Method Article
An Inexpensive Adaptation of a Commercial Microwave Reactor for Solid Phase Peptide Synthesis
Dans cet article
Résumé
A simple, inexpensive, and novel apparatus for performing solid phase peptide syntheses in a commercial microwave reactor is presented.
Résumé
A home-built apparatus to perform solid phase peptide synthesis (SPPS), assisted by microwave irradiation and heating, is presented. In contrast to conventional SPPS reaction vessels, which drain solvent and byproducts via a frit located at the bottom of the vessel, the presented apparatus employs a gas dispersion tube under vacuum to remove solvent, byproducts, and excess reagents. The same gas dispersion tube supplies nitrogen gas agitation of the SPPS beads during the reaction steps of coupling and deprotection. Microwave heating is beneficial for SPPS couplings of sterically hindered residues, such as alpha-aminoisobutyric acid (Aib), an alpha,alpha-dialkylated amino acid residue. This home-built apparatus has been used to prepare, via manual Fmoc SPPS methods, heptameric and octameric peptides dominated by the Aib residue, which is notoriously difficult to couple under standard room temperature conditions and reagents. Further, typical commercial microwave SPPS reactors are dedicated exclusively to SPPS synthesis rendering them inaccessible to non-SPPS users. In contrast, the presented apparatus preserves the versatility of the microwave reactor for conventional microwave acceleration of chemical reactions, as the apparatus is trivially removed from the commercial microwave reactor.
Introduction
Merrifield's introduction of solid phase peptide synthesis SPPS in the 1960s revolutionized peptide and chemical syntheses and was justly rewarded with a Nobel Prize in Chemistry1,2. In subsequent decades, many researchers have refined Merrifield's original techniques, leading to two alternatives that dominate SPPS practices: fluorenylmethoxycarbonyl (FMOC)-based versus tert-butyl oxycarbonyl (BOC)-based3. Final cleavage of the peptide from solid resin in FMOC requires a cocktail containing trifluoracetic acid, as compared to HF for BOC techniques, making FMOC-based methods the preferred choice of many laboratories.
State-of-the-art SPPS methods are occasionally challenged by desired sequences. In recent years, there have been advances overcoming some idiosyncratic concerns of aggregation4, diketopiperazine formation5, and N-methylated amino acid residues6. In an effort to optimize coupling yield, thousands of coupling reagents and additives have been explored7. Specifically, carboxylic acid activation via COMU8,9 or acid fluorides10,11,12, along with additives such as Oxyma13 and hydroxybenzotriazole (HOBt), have been developed for particularly challenging couplings such as those involving α,α-dialkylated residues.
Other non-reagent-based methods have been employed to increase yields for difficult couplings, including extended reaction times, "double coupling," and heating, especially microwave heating14,15,16. Indeed, commercially available automated peptide synthesizers employing microwave heating are among the most commercially successful units in the market as they speed reaction rates, appear to improve the final purity of peptides, and minimize solvent waste. Unfortunately, these units can be expensive, costing over $100,000 in some instances.
Our laboratory has interests in preparing variations of peptides containing the strongly helicogenic residue of alpha-aminoisobutryic acid (Aib)17,18,19. Due to the steric hindrance arising from the dialkylation of its alpha-carbon, Aib is notoriously difficult to couple. The methods mentioned above (acid fluorides, microwave heating20), as well as clever "dipeptide" SPPS chemistry21, have been used to adapt SPPS to Aib's coupling challenges. Off-resin preparation of Aib dimers, while improving overall yield, requires additional wet chemistry and elevated temperatures (50 °C)21. Moreover, we have avoided employing acid fluorides of Aib due to the toxic nature of fluorinating agents. Unfortunately, our laboratory lacks a dedicated microwave-based automated SPPS synthesizer, with dedicated reaction vessels for the necessary draining of SPPS byproducts. Nevertheless, a recent report from Clayden's group showing spectacular success in preparing Aib-based oligomers using SPPS and microwave irradiation22 stimulated us to adapt our laboratory's CEM Discover SP microwave unit for SPPS syntheses.
