developed a mouse model of dry eye disease by surgically removing both the extraorbital lacrimal glands (ELG) and intraorbital lacrimal glands (ILG) to investigate the ocular surface pathology in the context of aqueous deficiency dry eye. Two weeks post operation, the mice exhibited severe dry eye manifestations, including reduced tear secretion, corneal epithelial irregularities, positive fluorescein sodium staining, and neovascularization. Histological examination via hematoxylin and eosin staining revealed inflammatory cell infiltration and corneal epithelium dysplasia. Immunofluorescence staining and quantitative reverse-transcription polymerase chain reaction revealed decreased expression of the normal corneal epithelial biomarkers K12 and Pax6 and increased expression of Sprr1b in the corneal epithelium. These ocular manifestations indicated abnormal corneal epithelial differentiation. Furthermore, immunofluorescence staining of Ki67 revealed the increasing cell proliferation. In conclusion, the ELG plus ILG excision model proved suitable for studying changes in the ocular surface and elucidating the mechanisms underlying aqueous deficiency dry eye.
Introduction
Dry eye has become the most common ocular surface disease worldwide, with an estimated 5% to 50% of the population suffering from the disease1. Eye dryness and discomfort caused by dry eye, if persistent, can lead to visual disturbances and unstable tear films, further leading to ocular surface damage2. Some patients with dry eye may even suffer from chronic pain2.
According to the 2017 TFOS DEWS II Dry Eye Consensus, dry eye is a complex condition that involves multiple factors and is characterized by the disruption of the natural balance of the tear film. This condition is accompanied by a range of ocular symptoms, instability of the tear film, increased osmolarity of the tears, inflammation and damage to the ocular surface, and neuropathic pain, which are crucial in understanding the disease's progression3. In this feedback loop, dry eye can cause damage to corneal epithelial cells, decrease corneal stromal thickness and endothelial cell density, trigger immune cell activation and aggregation on the corneal surface, reduce mucin secretion by goblet cells, and cause immune dysfunction in the lacrimal gland, further exacerbating the condition4,5,6,7,8,9,10. The consensus delineates three primary forms of dry eye syndrome: aqueous deficiency dry eye, evaporative dry eye, and a combination of both, known as mixed dry eye3. Aqueous deficiency dry eye is associated with structural and functional changes in the lacrimal glands11. These alterations include acinar atrophy, ductal occlusion that blocks the flow of tears, lymphocyte infiltration indicating an immune response, and a reduction in the secretion of proteins that are essential for maintaining the health and stability of the tear film12,13.
The lacrimal gland is a tubular exocrine gland that is responsible for the production of water components in the tear film13,14, including water, electrolytes, and proteins, and contributes to the maintenance of the stability of the ocular surface microenvironment15,16. The health and visual clarity of the eye depend on the presence of the tear film, which forms a critical optical layer on the surface of the cornea, ensuring smooth lubrication of the cornea and conjunctiva17. The tear film promotes the metabolic activity of ocular surface cells and acts as a cleaner, effectively removing impurities and potential irritants from the eye surface, thereby maintaining the physiological balance and comfort of the eye18,19.
In rodents, such as rats and mice, the lacrimal glands consist of two lobes: the internal and external orbital lobes. The external orbital lobe is located below the ear, and its functional part is connected to the eye by an extended duct. This duct engages with the lacrimal gland lobe in the orbit before reaching the eye and is involved in tear production20. The internal orbital lobe, also known as the intraorbital lacrimal gland, is located under the bulbar conjunctiva of the outer canthus. The ILG is smaller than the ELG. Impaired lacrimal gland function can lead to aqueous deficiency dry eye, which, if left untreated, can progress to corneal ulcers and vision loss21.
Animal models of aqueous deficiency dry eye can be categorized into three intervention methods: surgical induction models, drug-induced models, and transgenic models. The surgical induction model involves the removal of the ELG. However, this model is not stable due to the presence of ILG22. The systemic injection of the cholinergic receptor blocker scopolamine is common in the drug-induced model23. Topical applications of atropine24 and benzalkonium chloride25 can also reduce mucin and tear secretion. Concanavalin-A can be injected locally into rabbit lacrimal glands to cause immune lacrimal adenitis, thereby establishing a model of aqueous deficiency dry eye26. Additionally, transgenic animals, such as NOD, Aly/aly, NFS/sld, IQI/Jic, Id3 KO, and other mouse strains, can mimic the symptoms and phenotypes of primary Sjögren syndrome27 to replicate aqueous deficiency dry eye.
In this study, the core experimental design involved a surgical procedure to painlessly and precisely remove the ELG and the ILG of the mouse. These lacrimal glands are essential for maintaining eye moisture and lubrication; their removal results in a significant reduction in tear secretion, mimicking aqueous deficiency dry eye in mice. This approach allows for the observation and analysis of mouse behavior, physiological responses, and ocular tissue changes in the dry eye state, providing a key experimental basis for pathology studies and the evaluation of treatment options for dry eye disease.
