Method Article
The Effect of Anti-Fatigue Decoction on the Behaviors and Serological Indicators in a Central Fatigue Rat Model
Dans cet article
Résumé
Here, we introduce a protocol to assess the effects of anti-fatigue decoction (AFD) on central fatigue in rats modeled by the modified multiple platform method (MMPM) by monitoring both their behavioral responses and serological markers.
Résumé
This study aimed to assess the effects of Anti-fatigue Decoction (AFD) against central fatigue by observing the behaviors and serological indicators of rats modeled by the modified multiple platform method (MMPM) after drug intervention. Grip strength measurements were used to evaluate the muscle strength of rats. The open field test was utilized to assess anxiety-like behavior, while the Morris water maze test was conducted to evaluate the memory function of the rats. Following the behavioral assessments, rat serum samples were collected to measure the concentrations of corticosterone (CORT) and lactic acid (LAC). The concentration of LAC was determined using the colorimetric method, while the concentration of CORT was measured using the enzyme-linked immunosorbent assay (ELISA) method. Compared to the blank control group, following MMPM modeling, rats exhibited significant reductions in grip strength and impaired ability to memory. The serum analysis revealed increased levels of LAC and CORT in the model group rats. AFD can noticeably reverse these adverse changes to a certain extent. These findings highlight the positive effects of AFD and coenzymeQ10 on physical and cognitive abilities and alterations in serum biomarker levels of central fatigue rats.
Introduction
Fatigue is a multifaceted and non-specific phenomenon that is typically characterized by feelings of tiredness and reduced ability to function1. It can be classified as either peripheral fatigue, which occurs at the muscular level, or central fatigue, originating in the central nervous system3,4. Prolonged central fatigue can be a significant contributor to psychological issues, including anxiety, depression, psychological distress, and memory problems5,6. Despite causing significant distress, there is a scarcity of specific medications targeting central fatigue7. While methylphenidate, a central nervous system stimulant, can provide temporary relief, its side effects, like insomnia and palpitations may worsen the condition8,9.
In previous clinical applications, traditional Chinese medicine has shown promising results in treating central fatigue, incorporating approaches such as oral decoctions, acupuncture, and Tai Chi10,11,12. Anti-fatigue decoction (AFD) is an effective formula developed by Professor Li Feng based on extensive clinical experience and has demonstrated positive therapeutic effects. It consists of Astragalus membranaceus (Huangqi), Fructus aurantii (Zhiqiao), Fructus crataegi (Shanzha), Schisandra chinensis (Wuweizi), Angelica sinensis (Danggui), and Dendrobium officinale (Shihu), in a ratio of 15: 15: 10: 5: 7: 8. AFD decoction was concentrated to 110 mL after boiling with ten times the volume of deionized water for 1 h thrice. In a previous study, we established an animal model of central fatigue using the Modified Multiple Platform Method (MMPM) and confirmed the manifestation of central fatigue via behavioral and central nervous system neurotransmitter evaluations13. In this study, we utilized the AFD intervention in the animal model of central fatigue to evaluate its pharmacological effects through behavioral assessments.
Protocole
This research work adheres to ethical guidelines for animal welfare. Proper care and housing conditions were maintained to ensure the health and well-being of the animals, and all procedures were approved by the institutional animal care and use committee of the Beijing University of Chinese Medicine (BUCM-4-2019041504-2094).
1. Animal raising and grouping
NOTE: Throughout the study, animal welfare was maintained following the 3Rs (Reduction, Refinement, and Replacement) principles.
- For this study, select 54 six-week-old specific pathogen-free (SPF) Wistar rats with a body weight of 210 g ± 10 g.
- House the rats in the animal facility under controlled conditions: temperature of 23 ± 2 °C, relative humidity of 50%, and a 12 h/12 h light/dark cycle. Allow the animals to acclimatize for 3 days before the experiment.
- Randomly divide the rats into a blank control group (labeled as "a" in the figures), a model group (labeled as "b" in the figures), an AFD-low dose (AFD-L; labeled as "c" in the figures) group, an AFD-medium dose (AFD-M; labeled as "d" in the figures) group, an AFD-high dose (AFD-H; labeled as "e" in the figures) group, a Coenzyme Q10 (CoQ10; labeled as "f" in the figures) group, with 9 rats in each group, using a random number table method.