We first investigated CEM's commercially available accessory to convert the microwave reactor into a manual SPPS/microwave unit23. Apart from the cost, this accessory would require the commitment of our microwave unit to only manual SPPS. Other laboratory users would no longer have access to the excellent capabilities of the microwave unit. Therefore, the implementation of the commercial accessory was deemed unacceptable in our case.
Instead, we assembled, through comparatively inexpensive components, an apparatus to perform microwave-assisted SPPS on the millimolar scale. The protocol below describes using simple and comparatively inexpensive components to effect manual, microwave-assisted SPPS.
Protocole
1. Assemble the apparatus (Figure 1)
NOTE: All the components for assembling the apparatus are found in the Table of Materials.
- Assemble the vacuum outlet.
- Connect 2" of 1/8" Teflon tubing to the right side of the "Tee" (Figure 1, Part labeled "1") made of ethylene tetrafluoroethylene (ETFE).
- Connect the 1/8" Teflon tube to a valve (Figure 1, Part labeled "2") made of ETFE.
- Attach 100 cm of Teflon tubing to the other side of the valve (Figure 1, Part labeled "2").
- Insert this tubing through the 1/8" opening of a rubber cork and use this cork to cap a side-armed Erlenmeyer flask (Figure 1A, Part labeled "8") connected to vacuum (house or modest pump).
- Assemble the nitrogen gas inlet.
- Connect 2" of 1/8" Teflon tubing to the left side of the ETFE "Tee" (Figure 1, Part labeled "1").
- Connect the 1/8" Teflon tube to an ETFE valve (Figure 1, Part labeled "3").
- Attach 100 cm of 1/8" Teflon tubing to the other side of the EFTE valve (Figure 1, Part labeled "3").
- Thread the far end of the 1/8" Teflon tubing through a rubber cork and use this cork to connect to a tygon tube attached to a nitrogen cylinder fitted with a regulator (Figure 1A, Part labeled "7").
- With the needle valve of the regulator closed, use the diaphragm valve of the regulator (Figure 1A, Part labeled "7"). to achieve 5-10 psi pressure.
- Assemble the aspirator/bubbler.
- Connect the stem of the "Tee" (Figure 1, Part labeled "1") to a 100 cm length of 1/8" OD Teflon tubing.
- Insert this tubing through a micro-septum (Figure 1, Part labeled "6") previously pierced with a needle.
- Insert the tubing all the way into a gas dispersion tube. Use the micro-septum to form a seal between the gas dispersion tube (Figure 1, Part labeled "4") and the Teflon tubing.
- Insert the gas dispersion tube into the test tube reaction vessel (Figure 1, Part labeled "5")., and place the test-tube into its holder (Pictured in Figure 1B), which is external to the microwave reactor.
NOTE: During chemical reactions, the test tube will be inserted into the microwave reactor. During the addition of reaction solutions and solvent washes, the test tube is held external to the microwave reactor.
- Attach the open vessel attenuator to the microwave reactor. The apparatus should now be ready for use.
2. Prepare reagents
- Prepare FMOC-amino acid solutions. For 0.100 mmol scale, prepare 0.2 M FMOC-AA-OH in DMF. Each coupling cycle will use 2.5 mL of the amino acid solutions.
- Prepare diisopropylcarbodiimide (DIC) coupling reagent. Prepare 1.0 M DIC in DMF. Each coupling will use 1.0 mL of DIC solution.
- Prepare Oxyma Pure additive solution. Prepare 0.5 M oxyma in DMF. Each coupling will use 1.0 mL of oxyma solution.
- Prepare deprotection solution. Prepare 20% v/v morpholine/DMF solution. Each deprotection run requires 7 mL of solution. Perform two deprotection runs for each deprotection of fmoc.
3. Preparation of peptide resin
- Measure the appropriate mass of SPPS Wang resin, 100-200 mesh, polystyrene crosslinked with 1% divinylbenzene, and place the dry resin into the test tube reaction vessel. For example, for a 0.100 mmol scale synthesis, a resin with a loading of 0.500 mmol g-1 would require 200 mg of resin.
