Method Article
A Protocol for Explant Cultures of IDH1-mutant Diffuse Low-grade Gliomas
Dans cet article
Résumé
IDH1-mutant diffuse low-grade gliomas are rare tumors that are difficult to culture. We introduce a simple, non-enzymatic explant method that preserves IDH1-mutant protein expression and sustains cultures for months. Importantly, this technique works with cryopreserved tumors, offering a valuable resource for glioma research.
Résumé
Diffuse gliomas, the most prevalent primary brain tumors, are categorized into low and high grades based on histopathological criteria and genetic mutations. Diffuse low-grade gliomas constitute a subset, predominantly afflicting young adults and predisposing to high-grade gliomas. There are two main types of diffuse low-grade gliomas: astrocytomas and oligodendrogliomas, each defined by distinct genetic and histological features. These tumors characteristically exhibit a mutated variant of the metabolic enzyme IDH1, disrupting cellular differentiation. They are heterogeneous, comprising both proliferative stem cells and more differentiated, quiescent glial cells.
Patient-specific, in vitro tumor models hold significant potential for unraveling oncogenic mechanisms and tailoring drug selection for personalized therapy. However, the absence of reliable in vitro models specifically for IDH1-mutant low-grade gliomas has hindered fundamental and translational research. Here, we present a simple method for culturing IDH1-mutant tumors as explants in defined media without needing enzymatic dissociation. These explants can be sustained for extended periods, maintaining IDH1-mutant protein expression as well as markers for oligodendrocyte progenitor cells such as ASCL1 (MASH1), OLIG1, and OLIG2 and for astrocytes (GFAP, SOX9). They can also be established from frozen tumors in DMSO. These cultures can serve as a valuable platform for drug screening, videomicroscopy, and gene studies, thus facilitating advancements in understanding and treating these challenging tumors. They also offer the potential to derive IDH1-mutant cell lines.
Introduction
Diffuse gliomas are the most frequent primary brain tumors. These tumors are currently incurable despite very intense treatment (surgery, radio, and chemotherapy)1. Increasing evidence supports the hypothesis that gliomagenesis originates from adult stem or glial progenitor cells resident in the brain2. Although glioblastomas represent the most aggressive type, diffuse low-grade gliomas (DLGG, grade 2 tumors) are also prevalent (15% of diffuse gliomas)1,3. DLGGs grow slowly and migrate along white matter tracts, a hallmark of this 'diffuse' neoplasm4. These DLGG very often recur despite treatment, ultimately progressing to high-grade malignancies (grades 3 and 4)5.
DLGG are characterized by a missense mutation in the isocitrate dehydrogenase 1 or 2 gene (IDH1 or IDH2), resulting in the aberrant production of the oncometabolite 2-hydroxyglutarate (2HG). This disrupts cell differentiation through epigenetic dysregulations6. IDH1-mutant gliomas are further classified into subtypes: astrocytomas typically have mutations in ATRX and P53, while oligodendrogliomas are characterized by 1p19q deletions7. These tumors exhibit a heterogeneity of tumoral cells. Single-cell RNA sequencing and immunocharacterization have revealed a diverse population of tumor cells, displaying stem-cell-like, astrocyte-like, and oligodendrocyte-like phenotypes within these tumors8,9.
In contrast to glioblastoma, which benefits from readily available, long-term cultures and cell lines, there exists a notable scarcity of cellular tools tailored for investigating IDH1-mutant tumors1. This presents a significant obstacle to advancing therapeutic development and deepening our understanding of IDH1-mutant gliomagenesis and disease progression. Traditional serum-grown cell lines, while long-utilized, inadequately replicate original tumors, displaying altered transcriptional profiles. Alternatively, serum-free culture conditions, whether in 2D or 3D tumoroid models, more effectively preserve the transcriptional profiles of tumor cells and their in vivo phenotypes, such as invasion into normal brain tissue10,11.
In addition, short-term cell cultures derived from a patient's surgical specimen preserve the molecular profile and cellular diversity of the tumor, thereby better representing the biological traits of tumors compared to cell lines12. Herein, we introduce a straightforward non-enzymatic method for culturing IDH1-mutant diffuse low-grade gliomas culture as explants derived from fresh or frozen patient resections, aiming to address the need for improved in vitro models for glioma research and therapeutic development. They also offer the potential to derive IDH1-mutant cell lines.
Protocole
The tumor fragments utilized in this protocol were obtained from a well-characterized and ethically approved collection, with patient consent. This research was conducted under the project titled Gliomacult: Characterization and Primary Cultures of Low-Grade Gliomas (Project No. RECH/P722/1-5) and was approved by the tumor biobank's scientific council on August 28, 2023.
1. Preparation of culture plates
NOTE: Perform these steps under a sterile tissue culture laminar flow hood.
- At least 1 h before culture, prepare poly-D-lysine (PDL) and laminin-coated 24 well plates.
