Method Article
Non-thermal Infrared Light Treatment of Ischemia/Reperfusion Injury and Subsequent Analysis of Macrophage Differentiation
Dans cet article
Résumé
We describe the reduction of reperfusion injury by 670 nm irradiation in a mouse model of ischemia and reperfusion by tourniquet placement. This 670 nm irradiation reduced the inflammatory response, decreased the number of proinflammatory macrophages, and increased the protective macrophages.
Résumé
Tissue damage and necrosis from inflammatory processes are a consequence of ischemia reperfusion injury (IRI). In skeletal muscle, ischemia reduces the aerobic energy capacity of muscle cells, leading to adverse biochemical alterations and inflammation. The goal of this study is to show that exposure to near-infrared light (NIR) during a period of ischemia reduces IRI by decreasing necrosis and inflammation in addition to decreasing proinflammatory M1 and increasing protective M2 macrophages. C57/Bl6 mice underwent unilateral tourniquet-induced hindlimb ischemia for 3 h followed by reperfusion for either 15 or 30 min. Mice were randomly assigned to 3 groups. Group 1 underwent IRI with 30 min reperfusion. Group 2 underwent IRI with a 15 min reperfusion. Each group consisted of 50% no-NIR and 50% NIR-treated mice with exposure of 50 mW/cm2 for 5 min/1 h after tourniquet closure. Group 3 were sham animals anesthetized for 3 h omitting IRI.
Laser doppler flow imaging was performed on all mice to confirm ischemia and reperfusion. Flow data were expressed as the ratio of ischemic limb and the contralateral control. The mice were euthanized after reperfusion, and the quadriceps and gastrocnemius were harvested. Immunoprecipitation and western blot of macrophage-markers CD68 (M1) and CD206 (M2) were performed and normalized to CD14 expression. The expression of the inflammatory markers CXCL1 and CXCL5 was significantly reduced by NIR in the IRI group. A significant decrease in CD68 and an increase in CD206 expression was observed in animals receiving IR and NIR. Tissue necrosis was decreased by NIR in the IRI group, as visualized by 2,3,5-triphenyltetrazolium chloride (TTC) staining. The findings demonstrate that exposure to NIR reduced IRI and improved tissue survival. NIR reduced inflammation, decreased proinflammatory M1, and increased protective M2 macrophages. Exposure to NIR reduced inflammation and enhanced regeneration, leading to tissue protection following ischemia.
Introduction
Ischemia reperfusion injury (IRI) is a clinical challenge seen following vascular injuries and the prolonged use of surgical tourniquets. Previous studies have shown that 60-90 min is the upper threshold for warm ischemia time, beyond which irreversible tissue damage can occur. More than any other single factor, the limitations of warm ischemia time limit the success and salvage of reimplantation of dysvascular limbs1,2.
In skeletal muscle, ischemia reduces the aerobic capacity of cells, leading to acute inflammation and adverse biochemical alterations. These effects are worsened by reperfusion, which stimulates the recruitment of neutrophils and the production of free radicals, further damaging the skeletal muscle. This can occur from vascular occlusion, whether the result of injury or the intentional use of a tourniquet to prevent hemorrhage. Some of the key mediators in this process are myeloperoxidase (MPO), an enzyme expressed by neutrophils that is integral to the respiratory burst function and production of free radicals3, and chemokines such as CXCL1 and CXCL5 that serve to recruit neutrophils to sites of acute inflammation4.
The femoral artery was not dissected to mimic an open tourniquet in an emergency. This approach is also based on the reproducibility of creating ischemia and reperfusion as well as a consistent blood-free area. Previous research has demonstrated that exposure to non-thermal infrared (NIR) light with a wavelength of 670 nm can increase vascular collateralization in a mouse ischemic hindlimb with NIR exposure over days, mitigating the effects of IRI5. Additionally, prior research has demonstrated that NIR light can induce the polarization of macrophages into either proinflammatory (M1) or prohealing (M2) phenotypes6.