- Add 3 mL of DMF solvent to the resin and swell the resin at room temperature for 15 min. For this step, make sure the nitrogen gas supply is "on/open" for agitation, and the vacuum valve (Figure 1, labeled "2") is "off/closed". Use the needle valve of the nitrogen supply (Figure 1A, labeled 7) to achieve a gentle bubbling within the solution.
NOTE: This setting should be appropriate for all further agitation by the nitrogen gas. - Following 15 min of swelling the resin, aspirate DMF solvent under vacuum: close the N2 valve (Figure 1, labeled "3"). Open the valve for vacuum (Figure 1, labeled "2") and aspirate DMF solvent. When the solvent is nearly removed, open the N2 valve (Figure 1, labeled "3").
- Repeat steps 3.2 and 3.3 2 times to fully swell the resin.
4. FMOC removal
NOTE: Resins are available preloaded with a variety of FMOC-protected amino acids preloaded as the C-terminal residues. Therefore, FMOC removal is usually the first step after swelling the resin.
- With the test tube reaction vessel external to the microwave reactor and the vacuum valve (Figure 1, labeled "2") closed, add 7 mL of 20% piperidine/DMF or 20% morpholine/DMF directly into the test tube.
- Turn on the nitrogen valve (Figure 1, labeled "3") to agitate the beads.
NOTE: SPPS resins are available preloaded with the C-terminal residue, protected by FMOC. - Insert the test tube containing the beads in the microwave reactor via the "open vessel" attenuator (Figure 1B, metal port of reactor).
- Heat to 90 °C for 2 min by using the microwave in dynamic mode: set the temperature target to 90 °C, 50 W power, hold time = 2.0 min (see Supplemental File 1 for a report on deprotection within the microwave). Monitor the temperature via the infrared sensor of the microwave; the microwave will reduce power to maintain the reaction temperature.
- Remove the test tube containing the beads from the microwave reactor.
- Aspirate DMF solution and reaction byproducts under vacuum: close the N2 valve (Figure 1, labeled "3"). Open the valve for vacuum (Figure 1, labeled "2"). When the solution is nearly removed, open the N2 valve (Figure 1, labeled "3").
- Close the vacuum valve (Figure 1, labeled "2"). Add 3 mL of DMF to rinse the test tube and gas dispersion tube.
- Aspirate DMF solvent wash under vacuum: close the N2 valve (Figure 1, labeled "3"). Open the valve for vacuum (Figure 1, labeled "2"). When the solution is nearly removed, open the N2 valve (Figure 1, labeled "3").
- Repeat steps 4.7 and 4.8 two times, for a total of three washes with DMF solvent.
- Repeat FMOC removal steps 4.1-4.9 for a total of two successive deprotection steps.
5. Coupling of FMOC amino acids
- Place the test tube reaction vessel in its holder external to the microwave reactor.
- Manually add 2.5 mL of 0.2 M FMOC amino acid (5x molar excess), 1.0 mL of 1.0 M DIC coupling agent solution (10x molar excess), and 1.0 mL of 0.5 M oxyma additive solution (5x molar excess) to the resin in the test tube.
- Open the nitrogen valve (Figure 1, labeled "3") to achieve agitation of beads.
- Insert the test tube containing the beads into the microwave reactor via the open vessel attenuator (Figure 1B, metal port of reactor). Heat the solution to 100 °C for 10 min. Using the microwave in dynamic mode, set the temperature target to 100 °C, 60 W power, hold time = 10.0 min (see Supplemental File 1 for a report on coupling within the microwave). Monitor the temperature via the infrared sensor of the microwave; the microwave will reduce power to maintain the reaction temperature.
- Remove the test tube containing the beads from the microwave reactor.
- Aspirate DMF solvent and reaction byproducts under vacuum: close the N2 valve (Figure 1, labeled "3"). Open the valve for vacuum (Figure 1, labeled "2"). When the solution is nearly removed, open the N2 valve (Figure 1, labeled "3").
- Close the vacuum valve (Figure 1, labeled "2"). Add 3 mL of DMF to rinse the test tube and gas dispersion tube.
- Aspirate DMF solvent wash under vacuum: close the N2 valve (Figure 1, labeled "3"). Open the valve for vacuum (Figure 1, labeled "2"). When the solution is nearly removed, open the N2 valve (Figure 1, labeled "3").