2. Modeling and intervention
NOTE: The establishment of this model was based on the previous literature13.
- Use the self-made modeling box (provided by the Neuroimmunology Laboratory of the Beijing University of Chinese Medicine).
NOTE: The modeling box has a length of 110 cm, a width of 60 cm, and a height of 40 cm. The bottom is fixed with 15 circular platforms (diameter 6.5 cm, height 8 cm). The horizontal spacing between the two platforms is 13 cm, and the vertical spacing is 10 cm. The open field box has a length, width, and height of 100 cm, 100 cm, and 35 cm, respectively. The bottom is black, and the wall is dark blue. The bottom of the open field box is divided into 25 square sections with dimensions of 25 cm, with the central 9 squares designated as the central zone and the outer 16 squares as the peripheral zone. - Ensure that the water level in the pool is approximately 1 cm above the platform and that the water temperature is maintained at 23 ± 2 °C.
- After the experiment starts, place the rat in the box covered with wire mesh on the surface and press it down with weights to prevent the animals from escaping. Suspend sufficient food beneath the wire mesh and provide clean drinking water.
- Place the rat in the model group in the box at 18:00 every day and take it out at 8:00 the next day. After each modeling session, dry the rat and place it back in a clean cage. Perform the modeling continuously for 21 days.
- During the experiment, clean the box, change the water daily, and provide sufficient food.
- On the 15th day of modeling, administer the agents via gastric lavage at 10:00 AM every day for a continuous period of 7 days.
- Administer the AFD-L group at a dosage of 3.24 g/kg/d, the AFD-M group at 6.48 g/kg/d, and the AFD-H group at 12.96 g/kg/d. Administer coenzyme Q10 at a dosage of 10 mg/kg/d.
- When administering the agents via gastric lavage, prepare a suspension by mixing the required dose in distilled water. The volume of administration for each group is 10 mL/kg. Give the blank and model groups the same amount of distilled water.
NOTE: The dosages administered to the rats were calculated using the body surface area (BSA) method and converted according to the normal therapeutic dosages for humans14.
3. Behavioral assessments
- Grip strength of rats
- Grasp the rat's tail and gently place it on the grip strength meter.
- Pull the rat backward with a uniform force while ensuring the computer system correctly records the data.
NOTE: Before experiments, technicians were trained to handle the rats to apply force consistently. - Repeat this process three times and calculate the average of the three measurements as the final grip strength value.
NOTE: To minimize errors caused by human factors, let one person conduct the entire experiment.
- Open field test in rats
- Conduct the behavior experiments in a dimly lit and quiet environment. Before the start of each experiment, place the rats in the behavior room for 1 h of adaptation.
- Ensure three experimenters are involved in the operation and wear black clothing when placing or guiding the rats. Throughout the experiment, ensure that the experimenters do not cross the box's boundaries with their bodies or arms.
- Open the analysis software.
- Click on File in the top menu, and select New Experiment to create a new project. In the Arena Settings, choose the shape corresponding to the arena where the rat will move.
- Using the mouse, draw the arena on the screen. Calibrate the camera and Save the arena settings.
- Allow a second experimenter, dressed in black clothing, to hold the rat by its back, sequentially place it in the center of the designated area, and quickly withdraw his hand.
- Start a new recording. Set the recording duration to 5 min.
- After the 5 min observation period, swiftly remove the rat from the open field box and clean its feces. Use 75% alcohol to clean the open field box.
- Repeat the process for all rats.
- Observe and record (1) the total distance moved, (2) the number of central zone crossings: number of times the rat's limbs cross into central grid squares, and (3) time spent in the central zone, i.e., time spent by the rat in the 9 central grid squares.
- Morris water maze in rats
- Conduct the behavior experiments in a dimly lit and quiet environment. Before starting each experiment, place the rats in the behavior room for 1 h of adaptation.
NOTE: The rats underwent 4 training days; on the 5th day, the formal experiment began. - Ensure that the water level in the pool is approximately 1 cm above the platform and that the water temperature is maintained at 23 ± 2 °C.
- Ensure three experimenters are involved in the operation and wear black clothing when placing or guiding the rats.