Protocole
Female C57BL/6 (C57) mice, aged 7-8 weeks, were used in this study. All procedures adhered to the ARVO guidelines for the use of animals in ophthalmic and vision research and were approved by the Animal Ethics Committee of Guizhou Medical University (Approval No. 2305193). No ocular surface lesions were observed under a slit lamp examination.
1. Preoperative steps
- Sterilize the instruments using a rapid sterilizer: needle holders, a 5-0 suture needle with thread, ophthalmic surgical scissors, and forceps.
- Anesthetize the mouse with 1.25% Avertin via intraperitoneal injection at a dose of 0.3 mL/100 g.
- After anesthesia is complete, place the mouse in the lateral decubitus position and remove the hair from the surgical area.
- Disinfect the skin and hair around the eyes with iodophor.
- Establishment of the dry eye model
- Place the mouse in the lateral decubitus position. Expose the surgical area under an operating microscope.
- Clamp the skin on the right side of the mouse's face with toothed forceps and make an incision at the midpoint of the line intersecting the external auricle and the mandibular line to the inner corner of the eye. Expose the muscle tissue; remove the ELG located on the muscle carefully.
- Extend the skin incision to the inner corner of the eye, bluntly separate the muscle, and locate the light red gland beneath the muscle. Peel off and remove the ILG.
- Suture the incision using 5-0 sutures with the help of a needle holder, ophthalmic surgical scissors, and forceps.
- Apply ofloxacin ointment at the end of the operation to prevent infection.
- After removing the unilateral ELG and ILG, place the mice in an environment with a rhythmic 12:12 h light-dark cycle with free access to food and water. This creates a unilateral dry eye model, while the contralateral eye with internal and external lacrimal glands serves as the normal control group.
2. Measurement of tear production
- Anesthetize the mouse with 1.25% Avertin intraperitoneally at a dose of 0.3 mL/100 g.
- Take a well-packaged phenol red thread for tear measurement.
- Gently pull down the lower eyelid to be measured. Position the top of the phenol red thread into the inner and outer thirds of the lower eyelid, initiating the timer promptly.
- Perform the test on one eye at a time for a duration of 15 s.
- After the allotted time, carefully retract the lower eyelid and remove the phenol red thread downward with caution.
- Utilize the scale provided on the outer bag of the phenol red thread to measure from the top of the thread to the entirety of the red portion.
- Employ an electronic stopwatch to accurately record the duration of the test.
3. Preparation of freezing tissue slices
- Perform rapid cervical dislocation on the mice following asphyxiation with CO2.
- Utilize scissors and forceps to carefully extract the eyeballs.
- Embed the eyeballs in optimal cutting temperature compound and utilize a frozen microtome to obtain freezing tissue slices of 5-7 µm thickness28.
- Store all slides in a specimen box at room temperature for approximately 30 min. Subsequently, transfer them to an ultra-low temperature freezer at -80 °C.
4. Hematoxylin and eosin (H&E) staining
- Remove frozen tissue slices from the ultra-low temperature freezer, lay them flat in a tissue box, and dry them in a fume hood.
- Wash slices with 1x PBS, cover for 5 min, and then remove the PBS. Cover the specimen with 4% paraformaldehyde, ensuring the lid is closed to prevent evaporation. After 15 min at room temperature, rinse the specimens for 3 x 5 min in 1x PBS.
- Place the specimen in a rack and immerse it in 0.5%hematoxylin dye for 5 min.
- Rinse the specimen in tap water for 5 min to wash off any excess dye. Subsequently, immerse it in 0.1% hydrochloric acid alcohol for 1-2 s to facilitate differentiation.
- Place the specimen holder in tap water for 15 min to remove the non-specific staining.
- Drain excess water and immerse the specimen in 0.05% eosin staining solution for 3 min.
- Wash the specimen in tap water and immerse it sequentially in 75%, 80%, 95%, and two rounds of 100% alcohol for 2 min each.
- Immerse the specimen holder in two rounds of xylene for 2 min each.
- Place the holder in a fume hood to dry and mount the specimen with mounting medium, avoiding damage and bubbles.
- Store the stained specimens in a box at room temperature and photograph them using a biomicrography system.
5. Immunofluorescence staining
- Retrieve the specimen from the freezer, lay it flat in the box, and dry it in the hood.
- Wash the specimen with 1x PBS, cover for 5 min, and aspirate the excess liquid. Cover the specimen with 4% paraformaldehyde, add water to the immunohistochemical wet box, and close the lid to prevent evaporation. Allow it to sit at room temperature for 15 min; then, rinse the specimen 3 x 5 min in 1x PBS.
NOTE: Handle the specimen with care during this process. - Remove excess PBS from the specimen surface and gently wipe the residual liquid around the tissue section with filter paper. Cover the specimen surface with cell permeabilization solution (0.2% Triton X-100) and incubate at room temperature for 20 min to permeabilize the membrane.