- Repeat steps 5.7 and 5.8 2 times for a total of three washes with DMF solvent.
6. Repeat SPPS peptide cycles
- Repeat all steps in sections 4 (FMOC removal) and 5 (Coupling of FMOC amino-acids) until a peptide of the desired sequence.
NOTE: Be sure to add reagent solutions outside the microwave reactor, at room temperature, directly to the test tube reaction vessel. Also make sure that nitrogen agitation is initiated prior to placing the test tube in the microwave reactor.
7. Peptide cleavage
- Transfer the resin to a fritted plastic syringe.
- Prepare a cleavage cocktail of 95% trifluoroacetic acid (TFA), 2.5% water, and 2.5% triisopropyl silane (TIPS) at a volume of 1 mL/100 mg resin.
- Cleave the peptide for 2 h at room temperature with either continual or occasional shaking.
- Precipitate the peptide product in 10 mL of ice-cold diethyl ether.
- Resuspend the pellet in a minimal amount of MeCN/0.1% aqueous TFA and lyophilize overnight.
Résultats
Examples of peptide sequences prepared with our apparatus are shown in Figure 2 and Figure 3. Table 1 summarizes the solutions, microwave parameters, and washes we employed. MALDI-TOF mass spectrometry confirmation is shown in the figures. Mass recovery of these peptides has been above 80%. Significantly, all these sequences have multiple couplings between adjacent alpha, alpha-dialkylated amino acids. These are among the most difficult residues to couple together via peptide bond formation.
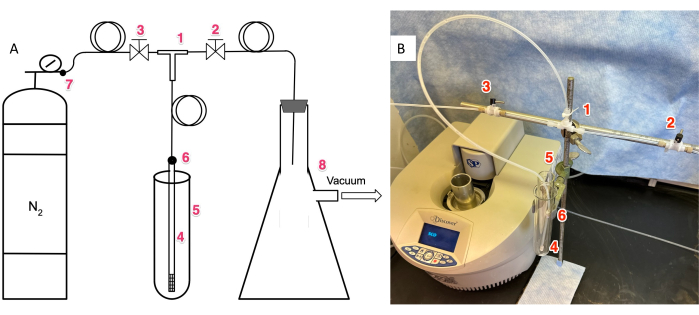
Figure 1: The home-built, microwave-assisted, manual SPPS apparatus. (A) Schematic showing 1) ETFE "tee"; 2) ETFE valve to vacuum; 3) ETFE valve to nitrogen gas; 4) gas dispersion tube, within 5) Pyrex test tube reaction vessel; 6) NMR-type septum to join 1/8" Teflon tubing to gas dispersion tube; 7) needle valve to control nitrogen gas flow; 8) side-arm flask to collect reaction waste. See Table of Materials for manufacturers and catalog numbers of components. (B) Photograph of assembled image. Numbering is identical to Figure 1A. Abbreviations: SPSS = solid phase peptide synthesis; ETFE = ethylene tetrafluoroethylene; NMR = nuclear magnetic resonance. Please click here to view a larger version of this figure.

Figure 2: Structure of peptide 1, synthesized via microwave SPPS methods and MALDI-TOF confirmation. (A) CHCA was employed as the matrix. The peak at m/z = 880.18 is potassium adduct, [MK+], calculated m/z=880.45. (B) HPLC trace of crude peptide 1. The solvent gradient is MeCN from 20% to 80% over 0.1% aqueous TFA, 20 min. Wavelength of observation = 222 nm. Abbreviations: SPSS = solid phase peptide synthesis; MALDI-TOF = matrix-assisted laser desorption ionization-time of flight; CHCA = alpha-cyano-4-hydroxycinnamic acid, HPLC = high-performance liquid chromatography; MeCN = acetonitrile; TFA = trifluoroacetic acid. Please click here to view a larger version of this figure.