- Divide the water pool into four quadrants. Fix a platform in the middle of the second quadrant. Attach different colored and shaped pieces of paper to the center of each quadrant's walls.
- Open the analysis software.
- Click on File in the top menu, and select New Experiment to create a new project. In the Arena Settings, choose the shape corresponding to the arena where the rat will move.
- Using the mouse, draw the arena on the screen. Calibrate the camera and Save the arena settings.
- During the learning period, let another experimenter sequentially place the rat facing the pool wall in each quadrant. The order of placement is different each day as follows:
- On the first day, place the rat in the following order: first quadrant, second quadrant, third quadrant, fourth quadrant.
- On the second day, place the rat in the following order: second quadrant, first quadrant, fourth quadrant, third quadrant.
- On the third day, place the rat in the following order: fourth quadrant, third quadrant, second quadrant, first quadrant;
- On the fourth day, place the rat in the following order: third quadrant, first quadrant, fourth quadrant, second quadrant.
- If the rat fails to find the platform within 120 s, guide it to the platform and allow it to stay on it for 10 s.
- After completing the daily experiment, let the third experimenter wipe off the water from the rat's body using a towel and dry it with a hairdryer. Perform this in another room to avoid noise interference.
- In the formal experiment, remove the platform and place the rat in the third quadrant. Record the rat's movement trajectory within 120 s.
- Observe and record (1) the time spent in the quadrant where the platform was located, and (2) the escape latency, which is the time it takes for the rat to reach the platform from entering the water pool for the first time.
- Conduct the behavior experiments in a dimly lit and quiet environment. Before starting each experiment, place the rats in the behavior room for 1 h of adaptation.
4. Serum biochemical analysis
- Sample processing
NOTE: A fast on food, but not water, was allowed the day before sampling.- Anesthetize the rats using a 2% (w/v) sodium pentobarbital solution via intraperitoneal injection at 0.5 mL/100 g body weight.
- After complete anesthesia, collect their blood from the abdominal aorta using a blood sampling vessel.
- Place the whole blood at room temperature (RT) for 2 h, then centrifuge it at 4 °C, 1522.38 x g for 20 min to obtain serum. Aliquote and store the serum at -80 °C for further use.
- Lactic acid (LAC) assay
NOTE: The concentration of LAC was determined using the colorimetric method.- Mix the enzyme reserve solution and enzyme dilution solution in a 1:100 volume ratio to prepare an enzyme working solution.
- Prepare the color developer according to the instructions in the kit.
- In a blank tube, add 20 µL of distilled water.
- In a standard tube, add 3 mmol/L standard solution.
- In a measurement tube, add 20 µL of the sample.
- Add 1 mL of enzyme working solution and 0.2 mL of color developer to each tube. Incubate at 37 °C for 10 min and then add 2 mL of the stop solution.
- Transfer 200 µL of the reaction solution to a 96-well ELISA plate. Measure the OD value at 530 nm. Calculate the LAC concentration based on the OD values.
NOTE: LAC concentration = (sample well OD value - blank well OD value)/( standard well OD value - blank well OD value) x standard solution concentration x dilution ratio factor.
- Corticosterone assay
NOTE: Commercially purchased ELISA kits are used to measure the concentration of cortisone in rat serum.- Prior to the experiment, dilute the antibodies conjugated with horseradish peroxidase (HRP) and washing solution to create a working solution. Add standard samples with concentrations of 0 nmol/L, 5 nmol/L, 15 nmol/L, 30 nmol/L, 60 nmol/L, 120 nmol/L, and 240 nmol/L to the coated plate in 20 µL volumes.
- Add the serum samples (20 µL) to the respective wells, followed by the addition of 200 µL of enzyme conjugate. Thoroughly shake the plate and incubate it for 60 min.
- Discard the reaction solution, and wash the plate three times with the wash solution (400 µL each time). After drying the plate on absorbent paper, add 100 µL of the substrate color reagent to each well.
- Incubate the plate at RT for 15 min, then add 50 µL of the stop solution to each well.
- Measure the OD values of each well at 450 nm. Use the OD values of the standard wells to generate a standard curve and then use it to calculate the concentrations of the sample wells.
5. Statistical analysis
- Perform statistical analysis using an appropriate software application. Represent the measurement data as mean ± standard deviation.