- Dilute 10 mg of PDL powder in 100 mL of borate buffer (pH 8.4) for a final concentration of 100 µg/mL. Once dissolved, filter the solution (0.22 µm) for sterility. PDL is ready to use; prepare 3 mL aliquots and store them at -20 °C.
- Thaw the 1-2 mg/mL laminin tube at 4 °C to avoid gel formation. Prepare 50 µL aliquots and store them at -20 °C.
NOTE: Repeating thawing and freezing should be avoided. - Plate coating
- Optional step: place sterile coverslips Ø, 13 mm, suitable for cell culture dishes, in a 24-well plate, one coverslip per well.
NOTE: This step is for further immunostaining of glioma explants. - For the coating of the wells, mix the 3 mL of the PDL solution in 9 mL of 1x PBS and add 50 µL of laminin. Add 500 µL of this solution to each well for a 1-2 µg/cm² coating concentration. Incubate the coated plates at room temperature, preferably with slow rocking (15 RPM), for at least 1 h.
- Immediately before the culture, remove the PDL-laminin solution and rinse twice with sterile 1x PBS.
NOTE: Do not let the wells dry out.
- Optional step: place sterile coverslips Ø, 13 mm, suitable for cell culture dishes, in a 24-well plate, one coverslip per well.
2. Media
- Culture IDH1-mutant explants in a defined medium comprising DMEM/F12 supplemented with L-glutamine, along with a serum-replacement blend consisting of N2 and B27 without Vitamin A. Use antibiotics, including ciprofloxacin, gentamicin, and fungin, to prevent bacterial and fungal contamination. Additionally, incorporate growth factors heparin, EGF, and FGF2 into the medium, filter it (0.2 µm), and store it for up to 2 weeks at 4 °C.
NOTE: For details on media ingredients and preparation, refer to Table 1 and the Table of Materials.
3. Explant preparation
- Before sample processing, prepare the workspace and sterile tools as depicted in Figure 1A. Sanitize a cool pack by spraying it with 70% ethanol. Sterilize scissors and forceps by immersing them in 70% ethanol and rinsing them with PBS.
- Promptly transfer the tumor resection for culture on ice, ideally within 2 h post surgical extraction (Figure 1B).
NOTE: The tumor sample should have a minimum diameter of 9-10 mm to ensure better results. The tissue is transported without culture medium as this method is preferred for histological purposes. The absence of liquid prevents ice crystal formation during flash freezing, thereby preserving tissue integrity for subsequent histological examination if necessary. - Place the culture dish with the tumor specimen over the cool pack and gently rinse it with 1X PBS to remove excess blood.
- The resected tumor often exhibits significant size and heterogeneity, comprising tumor mass, infiltrated tissue, and non-tumoral components (Figure 1C). Select portions displaying a gray color with a soft or jelly-like texture for tumor cell isolation, as they tend to contain more tumor cells. Conversely, avoid hard and white regions as they may contain fewer tumor cells suitable for culture (Figure 1D), as well as blackened areas, as they are likely a result of electrocautery and may not provide reliable results for analysis.
- Transfer the selected samples into a 1.5 mL microtube or cryovial for mincing. Add 1 mL of sterile media to the 1.5 mL microtube or cryovial and chop the sample with the sterile scissors until small pieces (1-2 mm3) are obtained.
NOTE: Approximately 1-2 min of chopping is necessary to obtain tiny fragments, typically 1-2 mm3 in size (Figure 1E). - With sterile scissors, carefully trim the end of a blue 1,000 µL pipette tip to create a wide opening, roughly 3-4 mm in diameter. Employing this modified tip, pipette 50-200 µL of the minced tissue fragments and distribute them evenly across each well. Because the smaller pieces tend to settle rapidly, gently re-homogenize the suspension in each well to ensure uniform distribution.
NOTE: Avoid overcrowding and maintain a balance by not introducing an excessive number of fragments into each well (Figure 1F). - Incubate the tumor fragments at 37°C in a 5% CO2 incubator with 100% humidity. Change the media every day if the tumor's metabolic activity (indicated by rapid yellowing of the media) and the number of fragments in each well requires it. During media changes, exercise care by delicately aspirating the media using a pipette equipped with a 200 µL tip, ensuring to avoid disturbing any adhering tumor fragments.

Figure 1: Tumor explant culture procedure. A diffuse low-grade glioma resection was obtained from a 44-year-old patient diagnosed with a grade II astrocytoma according to the WHO 2021 classification. The tumor exhibited an IDH1 mutation (Exon 4 c.395 G>A, p.Arg132His) and a TP53 mutation (Exon 8 c.843_844delinsGT, p.Asp281_Arg282delinsGluHis), along with the absence of ATRX staining. No mutations were detected in the Ets/TCF binding sites (C250T and C228T) of the TERT gene. (A) The working surface comprises a cool pack, providing an environment that minimizes sample degradation and maintains the viability of biological components. These precautions are important for preserving the quality and integrity of the tissue. (B) Transport of the sample from the hospital to the lab should be done on ice. (C) Tumor resection fragment. Arrows show the most suitable parts for culture. (D) Dissection of the tumor is performed to select the most relevant part to be minced. In our experience, white matter is generally less likely to generate successful explants. (E) Tumor fragments of approximately 1-2 mm³ of diameter in a 15 mL tube after being mechanically minced with scissors. (F) The number of tumor pieces required for the proper seeding is shown for wells on a 24-well plate. Please click here to view a larger version of this figure.