- Remove the permeabilization solution and rinse the specimen 3 x 5 min with 1x PBS.
- Pour out the PBS from the specimen surface and gently wipe residual liquid around the tissue section with filter paper. Cover the specimen with prewarmed blocking solution (2% BSA). Close the lid to prevent evaporation and incubate at room temperature for 1 h.
- During the blocking process, select the appropriate antibody according to the experimental needs, prepare the primary antibody with immunofluorescent antibody diluent (1% BSA) according to the recommended ratio, and centrifuge the prepared primary antibody in a 4 °C centrifuge at 2,000 × g, 10 min.
NOTE: All of the above are operated on ice or at low temperatures. It is recommended to use about 120 µL of primary antibody per sample. - Discard or pour off the primary antibody. Wash the specimen 3 x 10 min with 1x PBS buffer. After pouring off the PBS, rinse the specimen with fresh 1x PBS. Ensure that the PBS covers the slide during the first rinse.
- While the primary antibody is being rinsed, select the suitable secondary antibody based on its category. Dilute it with 1% BSA diluent at a 1:300 ratio. Spin the prepared antibody in a centrifuge at 4 °C, 2 000 × g for 10 min. Perform the entire process on ice or at a low temperature in the dark.
- Rinse the slide with PBS, gently wipe the liquid around the specimen with filter paper, use a suction pump to absorb any remaining liquid, and quickly add the prepared secondary antibody to the specimen. Cover it and incubate at room temperature for 1 h. Ensure all processes are conducted in the dark.
- Discard the secondary antibody, rinse with 1x PBS buffer, and wash 3 x 10 min. Ensure the PBS completely covers the slide. Handle the specimen gently to avoid rinsing it out.
- Pour off the 1x PBS, wipe away excess liquid with filter paper, use a suction pump to remove any remaining liquid, and mount the specimen with a mounting medium containing DAPI. Place the cover on the slide plate and store it in a 4° C freezer, protected from light with tin foil. Take care to avoid damaging the specimen and creating air bubbles.
- Photograph the stained sample using a laser confocal or upright fluorescence microscope system. Ensure that the imaging is done within a week to prevent fluorescence quenching.
6. Extraction of corneal mRNA
- Perform rapid cervical dislocation on the mice following asphyxiation with CO2. Then, utilize scissors and forceps to carefully extract the eyeballs, wash them 3x in 1x PBS, and place the eyeball in a Petri dish containing 1x PBS.
- Under the microscope, utilize scissors and forceps to cut the eyeball from the posterior pole of the eyeball to separate the lens. Clean up the excess sclera and iris, but leave the 0.5 mm white sclera and the entire cornea. Extract the mRNA from the mouse eyeballs using an RNA extraction kit according to the instructions.
7. Reverse transcription PCR
- Before performing reverse transcription, determine the concentration of RNA with a UV spectrophotometer.
- Take 1 mg of RNA based on the measured RNA concentration. Perform the reverse transcription reaction using a reverse transcription kit.
- After adding the reaction components, add the mixture to a 200 μL centrifuge tube, mix with gentle shaking, centrifuge at low speed to avoid air bubbles, and immediately put it into a normal thermal cycler for reaction.
- Analyze the cDNA from the reverse transcription process directly by real-time PCR or store it in a -20 °C freezer.
8. Real-time quantitative PCR reactions (qRT-PCR)
- Set up a 10 µL reaction system according to the instructions provided with real-time PCR kit29: 3.6 μL of DEPC-treated water, sense primer 0.2 μM, anti-sense Primer 0.2 μM, 1 μL of cDNA, 5 μL of Mix. See Table 1 for details about the primers.
- Depending on the grouping of experiments, add the reaction system to a 96-well plate or eight-strip tube dedicated to qRT-PCR. Once the qRT-PCR system is configured, make sure the mixture is well mixed and use a tabletop centrifuge for brief centrifugation to shake off the liquid from the tube wall. Always check the tube for air bubbles before qRT-PCR.
- Perform the reaction in triplicate and calculate the average Ct (cycle threshold).
Résultats
To investigate the effects and underlying mechanisms of aqueous deficiency dry eye on the ocular surface, we established a mouse model of aqueous deficiency dry eye by surgically removing both the ELG and ILG (Figure 1A). Following a 2-week period post tear gland resection, tear secretion in the dry eye (after removal of lacrimal glands) mice notably decreased compared to the normal group (Figure 1B,C). Evaluation of the cornea via slit lamp microscopy revealed significant dryness and fluorescein sodium staining exhibited diffuse punctate staining across the cornea, indicative of corneal epithelial defects. Moreover, neovascularization was observed in both the superficial epithelial and stromal layers of the cornea, extending diffusely towards the central cornea (Figure 1D).