- If the data followed a normal distribution and showed homogeneity of variances, perform an independent t-test.
- If the data followed a normal distribution but did not have equality of variances, perform an approximate t-test.
- If the data did not follow a normal distribution, use non-parametric tests for comparison.
- Use the Pearson's chi-square test for enumeration/count data analysis.
NOTE: A significance level of α = 0.05 was chosen, where P < 0.05 is referred to as statistically significant. - Generate the figures using an appropriate software application.
Résultats
Compared to the control group, the rats in the model group exhibited significant decreases in grip strength. However, the administration of AFD at low, medium, and high doses was all able to reverse this effect in a dose-dependent manner, as shown in Figure 1. Similarly, the positive control drug also demonstrated the ability to reverse the grip strength changes (Figure 1).
In the open field test, the model group rats showed reductions in total distance traveled, the number of central zone crossings, and the duration of central zone dwell time. The administration of AFD at low, medium, and high doses was able to reverse these effects in a dose-dependent manner, as depicted in Figure 2. At the same time, the positive control drug was also able to alleviate the symptoms (Figure 2). Heatmaps showing the superimposed trajectory frequencies from the open-field experiments of each group can be observed in Figure 3.
During the Morris water maze test, the model group rats exhibited prolonged escape latency and reduced swimming time in the target quadrant, indicating poorer spatial searchability. The positive control drug, Coenzyme Q10, was able to reverse the aforementioned changes. Noteworthily, AFD at low、medium and high doses reversed the prolonged escape latency, as illustrated in Figure 4. Representative heat maps of rat trajectories in the water maze experiment for each group can be observed in Figure 5.
In terms of serum levels, the rats in the model group had elevated levels of LAC and CORT. However, the administration of AFD at low, medium, and high doses reversed these changes in a dose-dependent manner similar to the positive control drug, as shown in Figure 6.
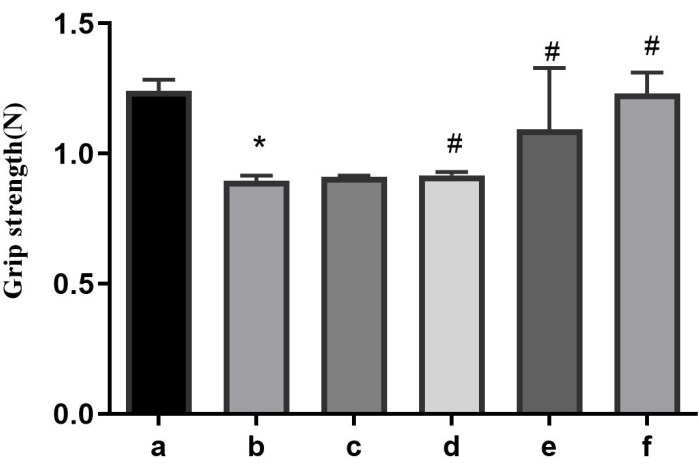
Figure 1: Results of the grip strength of each group. a: the control group; b: the model group; c: the AFD-L group; d: the AFD-M group; e: the AFD-H group; f: the CoQ10 group. *p < 0.05 vs. the control group; #p < 0.05 vs. the model group. Please click here to view a larger version of this figure.
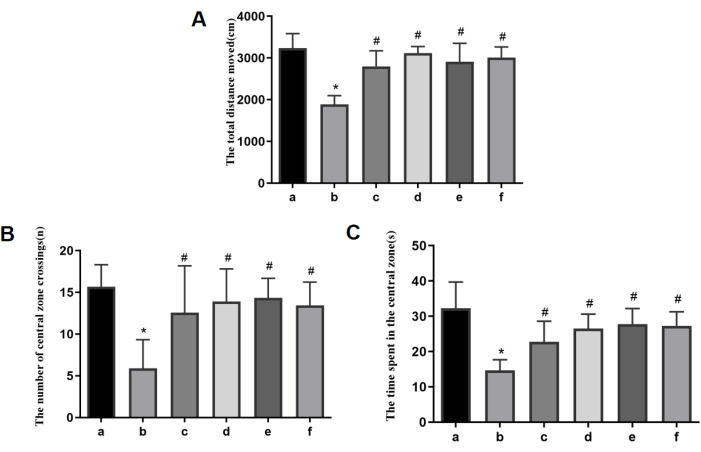
Figure 2: Open-field experiment and measurement of the level of locomotor activity. (A) The total distance traveled. (B) The number of central zone crossings. (C) The duration of the central zone dwells. a: the control group; b: the model group; c: the AFD-L group; d: the AFD-M group; e: the AFD-H group; f: the CoQ10 group. *p < 0.05 vs. the control group; #p < 0.05 vs. the model group. Please click here to view a larger version of this figure.