Any treatment that can minimize tissue damage and cellular death following hypoxia and reperfusion would be beneficial in increasing the success of limb salvage following vascular injuries. Therefore, the overall goal is to improve IRI by introducing 670 nm light treatment as a viable option to other treatment modalities. This paper is based on the hypothesis that exposure to NIR light during a period of ischemia decreases inflammation and tissue necrosis by decreasing the secretion of chemoattractant proteins and influx of inflammatory cells by inducing macrophages to take on an M2 phenotype.
Protocole
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee (Protocol: AUA#1517). All research involving mice was conducted in conformity with PHS policy.
1. Tourniquet placement
NOTE: A tourniquet was placed to induce ischemia and achieve a blood-free surgical field.
- Anesthetize a C57/Bl6 mouse with isoflurane in the induction chamber at a concentration of 3-5%.
- Remove the mouse from the induction chamber when it does not respond to toe pinches. Place the mouse in a nose cone for continued anesthesia at 1.5-2% on a heating pad with a non-reflective mat below a laser doppler imager (LDI).
- Place the tourniquet around the left hindlimb (Figure 1).
NOTE: Do not close the tourniquet yet. Measure the blood flow first (see section 2).
2. Blood flow measurement by LDI
NOTE: Blood flow was measured to confirm proper occlusion and reperfusion as described previously7.
- Place the anesthetized mouse below the LDI (26 cm) onto a heating pad with a non-reflective surface in a prone position with stretched legs and paws facing upward.
- Capture images by placing the mouse within the capturing area of the laser (16.1 cm x 16.1 cm).
- Adjust the laser to a scan speed of 4 ms/pixel. Capture images with the proprietary software by pushing the record button. Take images before, during occlusion, and during reperfusion by clicking on the record button.
NOTE: The images are flow-related map images; red is good blood flow; blue is no blood flow. Confirm in the image that the occluded hindlimb is blue. Similarly, check the image to confirm that the previously occluded hindlimb is red on reperfusion. It is imperative to have a non-reflective mat for the LDI; otherwise, the reflection will affect the images.
- Adjust the laser to a scan speed of 4 ms/pixel. Capture images with the proprietary software by pushing the record button. Take images before, during occlusion, and during reperfusion by clicking on the record button.
- Analyze the images with the provided software by defining a region of interest. Establish a ratio between the ischemic and control legs.
3. Ischemia and reperfusion protocol
- Close the tourniquet around the anesthetized mouse's hindlimb for 3 h.
NOTE: Tightening the tourniquet creates ischemia, interrupting the blood flow. - Open the tourniquet after 3 h of occlusion; open and perfuse the hindlimb for 15 or 30 min.
4. Tissue harvest
- Increase anesthesia to 5% isoflurane to deeply anesthetize the mice. Perform a pneumothorax by opening the chest cavity with sharp scissors to ensure death. Harvest the gastrocnemii and the quadriceps muscles8,9,10.
- Snap-freeze the collected tissue in liquid nitrogen and store at -80 °C for further analysis.
5. NIR application
NOTE: NIR is applied to lower inflammation and reduce reperfusion injury.
- Apply 670 nm NIR at an intensity of 100 mW for 5 min once per hour during occlusion.
- Target the hindlimb with the fiber optic light source at a distance of 2.5 cm between the hindlimb and the light. Do not expose the contralateral leg to NIR (see Figure 1 for the setup).
- Shine the NIR light for 5 min per hour for the duration of the ischemic period.
- Instrument the sham group the same way. Do not close the tourniquet or apply NIR to the hindlimb.
NOTE: The NIR light has a heat sink. The temperature does not increase within 5 min of light exposure11.
6. Necrosis TTC
NOTE: Tissue necrosis is assessed to visualize the reduction of necrosis in muscle tissue.
- Stain fresh quadriceps muscles with TTC at 37 °C for 20-30 min in 1% TTC in 0.1 M phosphate buffer adjusted to pH 7.4.
- Observe red, stained live tissue based on the presence of a formazan precipitate.