Histological analysis via H&E staining demonstrated squamous metaplasia in the corneal epithelium, accompanied by the accumulation of small and round inflammatory cells with dark blue-purple cell nuclei within the corneal stromal layer (Figure 2A). Furthermore, dysplastic changes were evident in the corneal epithelial basal layer of the experimental group (Figure 2B).
Immunofluorescence staining and qRT-PCR revealed that the expression levels of cornea-specific keratin12 (Figure 3A,B) and corneal epithelial cell-specific transcription factor Pax6 (Figure 4A,B) in corneal epithelial cells in the dry eye group were significantly lower than those in the normal group. Conversely, in the dry eye group, immunofluorescence of the abnormally differentiated corneal epithelial marker Sprr1b was significantly increased over that of the normal group (Figure 5A,B). Then,in immunofluorescence analysis, Ki67 cells in the dry eye group showed higher proliferative activity compared to the normal eye group (Figure 6A,B). These observed phenotypes closely resemble clinical presentations observed in patients with severe dry eye diseases such as Sjögren's syndrome and Stevens-Johnson syndrome30,31.
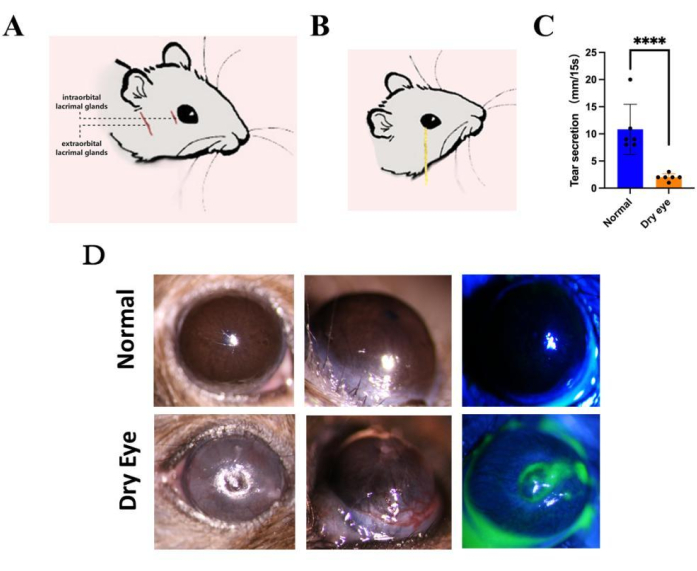
Figure 1: Establishment of the dry eye model. (A) Construction of a severe dry eye disease model in a low-humidity environment by surgically removing both the extraorbital and intraorbital lacrimal glands, followed by maintaining the mice for 14 days. After induction of the model, we (B,C) measured tear secretion with phenol cotton thread and (D) observed corneal defects and neovascularization with a slit lamp. (n = 6, ****P < 0.0001). Please click here to view a larger version of this figure.

Figure 2: H&E staining. We conducted H&E staining of the eyeballs to examine the morphology of corneal epithelial cells. (A) The results showed that the normal corneal epithelium showed squamous metaplasia, the corneal stromal layer gathered a large number of inflammatory cells that were small and round, and the cell nucleus was densely stained dark blue-purple; (B) the corneal epithelial base of the operation group showed dysplasia of dysplastic cells. Scale bar = 50 µm (20x), 20 µm (40x). Abbreviation: H&E = hematoxylin and eosin. Please click here to view a larger version of this figure.

Figure 3: Phenotypic changes of cells in the dry eye state. (A) The intensity of K12 immunostaining in the corneal epithelium of the dry eye group indicated a significant decrease in K12 expression compared to the normal group. (B) The expression of K12 in the corneal epithelial cells of the dry eye group related to the control group also showed a significant decrease. Scale bar = 50 µm. n = 3, ****P < 0.0001. Please click here to view a larger version of this figure.
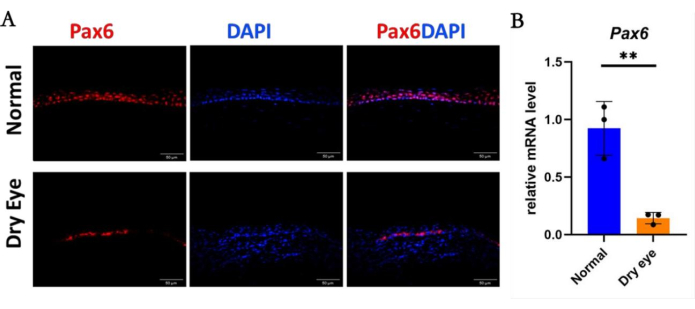
Figure 4: The cell lineage of corneal epithelium in the dry eye state. (A) Immunostaining and (B) mRNA level of Pax6 in the corneal epithelium of the dry eye group was significantly decreased compared to that of the normal group. Scale bar = 50 µm. n = 3, **P < 0.01. Please click here to view a larger version of this figure.