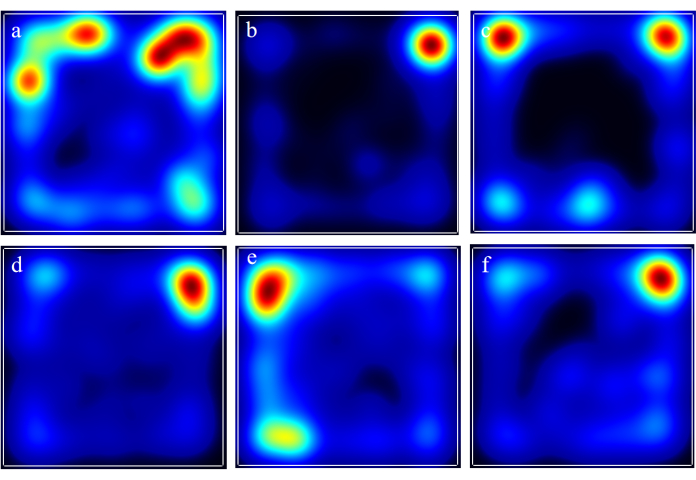
Figure 3: Heatmaps showing the superimposed trajectory frequencies from the open field experiments of each group. a: the control group; b: the model group; c: the AFD-L group; d: the AFD-M group; e: the AFD-H group; f: the CoQ10 group. Please click here to view a larger version of this figure.
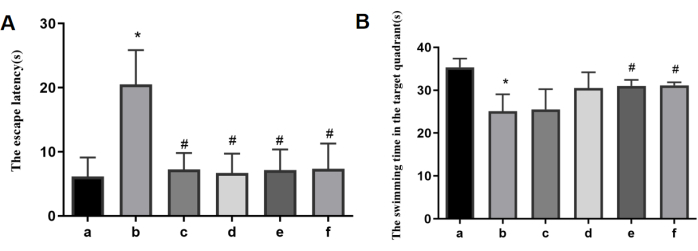
Figure 4: Results of the Morris water maze experimentin each group. (A) The escape latency. (B) The swimming time in the target quadrant. a: the control group; b: the model group; c: the AFD-L group; d: the AFD-M group; e: the AFD-H group; f: the CoQ10 group. *p < 0.05 vs. the control group; #p < 0.05 vs. the model group. Please click here to view a larger version of this figure.
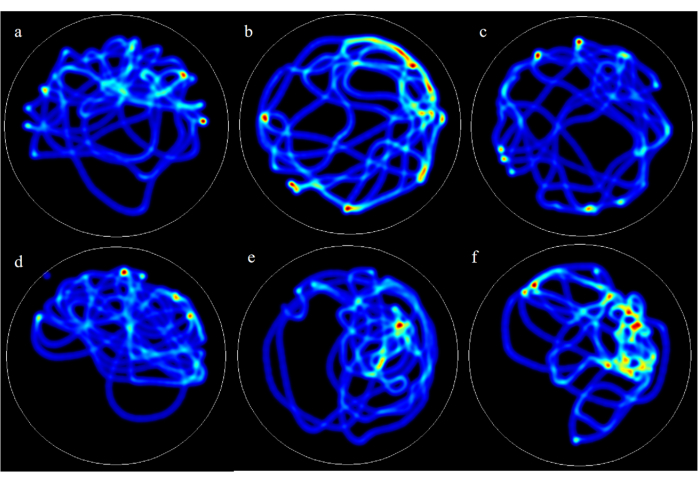
Figure 5: Representative heatmaps of rat trajectories in the Morris water maze experiment for each group. a: the control group; b: the model group; c: the AFD-L group; d: the AFD-M group; e: the AFD-H group; f: the CoQ10 group. Please click here to view a larger version of this figure.