Figure 3: Structure of peptide 2, synthesized via microwave SPPS methods and MALDI-TOF confirmation. (A) CHCA was employed as the matrix. The peak at m/z = 1022.7 is assigned to the potassium adduct, [MK+], calculated m/z=1023.5. The peak at m/z = 966.5 has been tentatively assigned to a C-terminal oxazolone via water loss24, calculated m/z = 967.5. (B) HPLC trace of crude peptide 2. The solvent gradient is MeCN from 20% to 80% for 20 min over 0.1% aqueous TFA. Wavelength of observation = 222 nm. Abbreviations: SPSS = solid phase peptide synthesis; MALDI-TOF = matrix-assisted laser desorption ionization-time of flight; CHCA = alpha-cyano-4-hydroxycinnamic acid, HPLC = high-performance liquid chromatography; MeCN = acetonitrile; TFA = trifluoroacetic acid, Please click here to view a larger version of this figure.
| Step | Solution/solvent | Volume (mL) | Time (Min) | Temp (°C) | |
| 1 | Deprotection | 20% Morpholine | 7 | 2 | 90 |
| 2 | Washes | DMF | 4, 4, 4 | - | RT |
| 3 | Deprotection | 20% Morpholine | 7 | 2 | 90 |
| 4 | Washes | DMF | 4, 4, 4 | - | RT |
| 5 | Coupling | Fmoc-AA-OH, OXYMA, DIC | 5, 2, 2 | 10 | 100 |
| 6 | Washes | DMF | 4, 4, 4 | - | RT |
| 7 | Repeat | ||||
| End | Cleavage | TFA/TIPS/H2O | 1 | >120 |
Table 1: Steps for one cycle of 0.1 mmol scale microwave-assisted SPPS: FMOC deprotection, washes, and FMOC-amino acid coupling. 0.2 M FMOC-AA-OH, 0.5 M OXYMA, 1.0 M DIC. Solutions added manually by micropipetter directly into the test tube for each step. Abbreviations: SPSS = solid phase peptide synthesis; FMOC = fluorenylmethoxycarbonyl; DIC = diisopropylcarbodiimide.
Supplemental File 1: CEM Discover SP Microwave Reactor Report for removal of Fmoc protecting group and CEM Discover SP Microwave Reactor Report for coupling an Fmoc-protected amino acid. Please click here to download this File.
Discussion
The apparatus presented here invokes a simple, novel, and inexpensive method for removing solvent, excess reagents and waste products, as well as adding nitrogen gas agitation, during microwave-assisted SPPS. In contrast to conventional SPPS vessels, which invoke a frit at the bottom of the vessel, the presented apparatus invokes a gas dispersion tube under vacuum to aspirate the vessel. The reaction vessel is therefore an ordinary test-tube, which is heated at the location recommended by the microwave reactor manufacturer within the "open vessel attenuator."
Our primary innovation is to remove solvents and reaction byproducts from the test tube via a "gas dispersion tube" under vacuum. During chemical transformations under microwave irradiation, as well as during washes with solvent (N,N-dimethylformamide (DMF) or N-methyl pyrrolidinone, NMP), the SPPS beads are agitated by nitrogen gas dispersed through the tube. The vacuum and nitrogen gas are controlled by ETFE valves to optimize resistance to solvent. SPPS waste is collected in a side-arm Erlenmeyer flask. All components are connected via 1/8" Teflon tubing. While we use "house" vacuum, a mechanical vacuum pump would serve as well. Figure 1, above, is a schematic of our apparatus.
Successful SPPS requires not only an apparatus, but chemical protocols of temperature, time, and chemical reagents. Employing the protocol of Clayden et al.22, we have prepared several peptides that would be difficult, if not impossible to prepare via conventional, room temperature SPPS methods. For coupling of amino acids, we use diisopropyl carbodiimide (DIC) and oxyma as the coupling cocktail and irradiate to 100 °C for 10 min. Oxyma is thought to suppress the racemization of residues during the coupling process13. For FMOC deprotection, we use 20% morpholine in DMF, irradiated at 90 °C for 2 min, and repeat once more. Between reactions, we use DMF to repeatedly (3x) rinse the SPPS beads (Wang resin).
Using this manual apparatus, we prepared difficult-to-synthesize peptides at the rate of about 40 min per residue. We have not yet studied possible optimizations of this rate. For example, we believe the time within the microwave reactor could likely be limited to "total reaction time," as opposed to "time at target temperature," without diminishing results. This is due to the amount of time spent asymptotically approaching the target temperature (see Supplemental File 1 for details.). Moreover, in principle, one could add all reagents and solvents without removing the reaction vessel from the microwave. However, we prefer to be able to inspect the resin at all steps.