NOTE: The red staining is based on the reduction of TTC by dehydrogenase enzymes in healthy tissue. Necrotic tissue is unstained10. - Capture images of TTC-stained slices for analysis and measure the necrotic region by defining a region of interest.
7. Western analysis for chlorotyrosine
- Subject mice to the ischemia/reperfusion protocol (section 3).
- Harvest tissues (section 4) and isolate protein as previously described12. Determine the protein concentration by the Bradford assay13.
- Use the total protein lysate to perform electrophoresis and blot for 3-chlorotyrosine as described previously12.
- Perform a western blot on lysates of the gastrocnemii.
- Add Laemmli sample buffer containing 5% mercaptoethanol to the samples to obtain a concentration of 25 µg of total protein in 40 µL.
- Run the samples on a 4-15% Tris-glycine extended gel (see the Table of Materials) at 50 mV for 5 min. Increase the voltage to 100 mV for 1 h until the dye front runs out.
- Incubate the gel in 1x transfer buffer (25 mM Tris, 190 mM glycine, 20% methanol; adjust the pH to 8.3 if necessary) for 10 min.
- Assemble the transfer sandwich (sponge, filter, gel, cellulose membrane, filter, sponge). Place a reusable ice pack in the transfer apparatus. Transfer for 30 min in the cold room at 100 mA.
NOTE: Ensure that no bubbles are trapped in the transfer sandwich, the blot is on the cathode and the gel on the anode. - Rinse the blot and block it in blocking buffer (see the Table of Materials) with 0.05% Tween for 30 min at room temperature. Wash the blot with Tris-buffered saline (TBS) containing 5% Tween three times for 5 min each before adding the primary antibody.
- Incubate overnight with the primary polyclonal antibodies to 3-chlorotyrosine. Dilute the antibodies in blocking buffer with 0.05% Tween (1:1,000).
- Use horseradish peroxidase (HRP)-conjugated donkey anti-mouse IgG and HRP-anti-goat IgG (1:10,000 dilution) as secondary antibodies.
- Visualize bands of identity in the blots using enhanced chemiluminescence (ECL) reagent and an imaging system.
- Mix components based on the manufacturer's protocol and incubate them with the blot for 3 min to bind the ECL reagents to the bands of interest.
- Image the blot with an imaging system (see the Table of Materials). Use imaging software such as ImageJ to measure the density and normalize the density values of the bands of interest to those of the control bands.
8. ELISA for CXCL1 and CXCL5
- Isolate total protein from the gastrocnemii muscles as described previously12.
- Assess the degree of inflammation by ELISA for CXCL1 and CXCL5 as previously described14 and according to the manufacturer's instructions.
9. Immunoprecipitation followed by western analysis
- Subject the mice to the ischemia/reperfusion protocol (section 3).
- Harvest the tissues (section 4) and isolate protein as previously described12. Determine the protein concentration by the Bradford Assay13.
- To concentrate the macrophage fraction of the total protein lysate, perform an immunoprecipitation for CD14 as described previously15.
- Use the CD14 immunoprecipitate to perform electrophoresis and blot for CD206 and CD68 as described previously12.
- Perform a western blot on lysates of the gastrocnemii as described in section 7.
- Incubate the immunoprecipitates overnight with the primary polyclonal antibodies to CD206 or CD68. Dilute the antibodies 1:200 in TBS.
- Use HRP-conjugated donkey anti-mouse IgG and HRP-anti-goat IgG (1:10,000 dilution) as secondary antibodies.
- Apply ECL reagent to the blot after mixing components based on the manufacturer's protocol and incubate for 3 min to visualize the bands of interest. Image the blot using an imaging system.
10. Statistical analysis
- Perform statistical analysis of data within and between groups with analysis of variance (ANOVA) for repeated measures followed by Bonferroni's modification of Student's t-test, p ≤ 0.05.
- Express data as mean ± standard error of the mean (SEM) or mean ± standard deviation (SD).