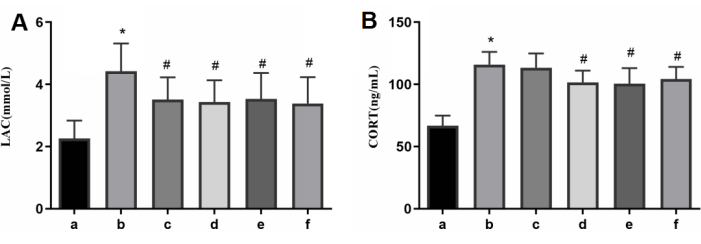
Figure 6: Results of serum levels of LAC and CORT in each group. (A) The serum levels of LAC. (B) The serum levels of CORT. a: the control group; b: the model group; c: the AFD-L group; d: the AFD-M group; e: the AFD-H group; f: the CoQ10 group. Please click here to view a larger version of this figure.
Discussion
AFD is composed of Astragalus membranaceus (Huangqi), Fructus aurantii (Zhiqiao), Fructus crataegi (Shanzha), Schisandra chinensis (Wuweizi), Angelica sinensis (Danggui), and Dendrobium officinale (Shihu), which is believed to have the function of invigorating the spleen and dispersing the stagnated liver-energy in traditional Chinese medicine. Also, all these herbs are considered to possess a favorable safety profile and are thus commonly used as food in China. In previous clinical practice, AFD has shown good efficacy in treating patients with central fatigue, but the specific therapeutic mechanism still needs further investigation. Therefore, we aim to validate the effects of AFD in improving central fatigue through animal experiments and further elucidate the underlying mechanisms.
The establishment of animal models that accurately represent disease pathogenesis and clinical characteristics is essential for conducting pharmacological research15. Our team has previously established a rat model of central fatigue through sleep deprivation, which results in reduced grip strength, anxiety, and impaired memory13. However, we failed to detect relevant serological indicators in the past study. Concurrently, the observation of significant alterations in serum biomarkers associated with fatigue reinforces the validity of this animal model as a reliable representation of central fatigue. In this study, different doses of AFD were used to intervene in the rat model of central fatigue, with coenzyme Q10 (a drug known to improve fatigue through enhancing energy metabolism)16 as the positive control. Behavioral assessment is a key step in studying fatigue-related animal experiments. Thus, it is imperative for researchers to ensure the preciseness of this procedure17. When an open field test was carried out, animals were placed in a closed platform, aiming to assess the changes in their mood by observing the relevant activities18,19. Morris water maze is a kind of experiment that forces laboratory animals to swim and learn to find hidden platforms in the water, which is mainly used to evaluate the learning and memory ability20,21. The models of central fatigue showed pronounced behavioral changes, and consequently, we employed the two tests to observe and assess the changes in rats' behaviors. The results demonstrate that the MMPM model rats exhibit conspicuous anxiety-like behavior and impaired capabilities to learn and memorize, which aligns with the previous research findings13.
Stress can lead to excessive activation of the hypothalamic-pituitary-adrenal (HPA) axis, resulting in the overproduction of glucocorticoids22. Long-term elevations in cortisol can inflict irreversible damage on neurons, which is a significant contributing factor to the development of insomnia, depression, and anxiety23,24. These processes are closely related to the occurrence and development of central fatigue, so it is believed that serum cortisol is associated with the degree of fatigue25. Animal models of central fatigue have demonstrated pronounced elevation in corticosterone levels, which can be effectively reversed through intervention with relevant medications26. The findings of this study indicate that AFD can effectively suppress the surge in serum corticosterone raised by stress-induced central fatigue, exhibiting a dose-dependent effect. This outcome also partially explains why AFD can improve anxiety-like behavior in a central fatigue model in rats.
Lactate has previously been considered a byproduct of the skeletal muscles. When exercise intensity increases, energy demands surpass the aerobic capacity, leading to an increased reliance on anaerobic metabolism for ATP production27. The accumulation of lactic acid is responsible for stimulating chemical receptors in the muscles, and this excitation is transmitted to the cerebrum and becomes a signal of pain and fatigue28. Some pathological factors also induce the overproduction of lactate, thereby activating this reflex arc. Recent clinical research described the close relationship between the level of lactic acid in serum and the degree of fatigue29. In our foregoing observation, MMPM rats showed a significant elevation in serum in comparison to blank control, potentially indicating a strong correlation between the increased lactate levels and muscle fatigue experienced while standing on a small platform. The application of AFD is able to remarkably reduce the levels of lactic acid in serum collected from WMPV rats to a certain extent, sequentially improving the degree of fatigue.