While our use-case is in SPPS, it should be noted that solid phase methods are increasingly being applied to a range variety of syntheses, such as dendrimer25, RNA26, and electrochemical syntheses27. A variety of use-cases might benefit from microwave assistance, for which this apparatus would be suitable. Last, while the apparatus was constructed to perform microwave-assisted SPPS, its foundational reaction vessel is a test tube. Therefore, the apparatus may be employed in conventional, room temperature SPPS, as well as SPPS conducted at elevated temperatures, for example, in an oil bath.
Déclarations de divulgation
The authors have no conflicts of interest to disclose.
Remerciements
The authors are grateful for the support of Fairfield University's INSPIRE grants, the support of the Department of Chemistry & Biochemistry at Fairfield University, and the assistance of Dr. Dorothy Szobcynski for her expertise in managing the laboratory. Additionally, the authors are grateful to Professors Jillian Smith-Carpenter and Aaron Van Dyke for discussions regarding peptide and organic synthesis. The authors appreciate the support of the Publication Fund of the College of Arts & Sciences of Fairfield University.
matériels
| Name | Company | Catalog Number | Comments |
| CEM Discover SP Microwave Reactor | CEM | Discontinued. Recently replaced in the product line by the Discover 2.0 | |
| Diisopropylcarbodiimide (DCC) | TCI America | ||
| Dimethylformamide (DMF) | Thermo Scientific Chemicals | ||
| Gas dispersion tube, micro, | Chemglass | CG-207-02 | medium porosity |
| micro septum | ChemGlass | CG-3022-20 | "NMR tube" type septum |
| Morpholine | Thermo Scientific Chemicals | ||
| N-Fmoc-protected Amino acids | |||
| Oyxma Pure | TCI America | ||
| Side-arm Ehrlenmeyer flask | Assorted vendors | Waste collection | |
| "Tee" | Idex | P-713 | ETFE |
| teflon tubing 1/8", | Restek | 25306 | OD x 0.063" ID, 3 m |
| Test tube (holder for reaction vessel external to microwave) | Assorted vendors | (30 x 175) | |
| Test tube (reaction vessel) | Corning Glass | 9820-25X | Pyrex 25 x 200 mm, rimless |
| Valve | Idex | P-721 | ETFE (2x) |
| Wang SPPS Resin, 1% crosslinked divinylbenzene, 100-200 mesh | Advanced ChemTech |
Références
- Merrifield, R. B. Solid Phase Peptide Synthesis. I. The Synthesis of a Tetrapeptide. J Am Chem Soc. 85 (14), 2149-2154 (1963).
- Sheppard, R. C. Nobel prize: Merrifield wins in chemistry. Nature. 311 (5988), 699-699 (1984).
- Jaradat, D. M. M. Thirteen decades of peptide synthesis: key developments in solid phase peptide synthesis and amide bond formation utilized in peptide ligation. Amino Acids. 50 (1), 39-68 (2018).
- Paradís-Bas, M., Tulla-Puche, J., Albericio, F. The road to the synthesis of "difficult peptides.". Chem Soc Rev. 45 (3), 631-654 (2016).
- Wang, J., et al. Mechanistic Study of diketopiperazine formation during solid-phase peptide synthesis of tirzepatide. ACS Omega. 7 (50), 46809-46824 (2022).
- Sharma, A., et al. N-methylation in amino acids and peptides: Scope and limitations. Biopolymers. 109 (10), e23110 (2018).
- El-Faham, A., Albericio, F. Peptide coupling reagents, more than a letter soup. Chem Rev. 111 (11), 6557-6602 (2011).
- Subirós-Funosas, R., Nieto-Rodriguez, L., Jensen, K. J., Albericio, F. COMU: scope and limitations of the latest innovation in peptide acyl transfer reagents. J Peptide Sci. 19 (7), 408-414 (2013).
- El-Faham, A., Albericio, F. COMU: A third generation of uronium-type coupling reagents. J Peptide Sci. 16 (1), 6-9 (2010).