Résultats
Flow measurements confirmed ischemia and reperfusion
NIR light placement and the experimental protocol are depicted in Figure 1. A murine hindlimb ischemia model was developed and employed to assess the effect of NIR exposure on skeletal muscle IRI. As was expected, laser doppler flux imaging (Figure 2A) verified that the tourniquet was effective at inducing ischemia along with a return in blood flow to near-baseline for both NIR-treated (n = 6) and non-light-treated hindlimbs (n = 6). Thermal imaging (Figure 2A) also demonstrated that there was no heating of the limbs from the LED arrays, suggesting that the observed effects were attributable to NIR light and not thermal heating.
NIR treatment reduces quadriceps infarction
Gross histological delineation of skeletal muscle tissue with TTC staining (Figure 3A) demonstrated a significant 1.5-fold decrease (p < 0.05) in the amount of necrotic tissue in NIR light-treated quadriceps (n = 3) after IRI compared to the non-NIR-exposed quadriceps (n = 3, Figure 3B).
NIR application reduces chlorotyrosine expression in gastrocnemii
Western blot analysis of 3-chlorotyrosine adducts (Figure 4) was performed as a surrogate marker for the presence of acute-phase neutrophils. It demonstrated a significant 2.9-fold decrease (p < 0.05) in chlorotyrosine adduct expression in the NIR light-treated gastrocnemius after IRI compared to the non-NIR-exposed gastrocnemius.
NIR application reduces the expression of CXCL1 and CXCL5 in gastrocnemii
An ELISA was employed to determine the expression levels of the two proinflammatory chemokines CXCL1 (Figure 5A) and CXCL5 (Figure 5B). NIR treatment reduced the expression of CXCL1 and CXCL5 significantly after 15 min of reperfusion. Only the expression level of CXCL5 was significantly reduced after 30 min of reperfusion.
Effects of NIR treatment on macrophage phenotype in gastrocnemii
Immunoprecipitation for the macrophage marker CD14 followed by western blot analysis for M1 marker CD68 and M2 marker CD206 was performed to understand the contribution of M1 and M2 macrophages to the propagation of inflammation. These data show that NIR treatment reduced the expression level of the inflammatory M1 macrophage marker (Ischemia + NIR 5752 ± 154, Ischemia - NIR 6464 ± 213, Control 6524 ± 202) (Figure 6A) and increased the expression level of the protective M2 macrophage marker (Figure 6B) normalized to the expression level of the total macrophage marker (Ischemia + NIR 7378.68 ± 425, Ischemia - NIR 5853.67 ± 215, Control 5542.53 ± 220).
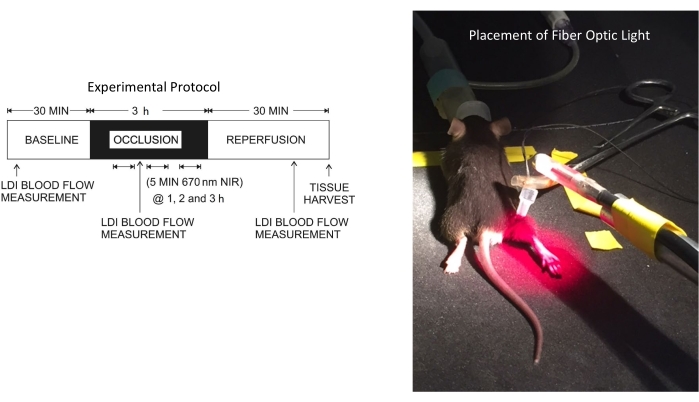
Figure 1: Experimental protocol and the position of the fiber optic light source. Abbreviations: LDI = laser doppler imager; NIR = near-infrared. Please click here to view a larger version of this figure.