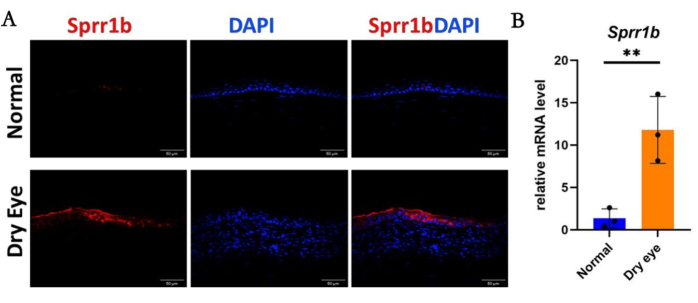
Figure 5: The abnormal differentiation of corneal epithelium in the dry eye state. (A) The immunostaining intensity of Sprr1b in corneal epithelial cells in the dry eye group was significantly higher than that in the normal group, which was also strongly supported by (B) qRT-PCR analysis. Scale bar = 50 µm. n = 3, **P < 0.01. Please click here to view a larger version of this figure.
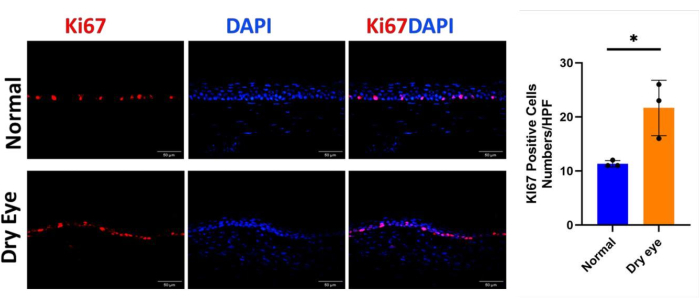
Figure 6: Cell proliferation of corneal epithelium in the dry eye state. (A, B) The immunostaining of corneal epithelial Ki67 in the dry eye group showed increased intensity compared to that in the normal group Scale bar = 50 µm. n = 3, *P < 0.05. Please click here to view a larger version of this figure.
| Gene | Sense Primer | Anti-Sense Primer |
| Mouse-Pax6 Mouse-Krt12 Mouse-Sprr1b | 5’- ACTTCAGTACCAGGGCAACC -3’ 5’- TGACCCTGACTAGAGCCGAC -3’ 5’- GACCACAGTACCTGTCCTCC -3’ | 5’- GCTTCATCCGAGTCTTCTCC -3’ 5’- ACATTGACCTCACCTGGACC -3’ 5’- CTGGCAAGGCTGTTTCACTT -3’ |
| Mouse-Actb | 5’- CTGTCGAGTCGCGTCCA -3’ | 5’- ACCCATTCCCACCATCACAC -3’ |
Table 1: Sequences of the primers used for quantitative RT-PCR.
Discussion
We successfully established a mouse model of aqueous deficiency dry eye disease by surgically excising both the ELG and ILG, aiming to investigate changes in the ocular surface under conditions of aqueous deficiency dry eye disease. We meticulously documented the morphological and physiological alterations observed in this mouse model of dry eye resulting from the double excision of the ELG and ILG. Two weeks post modeling, we observed and sampled the established mouse model of dry eye.
Considering that factors such as temperature, airflow velocity, relative humidity, seasonality, and pollutants can influence tear film evaporation rates and exacerbate or alleviate dry eye disease symptoms, we meticulously maintained the mouse's environment both before and after treatment. We ensured a rhythmic 12:12 h light-dark cycle for the mice, provided free access to food and water, and minimized potential confounding factors in the study. Before surgery, adequate anesthesia was administered to ensure the mouse remained immobile throughout the procedure, thereby reducing stress and physiological effects. Moreover, we maintained surgical instruments in a clean and sterile condition to prevent infection. During the procedure, particular attention was paid to the anatomical position of the ELG and ILG of the mouse, given their deep orbital location. Delicate surgical techniques were employed to identify and avoid surrounding blood vessels and nerves, thus minimizing unnecessary harm to the mouse. Surgical incision size, shape, and location were carefully considered as they significantly impacted surgical outcomes. Our primary principle was to minimize trauma to the mouse during surgery to avoid skin and mucous membrane damage, which could facilitate bacterial invasion and induce stress. Additionally, reducing trauma facilitated postoperative recovery in the mouse. Practical details such as gentle tissue handling during cutting and suturing were observed to prevent tissue overstretching or tearing. Postoperative mouse recovery was closely monitored, and any potential complications were promptly addressed.
Compared to excision of the ELG alone, excision of both the ELG and ILG was more challenging and invasive due to the deep orbital location of the ILG, necessitating extremely delicate surgical procedures. However, postoperative observation revealed no significant side effects such as bleeding, facial paralysis, or eyelid dysfunction. Moving forward, we intend to further explore the application potential of this model and endeavor to optimize surgical techniques to reduce invasiveness and pain in mice.