4. Minced tumor cryopreservation and thawing
NOTE: Cryopreservation ensures backup in case of contamination and loss of explant cultures or to process the tumor samples later.
- Cryopreservation: Prepare the freezing medium (20% v/v DMSO solution) by adding 2 mL of DMSO to 8 mL of cell culture medium.
- Combine 1 volume of the minced tumor suspension with an equal volume of the 20% DMSO freezing medium to achieve a final concentration of 10% DMSO. Carefully pipette the mixture up and down to ensure thorough blending, then dispense approximately 1 mL into cryovials.
- Rapidly transfer the cryovials into a cryogenic container for gradual temperature reduction, followed by overnight incubation at -80 °C before final storage in liquid nitrogen.
NOTE: This step must be performed immediately after the cells are placed in the freezing medium, as certain cryoprotectants, like DMSO, can be harmful if the cells are exposed to them at room temperature for longer than necessary. - Thawing: Remove the cryovials from the liquid nitrogen and immediately place them in a 37 °C water bath until around 80% of the cells have thawed.
- To remove the DMSO, quickly pipette the suspension into a 15 mL centrifuge tube containing 5 mL of prewarmed 1x PBS and centrifuge at 300 × g for 5 min.
- Remove the supernatant and repeat the wash with 1X PBS followed by centrifugation (step 4.5). Aspirate the supernatant, taking care not to disturb the pellet, and add the appropriate amount of medium to transfer into a precoated PDL-Laminin 24-well plate and place in the incubator.
- After 24 h, verify that the cells have attached. After a few days, remove non-adherent fragments by replacing the culture media.
5. Passage of the culture
NOTE: Once established, explants can be used for videomicroscopy or immunostaining. Alternatively, if tumor cells have migrated out of the explant and appear healthy, the explant can be passaged through enzymatic dissociation and reseeded in wells for further experimentation. However, it is important to note that this protocol is not designed for repeated passaging. Typically, only a single passage can be attempted before the tumor cells become senescent or die. Not all explant cultures will result in successful passage.
- To passage, remove old media, rinse with 1X PBS, and add (125 µL) 0.05% trypsin-EDTA to the wells and incubate for 3-4 min at 37 °C, followed by the addition of 6.5 µL of Trypsin Inhibitor, 12 µL of 20 mM CaCl2 (final dilution to 2mM), and 1 µL of DNAse I.
NOTE: The incubation time needed for dissociation may vary depending on the culture. It is important to check whether the cells are detached after at least 3 min. Typically, the explant may not be fully dissociated into a single-cell suspension, and small clusters are often retained, which can generate new explant cultures. - With a p1000 set on 500-600 µL, flush gently to disaggregate explants. Avoid bubble formation.
- Add 5 mL of 1x PBS and centrifuge for 5 min at 300 × g. Aspirate the supernatant, resuspend the pellet in fresh prewarmed medium, and count for seeding.
- Identify cultures with the potential to generate cell lines by observing dividing cells under a phase-contrast microscope. These dividing cells typically have a rounded morphology and visible chromosomes, as shown in Figure 2A.
Résultats
After a few days, some tumor fragments will adhere to the well surfaces, initiating the outgrowth of cells from the explants. The time between the initial plating and the appearance of tumoral growing cells is different from sample to sample. The initial cultures often contain substantial cellular debris, making it difficult to assess the presence of viable cells. Consequently, the culture should be maintained for a minimum of 4 weeks. Once the cells start to grow, elongated cells with a bipolar or multipolar appearance are observed (Figure 2B,C).
When thawing cryopreserved minced tumors, the success rate of explant culture may slightly decrease. Although we have not accurately quantified the impact of cryopreservation, the reduction in success appears to be minor. We have not observed any significant differences in cell morphology between cells derived from fresh tumors and those from cryopreserved tumors, as demonstrated in the three cases shown in Figure 2D. A pronounced and extensive migration of cells from the tumor explant is also normally observed with cryopreserved samples. As shown in Figure 2E, even after a single passage of the explant, tumor-derived cells with a bipolar appearance can be observed forming an intricate and extensive network. These explant cultures can be used for videomicroscopy, allowing detailed observation of cell migration from the explant. As an example, we present a video showing a cell appearing to migrate along a radial fiber (Figure 3 and Supplemental Video S1).