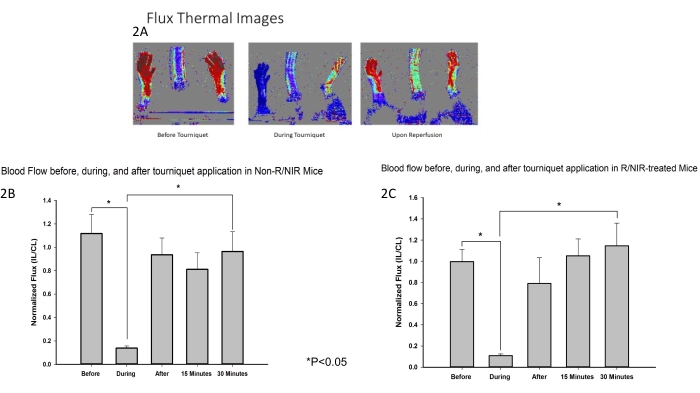
Figure 2: LDI measurements confirmed that the tourniquet was effective at inducing ischemia. (A) Flux Thermal images demonstrate interruption of blood flow and restoration of blood flow upon reperfusion. Analysis of flux images shows the interruption of blood flow during ischemia and the restoration of blood flow upon reperfusion in control (B) and R/NIR-treated mice (C). Abbreviations: LDI = laser doppler imager; R = reperfusion; NIR = near-infrared light. Please click here to view a larger version of this figure.
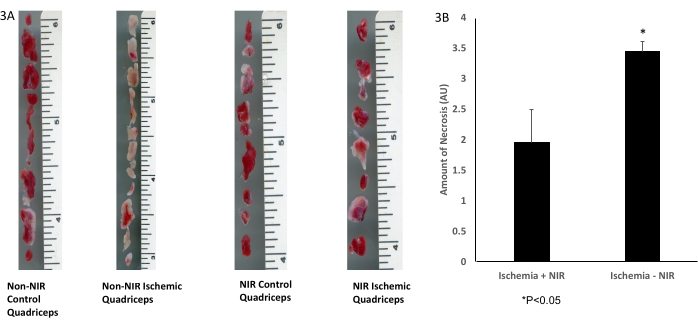
Figure 3: TTC staining of skeletal muscle tissue. (A) TTC staining; (B) analysis shows a significant decrease in infarcted skeletal muscle tissue. The scale bar (inches) depicts the size of the quadriceps muscle. *P ≤ 0.05. Abbreviations: NIR = near-infrared; TTC = 2,3,5-triphenyltetrazolium chloride. Please click here to view a larger version of this figure.
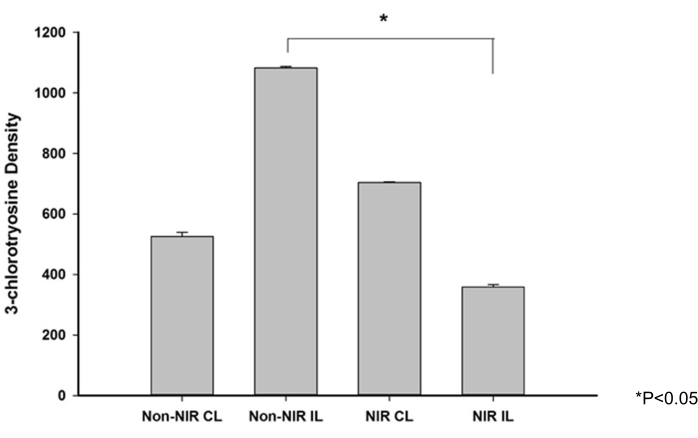
Figure 4: 3-Chlorotyrosine expression levels in skeletal muscle after NIR irradiation. Western blot analysis showing a significant decrease in 3-chlorotyrosine expression levels in skeletal muscle after NIR irradiation. *P ≤ 0.05. Abbreviations: NIR = near-infrared; IL = ischemic limb; CL = contralateral limb. Please click here to view a larger version of this figure.