However, when performing surgery to remove ELG and ILG from mice, the surgery is more invasive because both glands must be removed at the same time. This aggressiveness may result in significant trauma and stress responses in mice, as well as a higher risk of infection during and after surgery. In addition, ILG is located deep in the orbit and the surrounding blood vessels are dense, which increases the risk of intraoperative bleeding. Given the complexity of the surgery, the recovery period for mice is likely to be relatively long. Although the model can well simulate water-deficient dry eye syndrome, whether its applicability is broad enough to cover all types of dry eye studies still needs further validation.
Therefore, the following points must be taken care of during the procedure. All instruments must be sterilized before operation. Antibiotic eye drops should be applied the day before and on the day of surgery to reduce the risk of infection. Topical anesthesia is administered with appropriate analgesic drugs such as lidocaine after surgery to reduce stress response and pain in mice. More detailed postoperative care must be provided, including regular check-ups, prompt handling of complications, and provision of nutritious food and a clean environment. Through comparative studies with other dry eye syndrome models, the universality and applicability of the model are verified, and its reliability in different studies is ensured.
This method offers several advantages over traditional dry eye models, such as systemic injection of scopolamine, which may induce multiple systemic effects. Moreover, local injection effects are less durable, and it is challenging for transgenic animals to fully replicate dry eye lesion development in diseases like Sjögren's syndrome. Many previously reported experimental dry eye disease models exhibit drawbacks such as unstable symptoms, frequent treatment requirements, use of legally regulated toxic substances, and/or necessitate special breeding environments22. Therefore, we endeavored to construct a dry eye model that more accurately mirrors the reduction in water secretion by eliminating the secretory glands. However, the conventional excision of extraorbital lacrimal and Harderian glands models failed to attain the low tear secretion levels we anticipated. To address this, we employed a unilateral resection model devised by Shinomiya22 to remove both the ELG and ILG of mice, using the unaffected side as a control. Compared to the traditional animal model involving the resection of the extraorbital lacrimal gland and Harderian gland, this approach resulted in reduced tear secretion, a relatively straightforward procedure, and consistent outcomes.
Additionally, among the various animal models for dry eye, the rabbit model is considered relatively well-established. Rabbits are particularly suitable for ophthalmic examinations due to their larger eye size, which offers better access to the ocular surface32,33. In rabbit models, existing methods include the use of epithelial toxic drugs, atropine, lacrimal gland resection, and parasympathectomy. These techniques effectively simulate dry eye conditions. However, anatomically, the rabbit's lacrimal glands are widely distributed across the eyelid, anterior eyelid, upper orbit, and both the suborbital and postorbital regions. Adult rabbits have four or five distinct glandular structures in front of their orbits, including the upper palpebral lobe, upper orbital lobe, and larger infraorbital glands, as well as the Harderian gland34. Therefore, incomplete removal of the lacrimal glands during resection may result in an unsatisfactory dry eye model. Conversely, fully removing all secretory glands would cause significant trauma, potentially compromising the model's effectiveness. Moreover, existing studies often do not specifically address the creation of rabbit models for aqueous-deficient dry eye secondary to ocular surface scarring disorders (e.g., Stevens-Johnson syndrome, mucous membrane pemphigoid). In contrast, our mouse model closely mimics severe dry eye diseases, such as Sjögren's syndrome and Stevens-Johnson syndrome. By only removing the intraorbital and extraorbital lacrimal glands, our model successfully induces severe aqueous-deficient dry eye, while minimizing damage to the animal and simplifying the surgical procedure.
Overall, the model described herein offers advantages in terms of manipulation and functional properties, making it more convenient than other dry eye mouse models, such as those with ELG excision alone. Our model readily induces severe aqueous deficiency, resulting in severe dry eye lesions on the ocular surface, which could facilitate the development of novel therapeutics. In summary, the ELG plus ILG excision model demonstrates superiority over existing animal models in terms of convenience and stability.
Déclarations de divulgation
The authors have no conflicts of interest to disclose.
Remerciements
This study was supported in part by the Guizhou Provincial Science and Technology Projects (QKHJC-ZK[2024]ZD043), Fujian Provincial Science Fund for Distinguished Young Scholars (2023J06053 [to S.O]), the Natural Science Foundation of China (No.82101084 [to S.O.] and China Scholarship Council (CSC, 202306310049 [to Y.W.]).