Figure 2: Appearance of explant culture cells. (A) Brightfield microscopy images of an astrocytoma grade 2 explant culture at different time points. The black arrows highlight one rare proliferating cell. Dividing cells are recognizable by their round morphology and chromosomes aligned at metaphase (middle panel). (B) Illustration of a culture derived from an explant from a diffuse low-grade glioma resection. At the beginning of the culture, notably, there is a pronounced and extensive migration of cells from the tumor fragment (blue arrows), as well as the typical observation of round darker cells in such cultures (yellow arrows) referred to as gitter cells. (C) Cells will form an intricate and extensive network around the explants (blue arrows). (D) Examples of explant culture obtained after thawing three different minced cryopreserved tumors. After 2 weeks in culture, the presence of long bipolar cells is evident, indicating a healthy culture. (E) After being passaged, cells typically maintain their bipolar appearance. (F) In some cases where no explant attachment occurs within a 4 week period, the culture is deemed unsuccessful. Typically, these fragments have numerous gitter cells (red arrow), considered to be phagocytic microglial cells.13 A magnified view of these cells is provided in the inset. All scale bars: 10 µm. Please click here to view a larger version of this figure.

Figure 3: Images from explant videomicroscopy. Images captured from a videomicroscopy (see Supplemental Video S1) of a grade 3 astrocytoma explant over 11 s. The video illustrates an example of radial cell migration (indicated by the yellow circle), a phenomenon observed in gliomas16. Please click here to view a larger version of this figure.
The success of explant culture varies depending on the tumor, with either all wells forming a healthy culture or only a fraction of them yielding viable cells. If explants fail to attach within 3 weeks, the culture is typically discarded. These unsuccessful cultures often exhibit non-adherent, floating fragments containing numerous cells which we refer to here as gitter cells as illustrated in Figure 2F. These gitter cells display intense autofluorescence and bear resemblance to recently described lipid-laden macrophages13. They are likely enlarged phagocytic cells capable of engulfing substantial amounts of fatty material, such as breakdown products from myelin. Unpublished data from our lab based on RNA-seq indicate that these cells strongly express CD68, AIF1 (Iba1), and TMEM119, suggesting that they are microglial or macrophage cells. However, their complete identity and function remain to be elucidated. The success rate of explant culture is typically around 1 in 3 cultures. This likelihood is influenced by factors such as the number of tumoral cells present in the resected tissue and the surgical techniques employed, which may inadvertently damage cells, particularly with procedures like electrocautery.
The presence of mutant cells in these explant cultures is demonstrated by immunofluorescence targeting the mutant form of IDH1 R132H. For example, by deriving an explant culture from a typical grade II astrocytoma, we were able to detect cells both in the original tissue and the corresponding explant. This included the presence of IDH1 R132H-positive and ATRX-negative cells, which are hallmarks of these tumors, in the original sample and explant culture (Figure 4A). Even after passage through the explant, IDH1-mutant cells can still be detected (Figure 4B). To assess the proliferation of tumor cells, we conducted co-staining for IDH1 R132H and Ki67. It is important to note that the presence of KI67 alone does not guarantee cell proliferation, as cells may be arrested in the G2/S phase. We observed a significant decrease in Ki67-positive tumor cells, from approximately 13% in the primary explant to 4% after passage 1 (Figure 4B,C). This limited proliferation likely reflects the slow-growth nature observed in patients with DLGG, typically characterized by a low Ki67 proliferation index within the tumor14. By passage 2, most cells die or become senescent, indicating that the culture conditions are not optimal for the long-term propagation of IDH1 R132H tumor cells.
As we previously reported15, IDH1-mutant tumor cells in these explant cultures retain many of the markers expressed in the original tumors, such as DCX, EGFR, LIFR, MAG, MYT1, NG2, OLIG1/2, PTPRZ1, PDGFRA, SOX8, and SOX9 (Figure 5). We confirmed this by demonstrating that in one explant culture derived from a grade II astrocytoma, IDH1-mutant cells with a radial morphology express astrocytic (GFAP, SOX9) and oligodendrocyte lineage markers (ASCL1/MASH1, OLIG1, OLIG2; Figure 4D).

Figure 4: Explant cell characterization. The same tumor resection as in Figure 1 was used in this experiment. (A) Immunofluorescence staining of a section from an astrocytoma with IDH1 R132H and ATRX mutations and the corresponding explant culture. Green arrows indicate IDH1 R132H-positive, ATRX-negative tumor cells present in both the tissue (left images) and the explant culture (right images). Yellow arrows point to ATRX-positive, IDH1 R132H-negative cells, which are presumed to be non-tumoral. (B) Staining for Ki67 and IDH1 R132H in primary explant culture and after passage, derived from an astrocytoma. (C) Quantification of the staining shown in B, indicating a reduction in Ki67-positive tumor cells after passage (100 counted cells for primary culture and 60 cells for passaged). (D) Characterization of IDH1 R132H-positive tumor cells in a primary explant derived from an astrocytoma, using neural lineage cell markers (ASCL1, GFAP, OLIG1/2, SOX9) by immunofluorescence. For ASCL1 staining, the green arrow indicates one positive cell, while the blue arrow points to one negative or weakly stained cell. (E) Immunofluorescence staining for GFAP and OLIG2 in passaged explants, showing the maintenance of the cell phenotype. (F) Quantification of the staining shown in D and E for GFAP and OLIG2 (90 counted cells) in primary and passaged explant. Please click here to view a larger version of this figure.