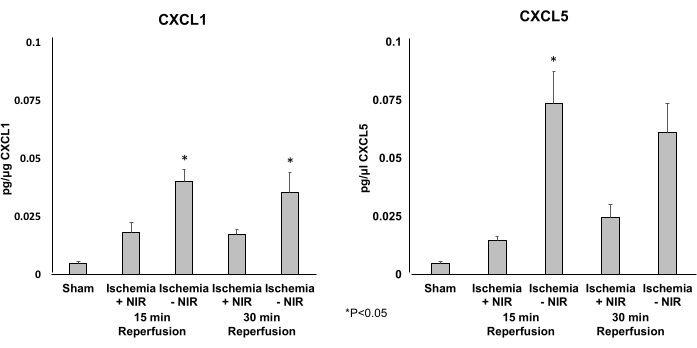
Figure 5: Expression levels of CXCL1 and CXCL5 determined by ELISA. (A) CXCL1 expression levels are significantly decreased at 15 min of reperfusion in the NIR group. This significant effect waned after 30 min when compared to the no-NIR control. (B) CXCL5 expression levels are significantly decreased at 15 and 30 min of reperfusion in the light group compared to the no-light group. *P ≤ 0.05. Abbreviations: NIR = near-infrared; ELISA = enzyme-linked immunosorbent assay. Please click here to view a larger version of this figure.
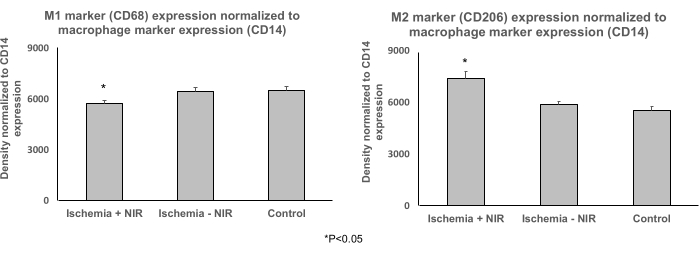
Figure 6: Expression levels of M1 and M2 normalized to the total macrophage marker CD14 determined by immunoprecipitation followed by western blot analysis. (A) Expression level of M1 macrophage marker CD68 normalized to the macrophage marker CD14 expression. Light treatment reduced the expression level of the proinflammatory M1 marker CD68 significantly when compared to no-light treatment and control. (B) Expression level of protective M2 macrophage marker CD206 normalized to the macrophage marker CD14 expression. Light treatment increased the expression of CD206 significantly compared to no-light treatment and control. *P ≤ 0.05. Abbreviation: NIR = near-infrared. Please click here to view a larger version of this figure.
Discussion
This paper describes one of the first studies to focus on the reduction of reperfusion injury by NIR light treatment by changing the inflammatory response in the hindlimb. Ischemia reperfusion injury and NIR light treatment are not entirely novel. Other studies focused on ischemia reperfusion by NIR light. NIR light treatment has been successfully used in the reduction of myocardial infract size and reduction of renal damage after ischemia reperfusion injury. Quirk et al. reported a reduction in myocardial infarct size and reduction of ischemia reperfusion after NIR application16,17,18.
The placement of a tourniquet is of great importance to stop bleeding after injury or create a blood-free area in a clinical setting during surgery and in emergency situations, including the battlefield19. Having prolonged ischemia followed by reperfusion raises the concern of reperfusion injury.
These data show that phototherapy in the form of a 670 nm LED light application reduces reperfusion injury by lowering the inflammatory response and changes macrophages from an inflammatory to an anti-inflammatory phenotype after tourniquet placement. The expression of the inflammatory marker, chlorotyrosine, is elevated after ischemia reperfusion, highlighting the injury (Figure 3). Chlorotyrosine is an oxidation end-product of MPO activity. Yu et al. reported an increase in chlorotyrosine expression as a marker of inflammation in an ischemia reperfusion model of murine stroke20.
Furthermore, Souza et al. described a macrophage phenotype change 24 h after photobiomodulation using the macrophage cell line J774, concluding that NIR treatment modulates the inflammatory phases and improves tissue repair6,21. In addition to recruiting macrophages and various other immune cells, CXCL1 and CXCL5 have significant chemoattractant effects on neutrophils, which are most directly responsible for IRI. MCP-1 was not included in this analysis as it is strictly a monocyte/macrophage chemoattractant. CXCL1 and CXCL5 are produced by various cells, including macrophages, neutrophils, and epithelial cells4,22
The limitation of this study is the penetration depth of NIR light. Hu et al. describe penetration of 5 cm in most tissues using cadavers and comparing different areas23. This paper's approach was to determine the polarization of M1 and M2 macrophages based on the concentration of all macrophages by immunoprecipitation followed by western blot analysis to determine changes in the contribution of M1 and M2 to inflammation after NIR treatment. Immunofluorescence, flow cytometry, and an entire panel of macrophage polarization markers were not performed. These findings show that inflammation in a reperfusion/injury setting was reduced by NIR light treatment and suggest a possible clinical application. The most critical steps in this protocol are the confirmation of occlusion and the application of NIR light once per hour for 5 min throughout the duration of ischemia.