matériels
| Name | Company | Catalog Number | Comments |
| 10 mL syringe | Zhejiang KDL Medical Devices Co., Ltd. China | ||
| 5-0 Suture Needle | Suzhou 66 Vision Co., Ltd. China | ||
| Absolute ethanol | Shanghai Sinopharm Chemical Reagent Co., Ltd., China | ||
| Alexa Fluor 488, Donkey anti mouse IgG | Invitrogen | 811493 | |
| Alexa Fluor 594, Donkey anti rabbit IgG | Invitrogen | A21207 | |
| Anti-Keratin 12 antibody EPR17882 | Abcam | ab185627 | |
| Anti-PAX6 antibody | Abcam | ab5790 | |
| Autoclave | Hirayama. Japan | ||
| C57BL/6 mouse | Shanghai Slack Laboratory Animal Co., Ltd. | ||
| Centrifuge at room temperature | Eppendorf. Germany | ||
| ddH2O | Shanghai Bioengineering Co., Ltd. China | ||
| Electronic balances | Shanghai Auhaus Biotech Co., Ltd. China | ||
| Fluorescence inverted phase contrast micrography system | TE-2000U,Nikon. Japan | ||
| Freeze the cassette | Jiangsu Shitai Experimental Equipment Co., Ltd. China | ||
| Freeze the slicing blade | Xiamen Taijing Biotechnology Co., Ltd. China | ||
| H-1200 with DAPI mounting medium | Xiamen Juin Biotechnology Co., Ltd. China | mounting medium containing DAPI | |
| H-5000 Tablet Mountant | Vector. USA | mounting medium | |
| HCl | Shanghai Sinopharm Chemical Reagent Co., Ltd., China | ||
| Hematoxylin-eosin stain kit | Auragene. USA | ||
| Iodine | |||
| Ki-67 antibody | Abcam | ab16667 | |
| liquid nitrogen | Xiamen Yidong Gas Co., Ltd. China | ||
| Microscopic needle holder | Suzhou 66 Vision Co., Ltd. China | ||
| Microscopic toothed forceps | Suzhou 66 Vision Co., Ltd. China | ||
| Microscopic toothless forceps | Suzhou 66 Vision Co., Ltd. China | ||
| Model 3050 frozen slicer | Leica, Deerfield, IL. USA | ||
| OCT | Shanghai Maokang Biotechnology Co., Ltd. China | ||
| Ofloxacin ointment | |||
| Ophthalmic sodium fluorescein test strips | Tianjin Jingming New Technology Development Co., Ltd. China | ||
| Paraformaldehyde powder | Sigma. USA | ||
| PBS | |||
| Phenol red cotton thread | |||
| Rapid sterilizer sterilizer | Suzhou 66 Vision Co., Ltd. China | ||
| Recombinant Human SPRR1b protein | Abcam | ab167925 | |
| Refrigerated tabletop centrifuge | Eppendorf. Germany | ||
| Slide holders | |||
| Slit lamp | Topcon. Japan | ||
| Specimen box | Lambolide (Fuzhou) Biotechnology Co., Ltd. China | ||
| Tert-Amyl alcohol | Shanghai. Macklin Biochemical, China | A800283 | 500 mL |
| Tribromoethanol | |||
| Triton X-100 | Sigma. USA | ||
| Ultra-low temperature freezer | Thermo Fisher Scientific. USA | ||
| Upright fluorescence micrograph | Leica DM2500. USA | ||
| Upright normal biomicrography system | Eclipse 50i, Nikon. Japan | ||
| Xylene | Shanghai Sinopharm Chemical Reagent Co., Ltd., China | ||
| Zeiss Surgical Microscope | VISU150, Carl ZEISS. Germany |
Références
- Stapleton, F., et al. Tfos dews ii epidemiology report. Ocul Surf. 15 (3), 334-365 (2017).
- Galor, A., Levitt, R. C., Felix, E. R., Martin, E. R., Sarantopoulos, C. D. Neuropathic ocular pain: An important yet underevaluated feature of dry eye. Eye (Lond). 29 (3), 301-312 (2015).
- Craig, J. P., et al. Tfos dews ii definition and classification report. Ocul Surf. 15 (3), 276-283 (2017).
- Hao, R., Ding, Y., Li, X. Alterations in corneal epithelial dendritic cell in Sjogren's syndrome dry eye and clinical correlations. Sci Rep. 12 (1), 11167 (2022).
- Wu, Y., et al. Evolution of therapeutic strategy based on oxidant-antioxidant balance for fuchs endothelial corneal dystrophy. Ocul Surf. 34, 247-261 (2024).
- Sekhon, A. S., He, B., Iovieno, A., Yeung, S. N. Pathophysiology of corneal endothelial cell loss in dry eye disease and other inflammatory ocular disorders. Ocul Immunol Inflamm. 31 (1), 21-31 (2023).
- Zuo, X., et al. Akr1c1 protects corneal epithelial cells against oxidative stress-mediated ferroptosis in dry eye. Invest Ophthalmol Vis Sci. 63 (10), 3 (2022).
- Akdemir, M. O., et al. The effect of pseudoexfoliation and pseudoexfoliation induced dry eye on central corneal thickness. Curr Eye Res. 41 (3), 305-310 (2016).
- Delcroix, V., et al. Lacrimal gland epithelial cells shape immune responses through the modulation of inflammasomes and lipid metabolism. Int J Mol Sci. 24 (5), 4309 (2023).
- Portal, C., Gouyer, V., Gottrand, F., Desseyn, J. L. Ocular mucins in dry eye disease. Exp Eye Res. 186, 107724 (2019).