- Wenschuh, H., Beyermann, M., Rothemund, S., Carpino, L. A., Bienert, M. Multiple solid-phase synthesis via Fmoc-amino acid fluorides. Tet Lett. 36 (8), 1247-1250 (1995).
- Wenschuh, H., Beyermann, M., Krause, E., Carpino, L. A., Bienert, M. Efficient solid phase assembly of peptides bearing contiguous highly hindered Aib residues via Fmoc Aib fluoride. Tetrahedron. 34 (23), 3733 (1993).
- Wenschuh, H., et al. Fmoc amino-acid fluorides - convenient reagents for the solid-phase assembly of peptides incorporating sterically hindered residues. J Org Chem. 59 (12), 3275-3280 (1994).
- Manne, S. R., De La Torre, B. G., El-Faham, A., Albericio, F. OxymaPure coupling reagents: beyond solid-phase peptide synthesis. Synthesis. 52 (21), 3189-3210 (2020).
- Collins, J. M., Hoz, A., Loupy, A. Microwave-enhanced synthesis of peptides, proteins, and peptidomimetics. Microwaves in Organic Synthesis. , 897-959 (2012).
- Pedersen, S. L., Tofteng, A. P., Malik, L., Jensen, K. J. Microwave heating in solid-phase peptide synthesis. Chem Soc Rev. 41 (5), 1826-1844 (2012).
- Pedersen, S. L., Sørensen, K. K., Jensen, K. J. Semi-automated microwave-assisted SPPS: Optimization of protocols and synthesis of difficult sequences. Peptide Sci. 94 (2), 206-212 (2010).
- Zeko, T., et al. FT-IR spectroscopy and density functional theory calculations of 13C isotopologues of the helical peptide Z-Aib 6 -OtBu. J Phys Chem B. 118 (1), 58-68 (2014).
- Gord, J. R., et al. Conformation-specific spectroscopy of capped, gas-phase Aib oligomers: Tests of the Aib residue as a 310-helix former. Phys Chem Chem Phys. 18 (36), 25512-25527 (2016).
- Fischer, J. L., et al. Single-conformation spectroscopy of capped aminoisobutyric acid dipeptides: the effect of C-terminal cap chromophores on conformation. J Phys Chem A. 123 (19), 4178-4187 (2019).
- Hjørringgaard, C. U., Pedersen, J. M., Vosegaard, T., Nielsen, N. C., Skrydstrup, T. An automatic solid-phase synthesis of peptaibols. J Org Chem. 74 (3), 1329-1332 (2009).
- Haynes, S. R., Hagins, S. D., Juban, M. M., Elzer, P. H., Hammer, R. P. Improved solid-phase synthesis of α,α-dialkylated amino acid-rich peptides with antimicrobial activity. J Peptide Res. 66 (6), 333-347 (2005).
- Zieleniewski, F., Woolfson, D. N., Clayden, J. Automated solid-phase concatenation of Aib residues to form long, water-soluble, helical peptides. Chem Commun. 56 (80), 12049-12052 (2020).
- Manual peptide synthesis. Discover SPSS Available from: https://cem.com/discover-spps (2023)
- Theis, C., Degenkolb, T., Brückner, H. Studies on the selective trifluoroacetolytic scission of native peptaibols and model peptides using HPLC and ESI-CID-MS. Chem Biodivers. 5 (11), 2337-2355 (2008).
- Ya-Ting Huang, A., Kao, C. -. L., Selvaraj, A., Peng, L. Solid-phase dendrimer synthesis: a promising approach to transform dendrimer construction. Materials Today Chemistry. 27, 101285 (2023).
- Flemmich, L., Bereiter, R., Micura, R. Chemical synthesis of modified RNA. Angew Chem Int Ed Engl. 63 (22), e202403063 (2024).
- Li, M., Li, Y. Solid-phase electrosynthesis. Acc Chem Res. 56 (24), 3694-3703 (2023).
Réimpressions et Autorisations
Demande d’autorisation pour utiliser le texte ou les figures de cet article JoVE
Demande d’autorisationThis article has been published
Video Coming Soon