Notably, after passaging, tumor cells tend to increase GFAP expression while reducing OLIG2 expression (Figure 4E,F). Additionally, we observed that after passaging, GFAP-positive cells become larger, suggesting a modification of their phenotype. We also identified heterogeneity among tumor cells, as seen in the presence of both ASCL1-positive and ASCL1-negative IDH1 R132H-positive cells (Figure 4D), as well as GFAP-high and GFAP-low IDH1 R132H-positive cells (Figure 6C). It is important to note that these explant cultures contain not only tumor cells but also non-tumoral cells. Specifically, we observed the presence of microglial cells (CD68+, IBA1+) (Figure 6A) and GFAP-positive, IDH1 R132H-negative cells, which may represent reactive astrocytes (Figure 6B).
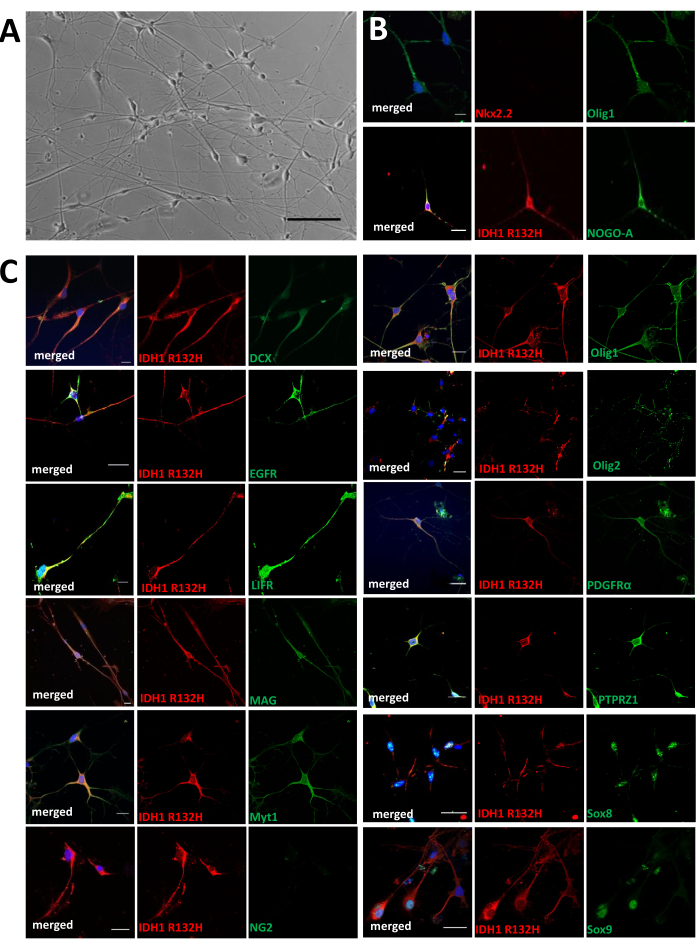
Figure 5: Characterization of explant cells in vitro. (A) Representative photographs of an explant culture obtained from one astrocytoma (scale bar = 50 µm). The culture contains cells with different morphologies. (B,C) Representative images of double immunofluorescences against IDH1R132H (red) and indicated marker (green) are shown. Tumoral cells express IDH1R132H and explored markers except NG2 and Nkx2.2. Scale bars = 20 µm. This figure was taken from Azar et al.15. Please click here to view a larger version of this figure.

Figure 6: Explant microenvironment and tumor cell heterogeneity. The same tumor resection used for Figure 1 was used here. (A) Immunofluorescence for CD68 and IBA1 indicates the presence of microglial cells in the culture. (B) Immunostaining for GFAP shows the presence of a double-positive IDH1 R132H/GFAP tumor cell (green arrow) and one IDH1 R132H-negative/GFAP-positive cell (yellow arrow), presumably a reactive astrocyte. (C) Tumor cell heterogeneity is demonstrated by GFAP staining, with one IDH1 R132H-positive cell showing high GFAP expression (green arrow) and another displaying weak or no GFAP expression (blue arrow). See also ASCL1/IDH1 R132H staining in Figure 4D. Please click here to view a larger version of this figure.