In conclusion, this study demonstrated that AFD effectively reversed grip strength decline, alleviated anxiety, and improved memory, potentially through its ability to reduce corticosterone levels and improve lactate metabolism. This paper is expected to offer a template for the experimental study of TCM against central fatigue in vivo. The study's limitation is that there is no advanced step to explore the underpinning of related molecular mechanisms. In the future, we will continue to explore the effects of AFD on the central nervous system and investigate its related mechanisms.
Déclarations de divulgation
The authors declare that there are no conflicts of interest regarding the publication of this paper. We have no financial or personal relationships with organizations or individuals that could inappropriately influence our research.
Remerciements
All authors would like to express their gratitude for the support from the National Natural Science Foundation of China (NO. 81874428) and Research project of Beijing University of Traditional Chinese Medicine (NO.2023-JYB-JBZD-001).
matériels
| Name | Company | Catalog Number | Comments |
| Corticosterone test kit | German DRG company | EIA4164 | Step 4.3 |
| Curve Expert 1.4 software | CurveExpert Professional | Version 1.4 | For calculation in corticosterone assay |
| Ethovision software | Noldus Information Technology , Netherlands | Version 15 | analysis software and video tracking system |
| Grip strength test device | Beijing Zhongshi Di Chuang limited company | ZS-ZL | Step 3.1 |
| Lactic acid test kit | Nanjing Jiancheng Bioengineering Research Institute | A019-2-1 | Step 4.2 |
| Modified multiple platform method | Neuroimmunology Laboratory of Beijing University of Chinese Medicine | None | Step 2.1 |
| Morris water maze test device | Beijing Zhongshi Di Chuang limited company | ZS-Morris | Step 3.3 |
| Open field test device | Beijing Zhongshi Di Chuang limited company | ZS-KC | Step 3.2 |
| Prism | GraphPad | Verson 8 | For generating figures |
| SPSS 26.0 | IBM | Verson 26.0 | Statistical analysis |
| Wistar rats | SiPeiFu (Beijing) Biotechnology Co., Ltd | license number: SCXK (Jing) 2019-0010 |
Références
- Dukes, J. C., Chakan, M., Mills, A., Marcaurd, M. Approach to fatigue: Best practice. Med Clin North Am. 105 (1), 137-148 (2021).
- Zargari Marandi, R., Madeleine, P., Omland, &. #. 2. 1. 6. ;., Vuillerme, N., Samani, A. Eye movement characteristics reflected fatigue development in both young and elderly individuals. Sci Rep. 8 (1), 13148 (2018).
- Tornero-Aguilera, J. F., Jimenez-Morcillo, J., Rubio-Zarapuz, A., Clemente-Suárez, V. J. Central and peripheral fatigue in physical exercise explained: A narrative review. Int J Environ Res Public Health. 19 (7), 3909 (2022).
- Dotan, R., Woods, S., Contessa, P. On the reliability and validity of central fatigue determination. Eur J Appl Physiol. 121 (9), 2393-2411 (2021).
- AlSaeed, S., et al. Fatigue, depression, and anxiety among ambulating multiple sclerosis patients. Front Immunol. 13, 844461 (2022).
- Lee, C. H., Giuliani, F. The role of inflammation in depression and fatigue. Front Immunol. 10, 1696 (2019).
- Meeusen, R., Watson, P., Hasegawa, H., Roelands, B., Piacentini, M. F. Central fatigue: the serotonin hypothesis and beyond. Sports Med. 36 (10), 881-909 (2006).
- Nourbakhsh, B., et al. Safety and efficacy of amantadine, modafinil, and methylphenidate for fatigue in multiple sclerosis: a randomised, placebo-controlled, crossover, double-blind trial. Lancet Neurol. 20 (1), 38-48 (2021).
- Rojí, R., Centeno, C. The use of methylphenidate to relieve fatigue. Curr Opin Support Palliat Care. 11 (4), 299-305 (2017).