Déclarations de divulgation
The authors have no conflicts of interest to declare.
Remerciements
We thank the Department of Orthopedic Surgery for financing this study. We also thank Brian Lindemer and Grant Broeckel for their technical support.
matériels
| Name | Company | Catalog Number | Comments |
| 2,3,5-Triphenyltetrazolium | Sigma Aldrich | 17779-10X10ML-F | 1% solution |
| 4–15% Criterio TGX Stain-Free Protein Gel | BioRad | #5678084 | Tris-glycine extended gels |
| 4x Laemmli Sample Buffer - 1610747 | BioRad | 16110747 | |
| 670 nm light source | NIR Technologies | custom made | |
| BCA Protein Assay Kit | Thermo Fisher | 23227 | |
| BioRad ChemiDoc | Bio-Rad | Imaging system | |
| Bio-Rad | |||
| β-mercaptoethanol | BioRad | 1610710 | |
| CXCL1 ELISA | R&D Systems | DY453-05 | |
| CXCL5 ELISA | R&D Systems | DX000 | |
| Forane | Baxter | 1001936040 | isoflurane inhalant |
| goat anti-rabbit IgG-HRP | Santa Cruz Biotechnologies | sc-2004 | 1:10,000 dilution |
| Ice Accu ice pack | |||
| Laser doppler Imager | Moor | MOORLDI2-HIR | |
| monoclonal CD14 antibody | Santa Cruz Biotechnologies | sc-515785 | 1:200 dilution |
| monoclonal CD206 antibody | Santa Cruz Biotechnologies | sc-58986 | 1:200 dilution |
| monoclonal CD68 antibody | Santa Cruz Biotechnologies | sc-20060 | 1:200 dilution |
| Pierce Protein free (TBS) blocking buffer | blocking buffer | ||
| polyclonal Chlorotyrosine Antibody | Hycult | HP5002 | 1:1,000 dilution |
| Protein A/G PLUS-Agarose | Santa Cruz Biotechnologies | sc-2003 | |
| Super Signal West Femto | ThermoFisher | 34095 | enhanced chemiluminescence reagent |
Références
- Drysch, M., et al. An optimized low-pressure tourniquet murine hind limb ischemia reperfusion model: Inducing acute ischemia reperfusion injury in C57BL/6 wild type mice. PLoS One. 14 (1), 0210961 (2019).
- Bonheur, J. A., Albadawi, H., Patton, G. M., Watkins, M. T. A noninvasive murine model of hind limb ischemia-reperfusion injury. Journal of Surgical Research. 116 (1), 55-63 (2004).
- Whiteman, M., Spencer, J. P. Loss of 3-chlorotyrosine by inflammatory oxidants: implications for the use of 3-chlorotyrosine as a bio-marker in vivo. Biochemical and Biophysical Research Communications. 371 (1), 50-53 (2008).
- Becker, S., Quay, J., Koren, H. S., Haskill, J. S. Constitutive and stimulated MCP-1, GRO alpha, beta, and gamma expression in human airway epithelium and bronchoalveolar macrophages. American Journal of Physiology. 266 (3), 278-286 (1994).
- Zaidi, M., et al. Transient repetitive exposure to low level light therapy enhances collateral blood vessel growth in the ischemic hindlimb of the tight skin mouse. Photochemistry and Photobiology. 89 (3), 709-713 (2013).
- Souza, N. H. C., et al. Photobiomodulation and different macrophages phenotypes during muscle tissue repair. Journal of Cellular and Molecular Medicine. 22 (10), 4922-4934 (2018).