Supplemental Video S1: Migration of a cell along a fiber in an astrocytoma grade II glioma explant. This 11 s video was captured using an inverted Axiovert microscope. Still images from this video are displayed in Figure 3. Please click here to download this File.
| Media component | Stock concentration | Volume to add | Final concentration |
| DMEM F12 | - | 50 mL | - |
| B27 | 50x | 125 µL-250 µL | 0.125x -0.250x |
| N2 | 100x | 500 µL | 1x |
| Heparin* | 2 mg/mL | 50 µL | 2 µg/mL |
| EGF** | 50 µg/mL | 10 µL | 10 ng/mL |
| FGF2** | 50 µg/mL | 10 µL | 10 ng/mL |
| L-Glutamine | 200 mM | 500 µL | 2 mM |
| Ciprofloxacine | 10 mg/mL | 10 µL | 2 µg/mL |
| Gentamycine | 50 mg/mL | 10 µL | 10 µg/mL |
| Fungin | 10 mg/mL | 10 µL | 2 µg/mL |
| *Heparin is resuspended in DPBS without Ca2+ / Mg2+ and filter with 0.22 µm filter | |||
| **Cytokines are resuspended in sterile DPBS without Ca2+/Mg2+ and containing crystalline BSA 1%, HEPES 1mM, pH 7.3 | |||
Table 1: Composition of glioma explant culture media.
Discussion
Developing in vitro and preclinical models for IDH1-mutant low-grade gliomas is particularly challenging due to the slow growth of these tumors and the complexity of replicating the extensive genomic and epigenomic alterations caused by the IDH mutation. Moreover, these infiltrative tumor cells actively interact with surrounding cells, including immune, endothelial, glial, and neuronal cells, potentially relying on them for survival. This complex interplay is difficult to replicate in a laboratory setting.
To uncover novel biological insights into IDH1-mutant low-grade gliomas and advance the development of innovative therapeutics, it is essential to establish models that faithfully replicate the intricacies of human disease. Compared to higher-grade gliomas, such as glioblastomas, there are currently relatively few in vitro models available for IDH-mutant gliomas. Most IDH1-mutant cell lines are derived from high-grade gliomas, such as anaplastic oligodendrogliomas (e.g., BT260 and BT237), which harbor additional mutations and only partially recapitulate the characteristics of diffuse low-grade gliomas with IDH1 mutations.
This method has some critical steps to consider. First, the resected tumor often exhibits significant size and heterogeneity, comprising tumor mass, infiltrated tissue, and non-tumoral components. Discerning these distinct parts can pose challenges, complicating the selection process for culturing (Figure 1C). As mentioned in protocol step 3.4, in our experience, higher numbers of tumor cells are obtained from portions displaying a gray color with a soft or jelly-like texture rather than from hard and white regions (Figure 1D) and blackened areas. Second, it is important to mince the tumor carefully into tiny fragments that are 1-2 mm3 in size. Third, while seeding with the tumor fragments, it is important to distribute the fragments evenly across each well without overcrowding (Figure 1F). Given that the smaller pieces tend to settle rapidly, it is imperative to gently re-homogenize the suspension in each well to ensure uniform distribution. Fourth, since the resection diagnosis will only be obtained a few weeks later from the pathology laboratory, cryopreservation of the cultures will allow for the subsequent culture of only those resections that have mutations of interest for a specific project (e.g., IDH1, ATRX, P53, SMARCA4). Next, thawing must be done quickly when recovering cells from liquid nitrogen. Finally, in our experience, explant cultures obtained from DLGG can be maintained for at least 2 months without a substantial increase in cell number and passage once at most.
The method presented here offers a simple and cost-effective approach to generating IDH1-mutant DLGG explant cell cultures directly from human brain tumor resections. This technique allows for the establishment of cultures even from small samples, as minimal as the size of a green pea. These explant cultures preserve the tumor's genetic makeup, including the mutation burden and some heterogeneity of both tumor and non-tumor cells within their microenvironment. Immunofluorescence characterization of the explants demonstrates that IDH1 R132H-positive cells retain the expression of key markers found in the tumors, such as GFAP, SOX9, ASCL1, and OLIG1/2. These results indicate that the tumor cells in the explants maintain important phenotypic characteristics despite the culture conditions.
A significant limitation of this method is that extensive resections, which can contain varying amounts of tumoral and non-tumoral tissues, may result in explant cultures with a wide range of IDH1 R132H-positive percentages, ranging from 5% to 90%. Additionally, another limitation of this explant culture method is its inherently slow growth rate, which restricts long-term expansion and propagation through passaging. However, although these explant cultures are not primarily intended for passaging, it can be performed once without major alterations to the culture. Explant cells can be passaged when a substantial number of cells have migrated from the tissue and spread across the coverslip or well. However, after passage, we observed a reduction in cell proliferation, as indicated by a decrease in Ki67-positive cells, and with subsequent passages, cells may cease to divide, die, or become senescent. We hypothesize that modifying the culture medium -- perhaps by introducing specific cytokines or chemicals -- could promote the long-term propagation of IDH1-mutant cultures by activating or inhibiting key signaling pathways, such as Notch signaling. It is noteworthy that cultivating cells in a low-oxygen environment may also promote the growth of diffuse low-grade glioma tumors11; however, this effect needs to be established in these explant cultures.