- Luo, C., et al. Natural medicines for the treatment of fatigue: Bioactive components, pharmacology, and mechanisms. Pharmacol Res. 148, 104409 (2019).
- Wang, T., Xu, C., Pan, K., Xiong, H. Acupuncture and moxibustion for chronic fatigue syndrome in traditional Chinese medicine: a systematic review and meta-analysis. BMC Complement Altern Med. 17 (1), 163 (2017).
- Xiang, Y., Lu, L., Chen, X., Wen, Z. Does Tai Chi relieve fatigue? A systematic review and meta-analysis of randomized controlled trials. PLoS One. 12 (4), e0174872 (2017).
- Zhang, W., et al. A rat model of central fatigue using a modified multiple platform method. J Vis Exp. (138), e57362 (2018).
- Drug Ther Bull. Body surface area for adjustment of drug dose. Drug Ther Bull. 48 (3), 33-36 (2010).
- Harrison, E. L., Baune, B. T. Modulation of early stress-induced neurobiological changes: a review of behavioural and pharmacological interventions in animal models. Transl Psychiatry. 4 (5), e390 (2014).
- Sanoobar, M., Dehghan, P., Khalili, M., Azimi, A., Seifar, F. Coenzyme Q10 as a treatment for fatigue and depression in multiple sclerosis patients: A double blind randomized clinical trial. Nutr Neurosci. 19 (3), 138-143 (2016).
- Grace, P. M., et al. Behavioral assessment of neuropathic pain, fatigue, and anxiety in experimental autoimmune encephalomyelitis (EAE) and attenuation by interleukin-10 gene therapy. Brain Behav Immun. 59, 49-54 (2017).
- Kraeuter, A. K., Guest, P. C., Sarnyai, Z. The open field test for measuring locomotor activity and anxiety-like behavior. Methods Mol Biol. 1916, 99-103 (2019).
- Jin, Q., et al. Network and experimental pharmacology to decode the action of wendan decoction against generalized anxiety disorder. Drug Des Devel Ther. 16, 3297-3314 (2022).
- Othman, M. Z., Hassan, Z., Che Has, A. T. Morris water maze: a versatile and pertinent tool for assessing spatial learning and memory. Exp Anim. 71 (3), 264-280 (2022).
- Bromley-Brits, K., Deng, Y., Song, W. Morris water maze test for learning and memory deficits in Alzheimer's disease model mice. J Vis Exp. (53), e2920 (2011).
- Richardson, A. E., VanderKaay Tomasulo, M. M. Stress-induced HPA activation in virtual navigation and spatial attention performance. BMC Neurosci. 23 (1), 40 (2022).
- Mikulska, J., Juszczyk, G., Gawrońska-Grzywacz, M., Herbet, M. HPA axis in the pathomechanism of depression and schizophrenia: New therapeutic strategies based on its participation. Brain Sci. 11 (10), 1298 (2021).
- Passos, G. S. Insomnia severity is associated with morning cortisol and psychological health. Sleep Sci. 16 (1), 92-96 (2023).
- Herane-Vives, A., et al. Cortisol levels in chronic fatigue syndrome and atypical depression measured using hair and saliva specimens. J Affect Disord. 267, 307-314 (2020).
- Kang, J. Y., et al. Korean red ginseng ameliorates fatigue via modulation of 5-HT and corticosterone in a sleep-deprived mouse model. Nutrients. 13 (9), 3121 (2021).
- Rabinowitz, J. D., Enerbäck, S. Lactate: the ugly duckling of energy metabolism. Nat Metab. 2 (7), 566-571 (2020).
- Proia, P., Di Liegro, C. M., Schiera, G., Fricano, A., Di Liegro, I. Lactate as a metabolite and a regulator in the central nervous system. Int J Mol Sci. 17 (9), 1450 (2016).
- Zu, Y., Lu, X., Yu, Q., Yu, L., Li, H., Wang, S. Higher postdialysis lactic acid is associated with postdialysis fatigue in maintenance of hemodialysis patients. Blood Purif. 49 (5), 535-541 (2020).
Réimpressions et Autorisations
Demande d’autorisation pour utiliser le texte ou les figures de cet article JoVE
Demande d’autorisationThis article has been published
Video Coming Soon