- Tang, G. L., Kim, K. J. Laser doppler perfusion imaging in the mouse hindlimb. Journal of Visualized Experiments: JoVE. (170), e62012 (2021).
- Ionescu, A., Zahavi, E. E., Gradus, T., Ben-Yaakov, K., Perlson, E. Compartmental microfluidic system for studying muscle-neuron communication and neuromuscular junction maintenance. European Journal of Cell Biology. 95 (2), 69-88 (2016).
- Adamovich, Y., Ezagouri, S., Dandavate, V., Asher, G. Monitoring daytime differences in moderate intensity exercise capacity using treadmill test and muscle dissection. STAR Protocols. 2 (1), 100331 (2021).
- Wang, C., Yue, F., Kuang, S. Muscle histology characterization using H&E staining and muscle fiber type classification using immunofluorescence staining. Bio-Protocol. 7 (10), 2279 (2017).
- Keszler, A., Lindemer, B., Hogg, N., Weihrauch, D., Lohr, N. L. Wavelength-dependence of vasodilation and NO release from S-nitrosothiols and dinitrosyl iron complexes by far red/near infrared light. Archives of Biochemistry and Biophysics. 649, 47-52 (2018).
- Weihrauch, D., et al. Vasodilation of isolated vessels and the isolation of the extracellular matrix of tight-skin mice. Journal of Visualized Experiments: JoVE. (121), e55036 (2017).
- Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 72, 248-254 (1976).
- Li, Z., Jiang, J., Gao, S. Potential of C-X-C motif chemokine ligand 1/8/10/12 as diagnostic and prognostic biomarkers in idiopathic pulmonary arterial hypertension. Clinical Respiratory Journal. , (2021).
- Weihrauch, D., et al. Intralipid increases nitric oxide release from human endothelial cells during oxidative stress. JPEN. Journal of Parenteral and Enteral Nutrition. 45 (2), 295-302 (2021).
- Liebert, A., Krause, A., Goonetilleke, N., Bicknell, B., Kiat, H. A role for photobiomodulation in the prevention of myocardial ischemic reperfusion injury: A systematic review and potential molecular mechanisms. Scientific Reports. 7, 42386 (2017).
- Asghari, A., Takhtfooladi, M. A., Hoseinzadeh, H. A. Effect of photobiomodulation on ischemia/reperfusion-induced renal damage in diabetic rats. Lasers in Medical Science. 31 (9), 1943-1948 (2016).
- Quirk, B. J., Sonowal, P., Jazayeri, M. A., Baker, J. E., Whelan, H. T. Cardioprotection from ischemia-reperfusion injury by near-infrared light in rats. Photomedicine and Laser Surgery. 32 (9), 505-511 (2014).
- Smith, E. R., Shapiro, G. L. Totally tourniquets. The facts & details about different types of tourniquets. JEMS: A Journal of Emergency Medical Services. 38 (11), 48-50 (2013).
- Yu, G., et al. Inhibition of myeloperoxidase oxidant production by N-acetyl lysyltyrosylcysteine amide reduces brain damage in a murine model of stroke. Journal of Neuroinflammation. 13 (1), 119 (2016).
- de Brito Sousa, K., et al. Differential expression of inflammatory and anti-inflammatory mediators by M1 and M2 macrophages after photobiomodulation with red or infrared lasers. Lasers in Medical Science. 35 (2), 337-343 (2020).
- Iida, N., Grotendorst, G. R. Cloning and sequencing of a new gro transcript from activated human monocytes: expression in leukocytes and wound tissue. Molecular and Cellular Biology. 10 (10), 5596-5599 (1990).
- Jani, S., Bergmann, S. R. Metabolic modulation of myocardial ischemia. Current Cardiology Reports. 8 (2), 123-130 (2006).
Réimpressions et Autorisations
Demande d’autorisation pour utiliser le texte ou les figures de cet article JoVE
Demande d’autorisationThis article has been published
Video Coming Soon