This protocol is not designed for repeated passaging. Typically, only a single passage can be attempted before the tumor cells become senescent or die. Not all explant cultures will result in successful passage. We usually proceed with passaging only when a significant number of cells have migrated from the tissue and spread across the coverslip. The primary criterion for passaging is the extent of cell migration from the explant, ensuring full coverage of the coverslip. We generally limit this to a single passage, unless there is a clear population of proliferating cells, in which case further passaging may facilitate the establishment of a cell line.
While most explant cultures do not lead to the establishment of cell lines, we have successfully derived three IDH1-mutant cell lines from these cultures, despite the very low success rate. One of these cell lines (LGG275) was partially characterized in our recent publication9 (Figure 7). Other cell lines are currently undergoing further characterization in the laboratory. It appears that only when tumor cells harbor additional mutations such as CDKN2A or RB1, they exhibit enhanced proliferative capabilities, allowing for the expansion and establishment of cell lines.

Figure 7: Phenotypic characterization of the LGG275 cell line. (A) Morphology of LGG275 cells. Scale bar=10 µm. (B ) Double immunofluorescences for indicated proteins. The tumoral status of these cells is indicated by the detection of IDH1 R132H and absence of nuclear ATRX (lanes 1, 2, 3). LGG275 cells show staining for CNP, EGFR, OLIG1 and SOX9 (lanes 2, 3, 4). Detection of SOX10 is weak in these cells (lane 1). Note that LGG275 cells show different nuclei size and shape. White arrowheads represent double positive cells while yellow arrowheads indicate single positive cells. Scale bars=20 µm. (C) Schematic illustration depicting the phenotype and mutation profile of LGG275 cells. This figure was taken from Augustus et al.9. Please click here to view a larger version of this figure.
Additionally, by avoiding enzymatic cell isolation procedures, this method likely preserves the intricate interactions between tumor cells and their surrounding environment. We observed microglial cells and non-tumoral IDH1 R132H-negative cells, indicating the presence of tumor-associated cells. This procedure enables further studies to address additional inquiries regarding diffuse low-grade gliomas, such as videomicroscopy for studying cell migration, in vitro drug screening, or orthotopic xenografting. By preserving cellular heterogeneity from primary patient specimens, we gain a valuable tool for enhancing our understanding of tumor biology. Unlike conventional cell culture methods, which often yield homogeneous tumor cell populations, this approach provides a more representative depiction of the complex tumor microenvironment. In summary, the establishment of these patient-derived, IDH1-mutant, diffuse, low-grade glioma cultures represents a significant advancement in the field, playing a crucial role in identifying the most effective therapeutic strategies.
Déclarations de divulgation
The authors have no conflicts of interest to declare.
Remerciements
The tumor fragments used here are part of the NEUROLOGY collection of fresh tissue samples maintained by the Biological Resource Center at Montpellier University Hospital (CRB@chu-montpellier.fr). This research was conducted under the project titled "Gliomacult: Characterization and Primary Cultures of Low-Grade Gliomas," led by Prof. J. P. Hugnot (Project No. RECH/P722/1-5). This work was supported by La ligue contre le cancer, ARC (Association for Cancer Research), ARTC, ARTC SUD, Les Etoiles dans la mer, EMBALL'ISO, Région Occitanie, INCA. K.A.C is supported by the National Council of Humanities Science and Technology (CONAHCYT), Mexico, and the Association for Brain Tumor Research (ARTC), France.
matériels
| Name | Company | Catalog Number | Comments |
| Anti-ASCL1 | BD Pharmingen™ | 556604 | Dilution 1:500 |
| Anti-ATRX | Sigma-Aldrich | HPA001906 | Dilution 1:100 |
| Anti-CD68 | Agilent | M081401-2 | Dilution 1:500 |
| Anti-GFAP | Aves Labs | AB_2313547 | Dilution 1:500 |
| Anti-IBA1 | Fujifilm Wako Antibodies | 019-19741 | Dilituion 1:1000 |
| Anti-IDH1 (R132H) mouse Monoclonal Antibody | Dianova | DIA-H09 | |
| Anti-Ki67 | BD Pharmingen™ | 556003 | Dilituion 1:1000 |
| Anti-OLIG1 | R&D Systems® | AF2417 | Dulituion 1:300 |