- Doctor, M. B., Basu, S. Lacrimal gland insufficiency in aqueous deficiency dry eye disease: Recent advances in pathogenesis, diagnosis, and treatment. Semin Ophthalmol. 37 (7-8), 801-812 (2022).
- Rocha, E. M., Alves, M., Rios, J. D., Dartt, D. A. The aging lacrimal gland: Changes in structure and function. Ocul Surf. 6 (4), 162-174 (2008).
- He, X., et al. Lacrimal gland microenvironment changes after obstruction of lacrimal gland ducts. Invest Ophthalmol Vis Sci. 63 (3), 15 (2022).
- Zoukhri, D. Mechanisms involved in injury and repair of the murine lacrimal gland: Role of programmed cell death and mesenchymal stem cells. Ocul Surf. 8 (2), 60-69 (2010).
- Li, W., Hayashida, Y., Chen, Y. T., Tseng, S. C. Niche regulation of corneal epithelial stem cells at the limbus. Cell Res. 17 (1), 26-36 (2007).
- Ou, S. K., et al. The role of ectodysplasin a on the ocular surface homeostasis. Int J Mol Sci. 23 (24), 12 (2022).
- Rolando, M., Zierhut, M. The ocular surface and tear film and their dysfunction in dry eye disease. Surv Ophthalmol. 45 (Suppl 2), S203-S210 (2001).
- Johnson, M. E., Murphy, P. J. Changes in the tear film and ocular surface from dry eye syndrome. Prog Retin Eye Res. 23 (4), 449-474 (2004).
- Masoudi, S. Biochemistry of human tear film: A review. Exp Eye Res. 220, 109101 (2022).
- Dartt, D. A. Neural regulation of lacrimal gland secretory processes: Relevance in dry eye diseases. Prog Retin Eye Res. 28 (3), 155-177 (2009).
- Garg, A., Zhang, X. Lacrimal gland development: From signaling interactions to regenerative medicine. Dev Dyn. 246 (12), 970-980 (2017).
- Shinomiya, K., Ueta, M., Kinoshita, S. A new dry eye mouse model produced by exorbital and intraorbital lacrimal gland excision. Sci Rep. 8 (1), 1483 (2018).
- Viau, S., et al. Time course of ocular surface and lacrimal gland changes in a new scopolamine-induced dry eye model. Graefes Arch Clin Exp Ophthalmol. 246 (6), 857-867 (2008).
- Burgalassi, S., Panichi, L., Chetoni, P., Saettone, M. F., Boldrini, E. Development of a simple dry eye model in the albino rabbit and evaluation of some tear substitutes. Ophthalmic Res. 31 (3), 229-235 (1999).
- Lin, Z., et al. A mouse dry eye model induced by topical administration of benzalkonium chloride. Mol Vis. 17, 257-264 (2011).
- Honkanen, R. A., Huang, L., Rigas, B. A rabbit model of aqueous-deficient dry eye disease induced by concanavalin a injection into the lacrimal glands: Application to drug efficacy studies. J Vis Exp. (155), e59631 (2020).
- Barabino, S., Dana, M. R. Animal models of dry eye: A critical assessment of opportunities and limitations. Invest Ophthalmol Vis Sci. 45 (6), 1641-1646 (2004).
- Wu, H., et al. A su6668 pure nanoparticle-based eyedrops: Toward its high drug accumulation and long-time treatment for corneal neovascularization. J Nanobiotechnol. 22 (1), 14 (2024).
- Zhu, H. M., et al. A synergistic therapy with antioxidant and anti-vegf: Toward its safe and effective elimination for corneal neovascularization. Adv Healthc Mater. 13 (5), 10 (2024).
- Mao, Y., et al. Downregulation of p38 mapk signaling pathway ameliorates tissue-engineered corneal epithelium. Tissue Eng Part A. 28 (23-24), 977-989 (2022).
- Mcnamara, N. A., Gallup, M., Porco, T. C. Establishing pax6 as a biomarker to detect early loss of ocular phenotype in human patients with sjogren's syndrome. Invest Ophthalmol Vis Sci. 55 (11), 7079-7084 (2014).
- Thacker, M., et al. Benzalkonium chloride-induced dry eye disease animal models: Current understanding and potential for translational research. Indian J Ophthalmol. 71 (4), 1256-1262 (2023).
- Singh, S., et al. Developing a model for aqueous deficient dry eye secondary to periglandular cicatrizing conjunctivitis. Exp Eye Res. 244, 109949 (2024).
- Singh, S., Sharma, S., Basu, S. Rabbit models of dry eye disease: Current understanding and unmet needs for translational research. Exp Eye Res. 206, 108538 (2021).
Réimpressions et Autorisations
Demande d’autorisation pour utiliser le texte ou les figures de cet article JoVE
Demande d’autorisationExplorer plus d’articles
This article has been published
Video Coming Soon