| Anti-OLIG2 | Sigma-Aldrich | MABN50 | Dilution 1:250 |
| Anti-SOX9 | Abcam® | EPR14335-78 | Dilution 1:100 |
| B-27 Supplement (50x), minus vitamin A | Thermo Fisher Scientific | 12587010 | |
| Bovine Serum Albumin (BSA) | CiteAb | A-421-100 | |
| Ciprofloxacin HCl | Sigma-Aldrich | PHR1044-1G | |
| CoolCell | Corning® | 432000 | Cell Freezing Container, for 12 x 1 mL or 2 mL Cryogenic Vials |
| Cover slip Ø, 13 mm | Karl Hecht™ Assistent™ | 328017 | |
| Dimethyl Sulfoxide (DMSO) | Sigma-Aldrich | D8418 | |
| DMEM/F-12, no glutamine | Thermo Fisher Scientific | 21331020 | |
| DNAse I | Sigma-Aldrich | 10104159001 | |
| Dulbecco′s Phosphate Buffered Saline (PBS) 1x | Sigma-Aldrich | D8537 | |
| Falcon 100 mm TC-treated Cell Culture Dish | Corning® | 353003 | |
| Falcon 24-well Clear Flat Bottom | Corning® | 353047 | TC-treated Multiwell Cell Culture Plate, with Lid, Sterile |
| Fungin | InvivoGen | ant-fn-1 | |
| Gentamicin | Thermo Fisher Scientific | 15750-037 | |
| Heparin | Sigma-Aldrich | H3149-100KU | |
| HEPES | Sigma-Aldrich | H-3375 | |
| Human EGF | PeproTech | AF-100-15-1MG | |
| Human FGF-basic | PeproTech | 100-18B-1MG | |
| Laminin | Sigma-Aldrich | L2020 | |
| L-Glutamine | Thermo Fisher Scientific | 25030-024 | |
| N-2 Supplement (100x ) | Thermo Fisher Scientific | 17502-048 | |
| PDL | Sigma-Aldrich | P7886 | |
| Serum acrodisc 37 mm syringe filter with GF/0.2 µm Supor | PALL Corporation | 4525 | |
| Trypsin Inhibitor | Thermo Fisher Scientific | 17075-029 | |
| Trypsin-EDTA (0.05%), phenol red | Thermo Fisher Scientific | 25300054 | |
| Zeiss Z1 Apotome 3 microscope | Zeiss | straight widefield epifluorescence microscope |
Références
- Dasgupta, P., Balasubramanyian, V., De Groot, J. F., Majd, N. K. Preclinical models of low-grade gliomas. Cancers (Basel). 15 (3), 596 (2023).
- Tirosh, I., et al. Single-cell RNA-seq supports a developmental hierarchy in human oligodendroglioma. Nature. 539 (7628), 309-313 (2016).
- Jooma, R., Waqas, M., Khan, I. Diffuse low-grade glioma - changing concepts in diagnosis and management: A review. Asian J Neurosurg. 14 (2), 356-363 (2019).
- Louis, D. N. Molecular pathology of malignant gliomas. Annu Rev Pathol. 1, 97-117 (2006).
- Adenis, L., Plaszczynski, S., Grammaticos, B., Pallud, J., Badoual, M. The effect of radiotherapy on diffuse low-grade gliomas evolution: Confronting theory with clinical data. J Pers Med. 11 (8), 818 (2021).
- Pirozzi, C. J., Yan, H. The implications of idh mutations for cancer development and therapy. Nat Rev Clin Oncol. 18 (10), 645-661 (2021).
- Van Den Bent, M. J., Smits, M., Kros, J. M., Chang, S. M. Diffuse infiltrating oligodendroglioma and astrocytoma. J Clin Oncol. 35 (21), 2394-2401 (2017).
- Suva, M. L., Tirosh, I. The glioma stem cell model in the era of single-cell genomics. Cancer Cell. 37 (5), 630-636 (2020).
- Augustus, M., et al. Identification of CRYAB+ KCNN3+ SOX9+ astrocyte-like and EGFR+ PDGFRA+ OLIG1+ oligodendrocyte-like tumoral cells in diffuse idh1-mutant gliomas and implication of NOTCH1 signalling in their genesis. Cancers (Basel). 13 (9), 2107 (2021).
- Hubert, C. G., Rich, J. N. Patient-derived explants as tumor models. Cancer Cell. 40 (4), 348-350 (2022).
- Abdullah, K. G., et al. Establishment of patient-derived organoid models of lower-grade glioma. Neuro Oncol. 24 (4), 612-623 (2022).
- Yuzhakova, D. V., et al. Development of a 3d tumor spheroid model from the patient's glioblastoma cells and its study by metabolic fluorescence lifetime imaging. Sovrem Tekhnologii Med. 15 (2), 28-38 (2023).
- Kloosterman, D., et al. Morphology of microglia across contexts of health and disease. Cell. 187, 5336-5356.e30 (2024).
- Thomas, D. L. 2021 updates to the World Health Organization classification of adult-type and pediatric-type diffuse gliomas: A clinical practice review. Chin Clin Oncol. 12 (1), 7 (2023).
- Azar, S., et al. Cellular and molecular characterization of IDH1-mutated diffuse low grade gliomas reveals tumor heterogeneity and absence of EGFR/PDGFRalpha activation. Glia. 66 (2), 239-255 (2018).
- Bhaduri, A., et al. Outer radial glia-like cancer stem cells contribute to heterogeneity of glioblastoma. Cell Stem Cell. 26 (1), 48-63.e6 (2020).
Réimpressions et Autorisations
Demande d’autorisation pour utiliser le texte ou les figures de cet article JoVE
Demande d’autorisationExplorer plus d’articles
This article has been published
Video Coming Soon